Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Design and Breeding
2.3. Sampling and Growth Performance
2.4. Biochemical Assay
2.5. Intestinal Microbiota Analysis
2.6. Transcriptomic Analysis
2.7. Statistical Analysis
3. Results
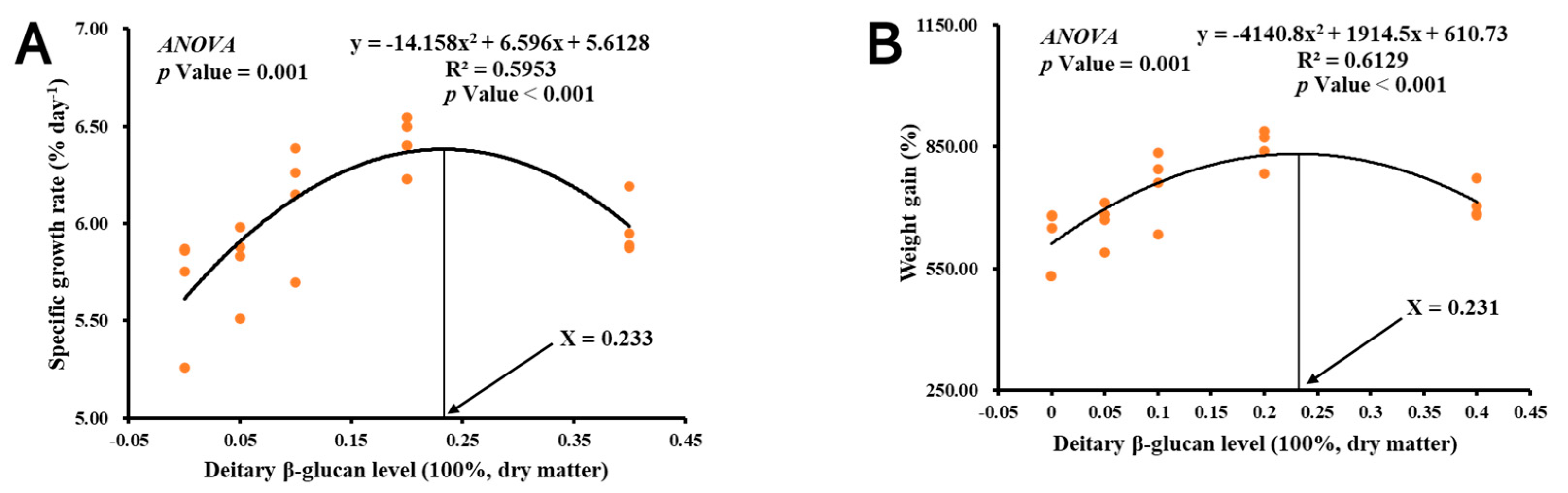
3.1. Growth Performance
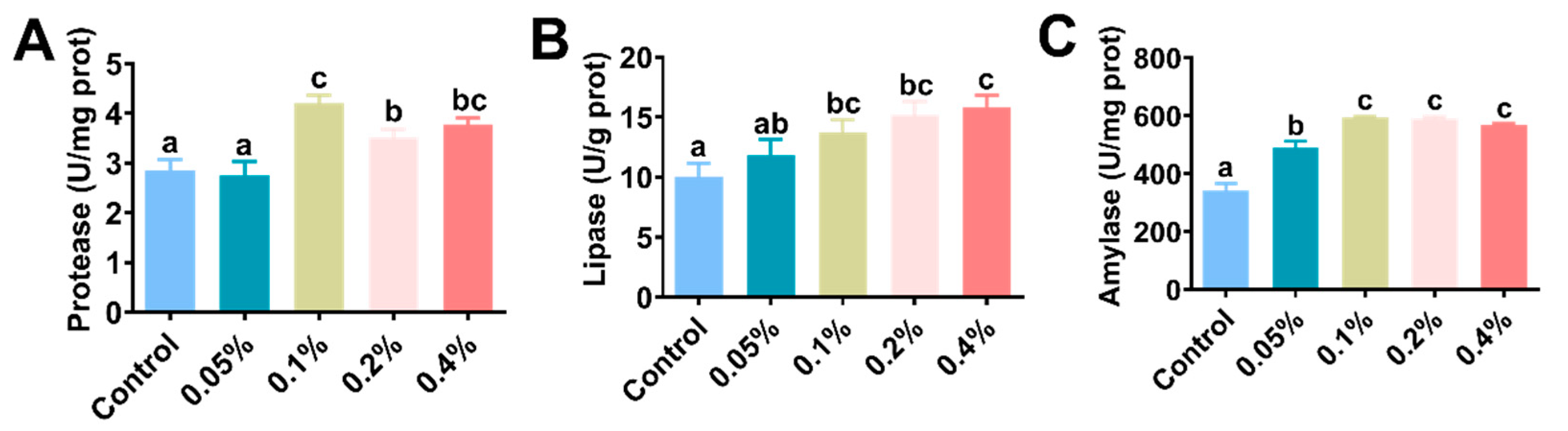
3.2. Hepatopancreatic Digestive Enzyme Activity
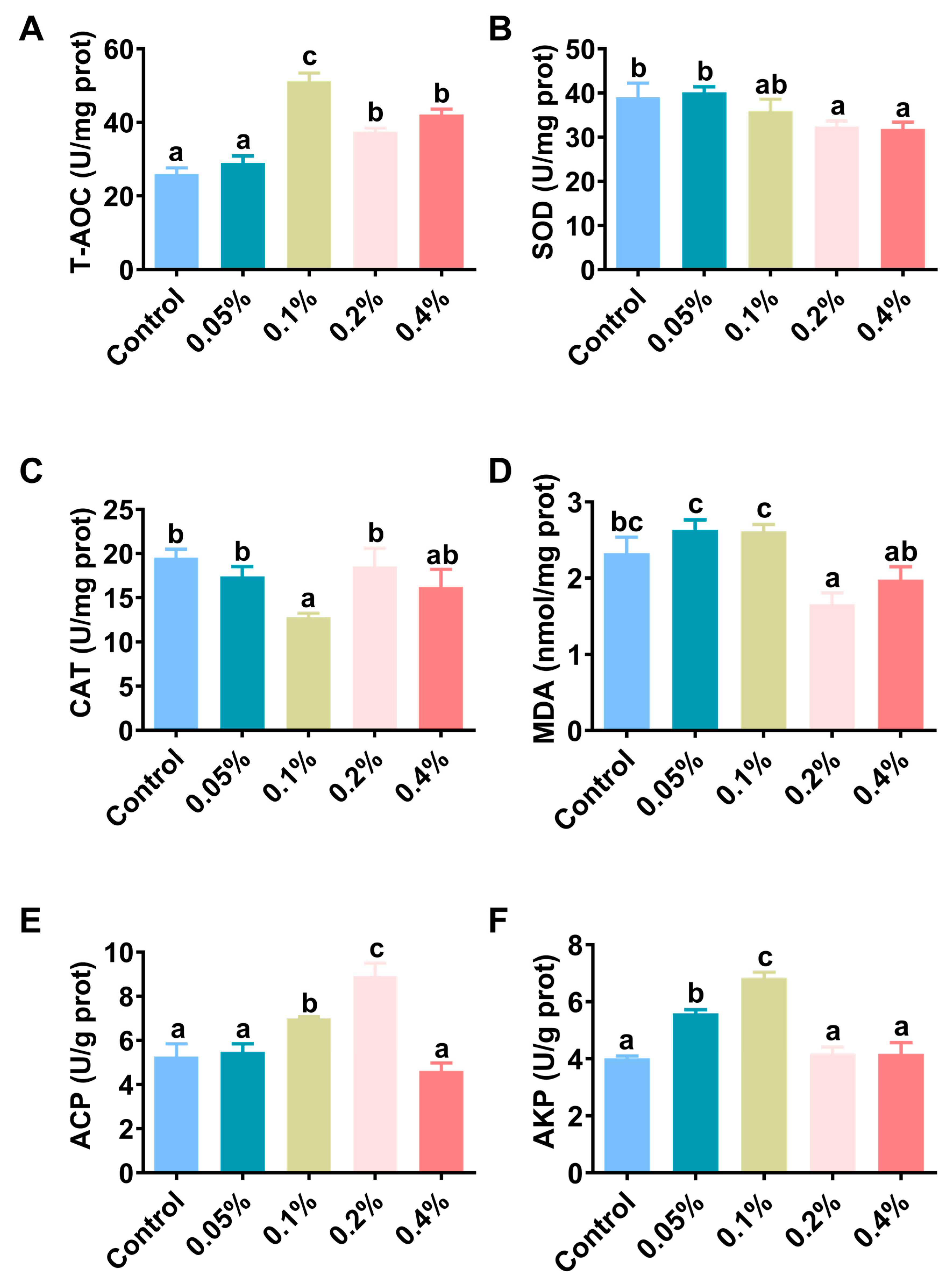
3.3. Antioxidant Capacity and Immunity
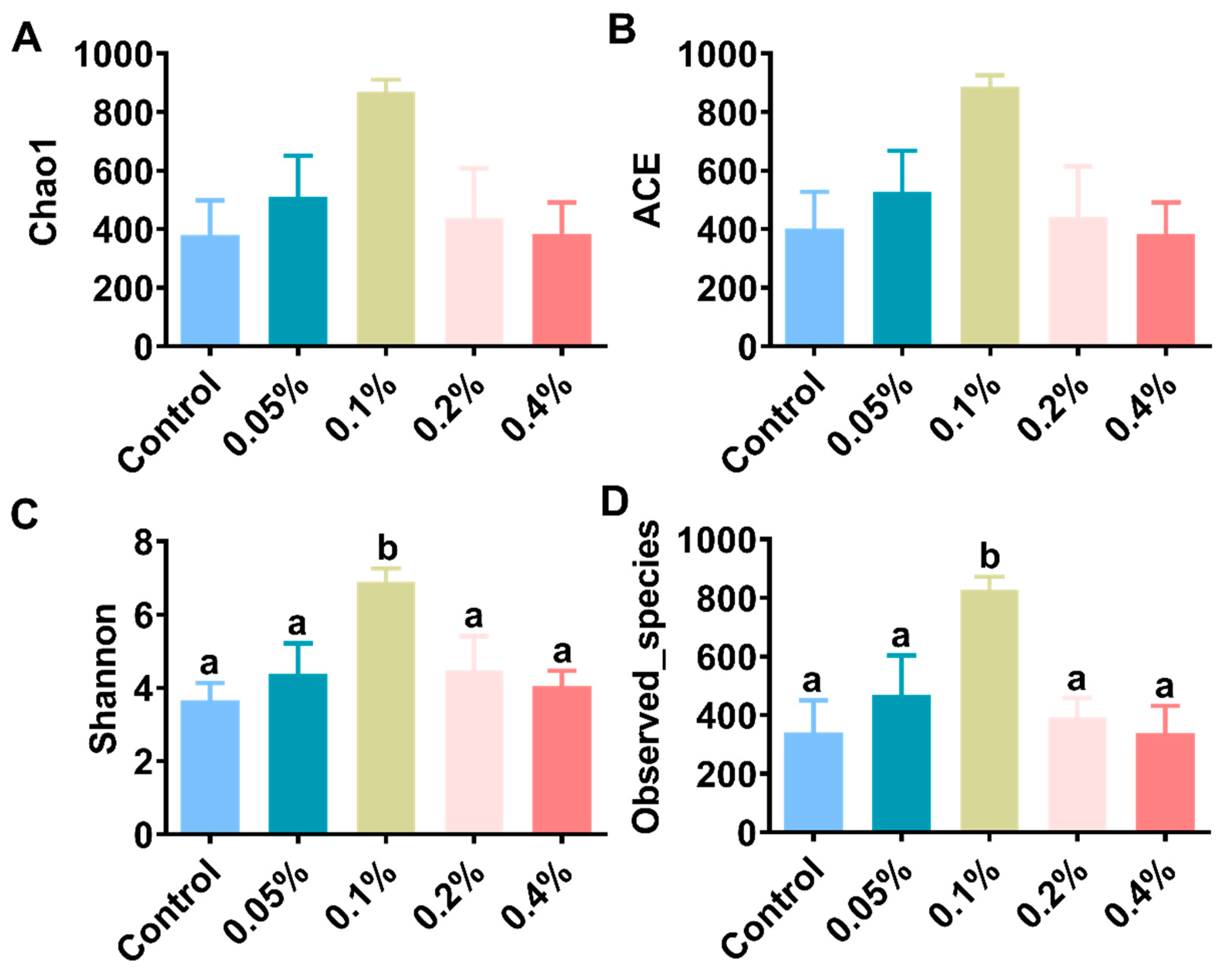
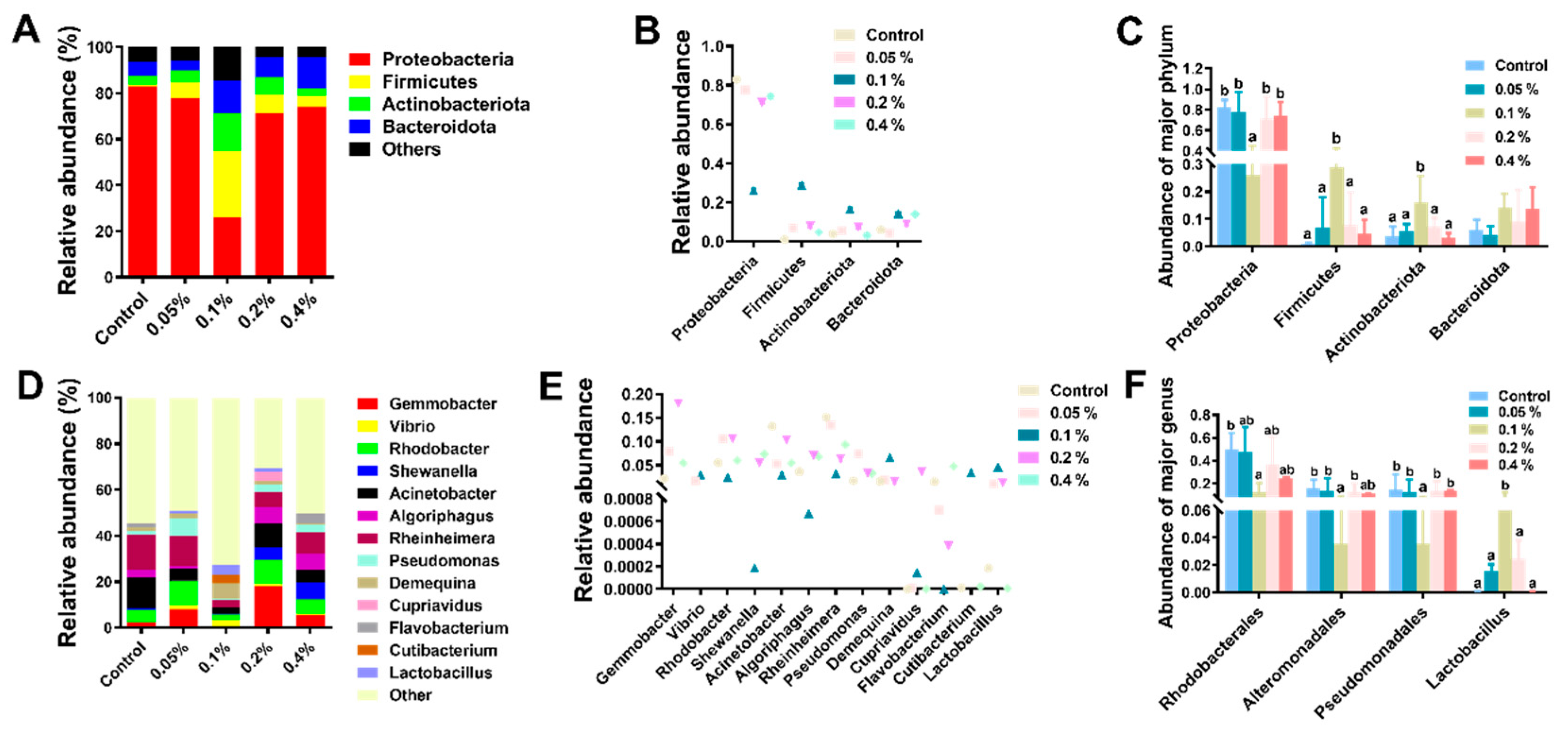
3.4. Intestinal Microbiota Analysis
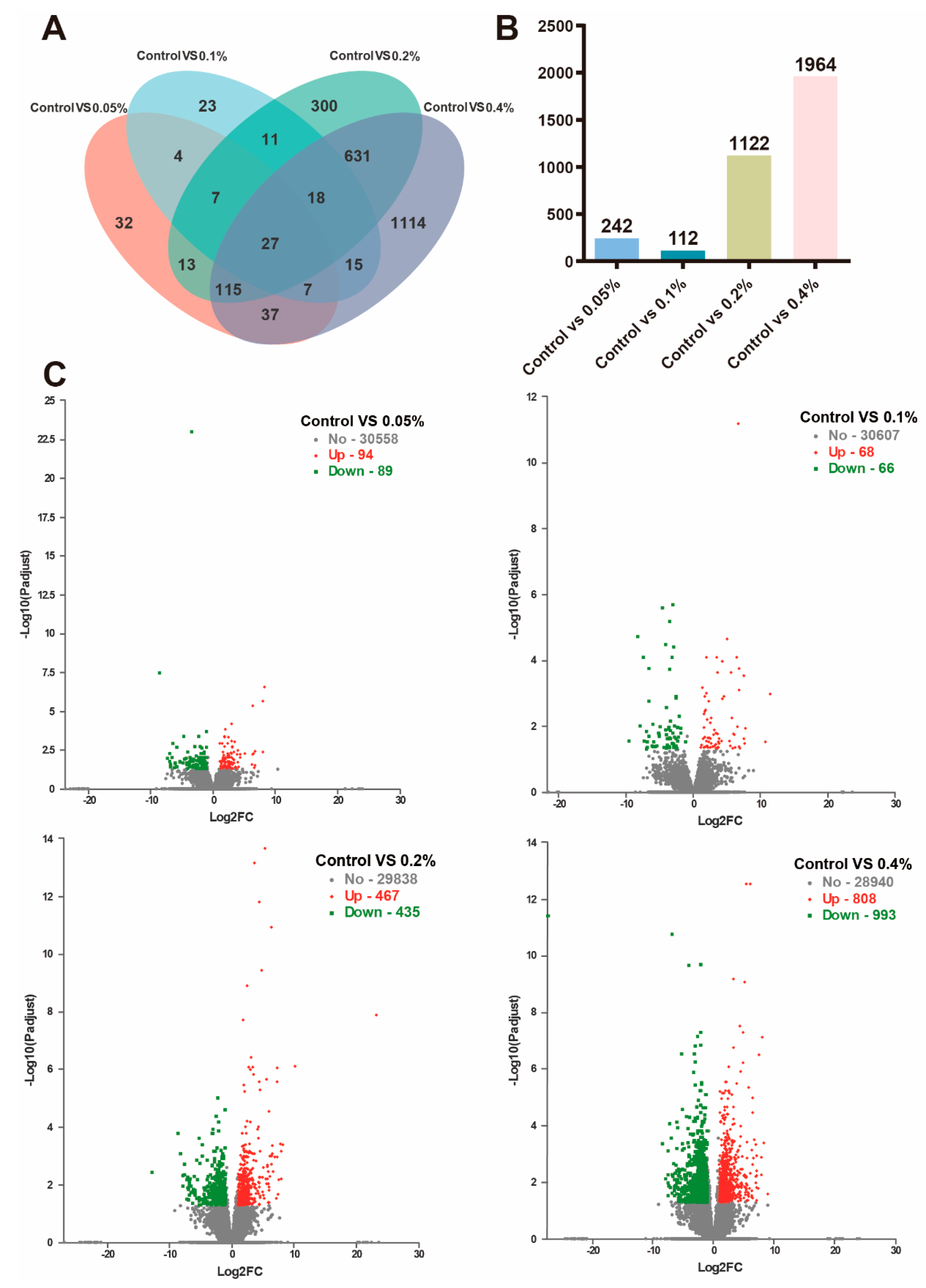
3.5. Transcriptome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saoud, I.; Davis, D.; Rouse, D. Suitability studies of inland well waters for Litopenaeus vannamei culture. Aquaculture 2003, 217, 373–383. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Osmoregulation in decapod crustaceans: Implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 2012, 334–337, 12–23. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture PRC. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Ye, L.; Jiang, S.; Zhu, X.; Yang, Q.; Wen, W.; Wu, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 2009, 290, 140–144. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Chen, X.; Yu, N.; Lai, Q.; Qin, J. Growth, body composition, respiration and ambient ammonia nitrogen tolerance of the juvenile white shrimp, Litopenaeus vannamei, at different salinities. Aquaculture 2007, 265, 385–390. [Google Scholar] [CrossRef]
- Esparza-Leal, H.; Ponce-Palafox, J.; Cervantes-Cervantes, C.; Valenzuela-Quiñónez, W.; Luna-González, A.; López-Álvarez, E.; Vázquez-Montoya, N.; López-Espinoza, M.; Gómez-Peraza, R. Effects of low salinity exposure on immunological, physiological and growth performance in Litopenaeus vannamei. Aquac. Res. 2019, 50, 944–950. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J.; Li, C.; Morni, W.; Suhaili, A.; Kuo, Y.; Chang, Y.; Chen, L.; Tsui, W.; Chen, Y.; et al. Modulation of the innate immune system in white shrimp Litopenaeus vannamei following long-term low salinity exposure. Fish Shellfish Immunol. 2012, 33, 324–331. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J. Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 2003, 224, 193–201. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Ramos-Carreño, S.; Valencia-Yáñez, R.; Correa-Sandoval, F.; Ruíz-García, N.; Díaz-Herrera, F.; Giffard-Mena, I. White spot syndrome virus (WSSV) infection in shrimp (Litopenaeus vannamei) exposed to low and high salinity. Arch. Virol. 2014, 159, 2213–2222. [Google Scholar] [CrossRef]
