Combined Treatment (Ultraviolet-C/Physapruin A) Enhances Antiproliferation and Oxidative-Stress-Associated Mechanism in Oral Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. UVC Irradiation and Drug Treatments
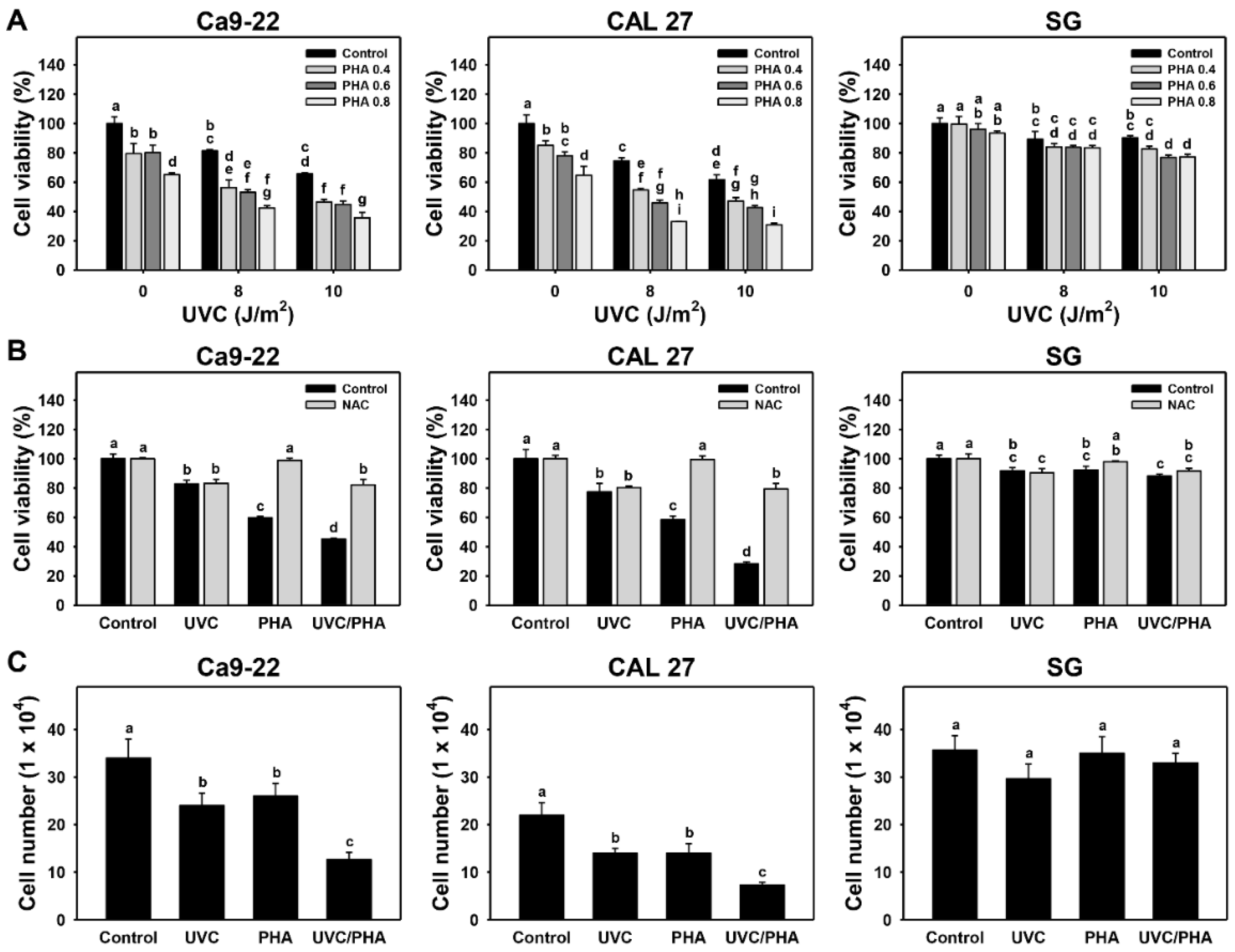
2.3. Cell Survival and Cell Number Assays and Synergy Determination
2.4. Cell Cycle Assays
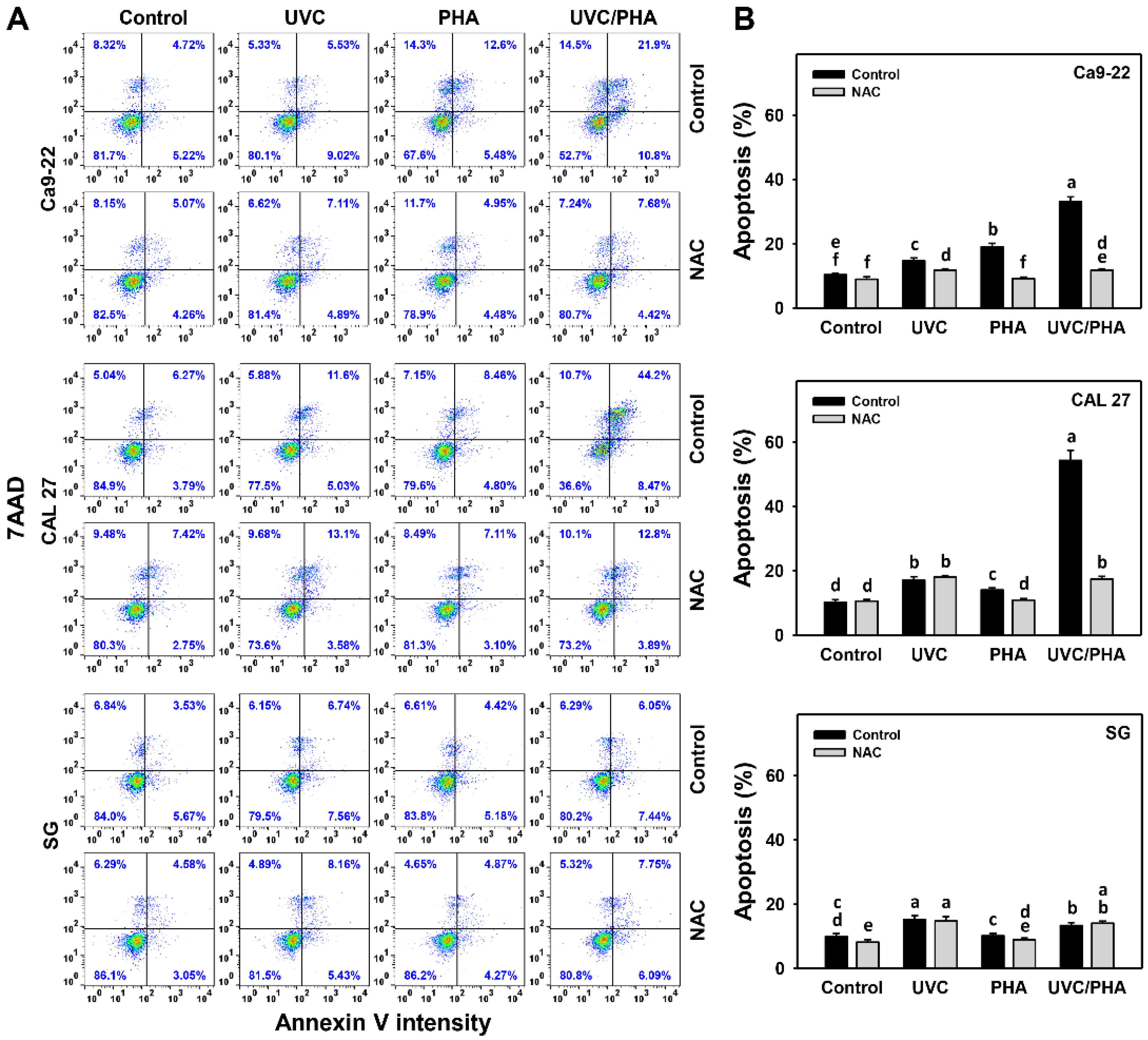
2.5. Annexin V Assay and Caspase 3/7 (Cas 3/7) Activity