- Chaiyapechara, S.; Uengwetwanit, T.; Arayamethakorn, S.; Bunphimpapha, P.; Phromson, M.; Jangsutthivorawat, W.; Tala, S.; Karoonuthaisiri, N.; Rungrassamee, W. Understanding the host-microbe-environment interactions: Intestinal microbiota and transcriptomes of black tiger shrimp Penaeus monodon at different salinity levels. Aquaculture 2022, 546, 737371. [Google Scholar] [CrossRef]
- Tian, L.; Tan, P.; Yang, L.; Zhu, W.; Xu, D. Effects of salinity on the growth, plasma ion concentrations, osmoregulation, non-specific immunity, and intestinal microbiota of the yellow drum (Nibea albiflora). Aquaculture 2020, 528, 735470. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Zhou, L.; Dong, Y.; Li, E. Beneficial effects of dietary β-glucan on growth and health status of Pacific white shrimp Litopenaeus vannamei at low salinity. Fish Shellfish Immunol. 2019, 91, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.; Das, P.; Kumar, S.; Mandal, S.; Prusty, A.; Singh, S.; Akhtar, M.; Behera, B.; Kumar, K.; Pal, A. Beta-glucan: An ideal immunostimulant in aquaculture (a review). Fish Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirano, R.; Sakai, Y.; Choi, M.; Sakanaka, M.; Kurihara, S.; Iino, H.; Xiao, J.; Katayama, T.; Odamaki, T. Bifidobacterium response to lactulose ingestion in the gut relies on a solute-binding protein-dependent ABC transporter. Commun. Biol. 2021, 4, 541. [Google Scholar] [CrossRef]
- Xie, S.; Wei, D.; Yin, P.; Zheng, L.; Guo, T.; Liu, Y.; Tian, L.; Niu, J. Dietary replacement of fish-meal impaired protein synthesis and immune response of juvenile Pacific white shrimp, Litopenaeus vannamei at low salinity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 228, 26–33. [Google Scholar] [CrossRef]
- Abdel-Latif, H.; El-Ashram, S.; Yilmaz, S.; Naiel, M.; Abdul Kari, Z.; Hamid, N.; Dawood, M.; Nowosad, J.; Kucharczyk, D. The effectiveness of Arthrospira platensis and microalgae in relieving stressful conditions affecting finfish and shellfish species: An overview. Aquac. Rep. 2022, 24, 101135. [Google Scholar] [CrossRef]
- Tang, S.; Han, F.; Zhou, L.; Liu, S.; Xu, C.; Chen, L.; Li, E. Regulation of mannan oligosaccharide on growth, health and intestinal microbiota of Litopenaeus vannamei at low salinity. J. Fish China 2021, 45, 2044–2060. [Google Scholar]
- Kuo, H.; Chang, C.; Cheng, W. Synbiotic combination of prebiotic, cacao pod husk pectin and probiotic, Lactobacillus plantarum, improve the immunocompetence and growth of Litopenaeus vannamei. Fish Shellfish Immunol. 2021, 118, 333–342. [Google Scholar] [CrossRef]
- Dawood, M.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.; Hossain, M.; Nhu, T.; Moss, A.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Rajan, D. Potential uses of fungal polysaccharides as immunostimulants in fish and shrimp aquaculture: A review. Aquaculture 2019, 500, 250–263. [Google Scholar] [CrossRef]
- Luan, L.; Vu, N.; Nghia, N.; Thao, N. Synergic degradation of yeast β-glucan with a potential of immunostimulant and growth promotor for tiger shrimp. Aquac. Rep. 2021, 21, 100858. [Google Scholar] [CrossRef]
- Pooljun, C.; Jariyapong, P.; Wongtawan, T.; Hirono, I.; Wuthisuthimethavee, S. Effect of feeding different types of β-glucans derived from two marine diatoms (Chaetoceros muelleri and Thalassiosira weissflogii) on growth performance and immunity of banana shrimp (Penaeus merguiensis). Fish Shellfish Immunol. 2022, 130, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.; Li, P.; Lawrence, A.; Gatlin, D. Dietary β-glucan and nucleotide effects on growth, survival and immune responses of pacific white shrimp, Litopenaeus vannamei. J. Appl. Aquac. 2009, 21, 160–168. [Google Scholar] [CrossRef]
- Do Huu, H.; Sang, H.; Thuy, N. Dietary β-glucan improved growth performance, Vibrio counts, haematological parameters and stress resistance of pompano fish, Trachinotus ovatus Linnaeus, 1758. Fish Shellfish Immunol. 2016, 54, 402–410. [Google Scholar] [CrossRef]
- da Silva, B.; do Nascimento Vieira, F.; Mouriño, J.; Ferreira, G.; Seiffert, W. Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture 2013, 384, 104–110. [Google Scholar] [CrossRef]
- Wongsasak, U.; Chaijamrus, S.; Kumkhong, S.; Boonanuntanasarn, S. Effects of dietary supplementation with β-glucan and synbiotics on immune gene expression and immune parameters under ammonia stress in Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2015, 436, 179–187. [Google Scholar] [CrossRef]
- Miest, J.; Arndt, C.; Adamek, M.; Steinhagen, D.; Reusch, T. Dietary β-glucan (MacroGard®) enhances survival of first feeding turbot (Scophthalmus maximus) larvae by altering immunity, metabolism and microbiota. Fish Shellfish Immunol. 2016, 48, 94–104. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Gul, Y.; Li, Q.; Song, J.; Hu, M. Effects of copper supplement on the immune function and blood-chemistry in adult Chinese horseshoe crab Tachypleus tridentatus. Aquaculture 2020, 515, 734576. [Google Scholar] [CrossRef]
- Yu, Q.; Fu, Z.; Huang, M.; Xu, C.; Wang, X.; Qin, J.; Chen, L.; Han, F.; Li, E. Growth, physiological, biochemical, and molecular responses of Pacific white shrimp Litopenaeus vannamei fed different levels of dietary selenium. Aquaculture 2021, 535, 736393. [Google Scholar] [CrossRef]
- Davis, M. Contrast Coding in Multiple Regression Analysis: Strengths, Weaknesses, and Utility of Popular Coding Structures. J. Dairy Sci. 2010, 8, 61–73. [Google Scholar] [CrossRef]
- Chen, K.; Li, E.; Xu, C.; Wang, X.; Lin, H.; Qin, J.; Chen, L. Evaluation of different lipid sources in diet of pacific white shrimp Litopenaeus vannamei at low salinity. Aquac. Rep. 2015, 2, 163–168. [Google Scholar] [CrossRef]
- Felix, N.; Jeyaseelan, M.; Kirubakaran, C. Growth improvement and enhanced disease resistance against Vibrio alginolyticus using-glucan as a dietary supplement for Penaeus monodon (Fabricius). Indian J. Fish 2008, 55, 247–250. [Google Scholar]
- Xu, B.; Wang, Y.; Li, J.; Lin, Q. Effect of prebiotic xylooligosaccharides on growth performances and digestive enzyme activities of allogynogenetic crucian carp (Carassius auratus gibelio). Fish Physiol. Biochem. 2009, 35, 351–357. [Google Scholar] [CrossRef]
- Akter, M.; Sutriana, A.; Talpur, A.; Hashim, R. Dietary supplementation with mannan oligosaccharide influences growth, digestive enzymes, gut morphology, and microbiota in juvenile striped catfish, Pangasianodon hypophthalmus. Aquac. Int. 2016, 24, 127–144. [Google Scholar] [CrossRef]
- Li, E.; Wang, X.; Chen, K.; Xu, C.; Qin, J.; Chen, L. Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev. Aquac. 2017, 9, 57–75. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, A.; Wang, J.; Sun, R. Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei (Boone, 1931) exposed to acute salinity changes. Aquaculture 2007, 265, 351–358. [Google Scholar] [CrossRef]
- Köhrle, J. The deiodinase family: Selenoenzymes regulating thyroid hormone availability and action. Cell Mol. Life Sci. 2000, 57, 1853–1863. [Google Scholar] [CrossRef]
- Chang, C.; Su, M.; Chen, H.; Liao, I. Dietary β-1, 3-glucan effectively improves immunity and survival of Penaeus monodon challenged with white spot syndrome virus. Fish Shellfish Immunol. 2003, 15, 297–310. [Google Scholar] [CrossRef]
- Xing, J.; Lin, T.; Zhan, W. Variations of enzyme activities in the haemocytes of scallop Chlamys farreri after infection with the acute virus necrobiotic virus (AVNV). Fish Shellfish Immunol. 2008, 25, 847–852. [Google Scholar] [CrossRef]
- Chang, C.; Huang, S.; Chen, S.; Chen, S. Innate immune responses and efficacy of using mushroom beta-glucan mixture (MBG) on orange-spotted grouper, Epinephelus coioides, aquaculture. Fish Shellfish Immunol. 2013, 35, 115–125. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, G.; Wang, L.; Sagada, G.; Zhang, J.; Shao, Q. The influence of dietary β-1, 3-glucan on growth performance, feed utilization, antioxidative and immune status of Pacific white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2021, 27, 1590–1601. [Google Scholar] [CrossRef]
- Sonnenburg, E.; Sonnenburg, J.; Manchester, J.; Hansen, E.; Chiang, H.; Gordon, J. A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism. Proc. Natl. Acad. Sci. USA 2006, 103, 8834–8839. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Mithieux, G. Gut-brain signaling in energy homeostasis: The unexpected role of microbiota-derived succinate. J. Endocrinol. 2018, 236, R105–R108. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.; Chen, L. Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Rev. Fish Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Pinoargote, G.; Flores, G.; Cooper, K.; Ravishankar, S. Effects on survival and bacterial community composition of the aquaculture water and gastrointestinal tract of shrimp (Litopenaeus vannamei) exposed to probiotic treatments after an induced infection of acute hepatopancreatic necrosis disease. Aquac. Res. 2018, 49, 3270–3288. [Google Scholar] [CrossRef] [Green Version]
- Boonanuntanasarn, S.; Wongsasak, U.; Pitaksong, T.; Chaijamrus, S. Effects of dietary supplementation with β-glucan and synbiotics on growth, haemolymph chemistry, and intestinal microbiota and morphology in the Pacific white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2016, 22, 837–845. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Duan, F.; Liu, A.; Li, S.; Zhong, W.; Sheng, W.; Chen, J.; Xu, J.; Xiao, S. Prebiotics enhance the biotransformation and bioavailability of ginsenosides in rats by modulating gut microbiota. J. Ginseng Res. 2021, 45, 334–343. [Google Scholar] [CrossRef]
- Nie, L.; Zhou, Q.; Qiao, Y.; Chen, J. Interplay between the gut microbiota and immune responses of ayu (Plecoglossus altivelis) during Vibrio anguillarum infection. Fish Shellfish Immunol. 2017, 68, 479–487. [Google Scholar] [CrossRef]
- Siriyappagouder, P.; Galindo, J.; Lokesh, J.; Mulero, V.; Fernandes, J.; Kiron, V. Exposure to yeast shapes the intestinal bacterial community assembly in zebrafish larvae. Front. Microbiol. 2018, 9, 1868. [Google Scholar] [CrossRef] [Green Version]
- Ringø, E.; Olsen, R.; Gifstad, T.; Dalmo, R.; Amlund, H.; Hemre, G.; Bakke, A. Prebiotics in aquaculture: A review. Aquac. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Hornett, E.; Wheat, C. Quantitative RNA-Seq analysis in non-model species: Assessing transcriptome assemblies as a scaffold and the utility of evolutionary divergent genomic reference species. BMC Genom. 2012, 13, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Yang, H.; Sun, L.; Chen, M. Transcription profiling using RNA-Seq demonstrates expression differences in the body walls of juvenile albino and normal sea cucumbers Apostichopus japonicus. China J. Oceanol. Limnol. 2014, 32, 34–46. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Li, C.; Chen, K.; Qin, J.; Chen, L.; Li, E. Molecular pathway and gene responses of the pacific white shrimp Litopenaeus vannamei to acute low salinity stress. J. Shellfish Res. 2015, 34, 1037–1048. [Google Scholar] [CrossRef]
- Zhuo, X.; Qin, Y.; He, P.; Wei, P.; Zhang, B.; Chen, X.; Peng, J. Transcriptomic analysis of Litopenaeus vannamei hepatopancreas under cold stress in cold-tolerant and cold-sensitive cultivars. Gene 2021, 764, 145090. [Google Scholar] [CrossRef]
- Nappi, A.; Vass, E.; Frey, F.; Carton, Y. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol. 1995, 68, 450–456. [Google Scholar]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Dev. Comp. Immunol. 2018, 80, 81–93. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, H.; Zhu, K.; Guo, L.; Zhang, N.; Liu, B.; Yang, J.; Liu, B.; Jiang, S.; Zhang, D. Molecular characterization of Na+/K+/2Cl− cotransporter 1 alpha from Trachinotus ovatus (Linnaeus, 1758) and its expression responses to acute salinity stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 223, 29–38. [Google Scholar] [CrossRef]
- Lee, J.; Cho, B.; Park, J. Transcriptomic analysis of brine shrimp Artemia franciscana across a wide range of salinities. Mar. Genom. 2022, 61, 100919. [Google Scholar] [CrossRef]
- Gobinath, D.; Madhu, A.; Prashant, G.; Srinivasan, K.; Prapulla, S. Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br. J. Nutr. 2010, 104, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Lokesh, J.; Ghislain, M.; Reyrolle, M.; Bechec, M.; Pigot, T.; Terrier, F.; Roy, J.; Panserat, S.; Ricaud, K. Prebiotics modify host metabolism in rainbow trout (Oncorhynchus mykiss) fed with a total plant-based diet: Potential implications for microbiome-mediated diet optimization. Aquaculture 2022, 561, 738699. [Google Scholar] [CrossRef]

| Ingredients (%) | Dietary β-Glucan Concentrations | ||||
|---|---|---|---|---|---|
| 0 | 0.05% | 0.1% | 0.2% | 0.4% | |
| Fish meal | 26 | 26 | 26 | 26 | 26 |
| Soybean meal | 28 | 28 | 28 | 28 | 28 |
| Com starch | 23 | 23 | 23 | 23 | 23 |
| Shrimp meal | 4 | 4 | 4 | 4 | 4 |
| Calcium dihydrogen phosphate | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin premix a | 2 | 2 | 2 | 2 | 2 |
| Mineral premix b | 2 | 2 | 2 | 2 | 2 |
| Choline chloride | 1 | 1 | 1 | 1 | 1 |
| Fish oil | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean oil | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean lecithin | 1 | 1 | 1 | 1 | 1 |
| Cholesterol | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Carboxymethylcellulose (CMC) | 3 | 3 | 3 | 3 | 3 |
| Butylated hydroxytoluene (BHT) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Microcrystalline cellulose | 2.9 | 2.85 | 2.8 | 2.7 | 2.5 |
| β-1,3-Glucan c | 0 | 0.05 | 0.1 | 0.2 | 0. 4 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Nutrient levels (%) | |||||
| Crude protein | 35.2 | 35.4 | 35.5 | 35.5 | 35.4 |
| Crude lipid | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 |
| Ash | 10.3 | 10.4 | 10.7 | 10.7 | 10.6 |
| Moisture | 9.2 | 9.2 | 9.2 | 9.2 | 9.3 |
| Dietary β-Glucan Levels (%) a | IW (g) | FW (g) | WG (%) | SGR (% Day−1) | Survival (%) |
|---|---|---|---|---|---|
| 0 | 0.27 | 1.91 ± 0.07 a | 634.08 ± 35.42 a | 5.68 ± 0.14 a | 90 ± 4.56 |
| 0.05 | 0.27 | 2.01 ± 0.09 a | 663.15 ± 26.37 ab | 5.80 ± 0.10 ab | 90 ± 2.04 |
| 0.1 | 0.26 | 2.23 ± 0.09 b | 756.20 ± 43.09 bc | 6.12 ± 0.15 bc | 95 ± 3.54 |
| 0.2 | 0.25 | 2.36 ± 0.04 b | 845.86 ± 22.82 c | 6.42 ± 0.07 c | 90 ± 1.44 |
| 0.4 | 0.25 | 1.92 ± 0.02 a | 710.36 ± 21.32 ab | 5.98 ± 0.07 ab | 92.5 ± 1.25 |
| ANOVA (p value) | 0.533 | 0.001 | 0.002 | 0.003 | 0.444 |
| SOP (n = 4) | |||||
| Adj. R2 | 0.6423 | 0.5365 | 0.0257 | ||
| p Value | <0.001 | <0.001 | <0.001 |
| Enzyme a | Regression Analysis (n = 4) b | Enzyme | Regression Analysis (n = 4) | ||
|---|---|---|---|---|---|
| SOP | SOP | ||||
| Adj. R2 | p Value | Adj. R2 | p Value | ||
| T-AOC | 0.3935 | 0.046 | Protease | 0.326 | 0.035 |
| SOD | 0.3979 | 0.695 | Lipase | 0.5556 | 0.002 |
| CAT | 0.056 | 0.567 | Amylase | 0.8093 | 0.005 |
| MDA | 0.2971 | 0.023 | |||
| ACP | 0.7077 | 0.765 | |||
| AKP | 0.2194 | 0.193 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Zhou, L.; Qu, Y.; Lu, K.; Han, F.; Li, E. Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity. Antioxidants 2022, 11, 2282. https://doi.org/10.3390/antiox11112282
Qiao Y, Zhou L, Qu Y, Lu K, Han F, Li E. Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity. Antioxidants. 2022; 11(11):2282. https://doi.org/10.3390/antiox11112282
Chicago/Turabian StyleQiao, Yanbing, Li Zhou, Yayu Qu, Kunyu Lu, Fenglu Han, and Erchao Li. 2022. "Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity" Antioxidants 11, no. 11: 2282. https://doi.org/10.3390/antiox11112282
APA StyleQiao, Y., Zhou, L., Qu, Y., Lu, K., Han, F., & Li, E. (2022). Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity. Antioxidants, 11(11), 2282. https://doi.org/10.3390/antiox11112282









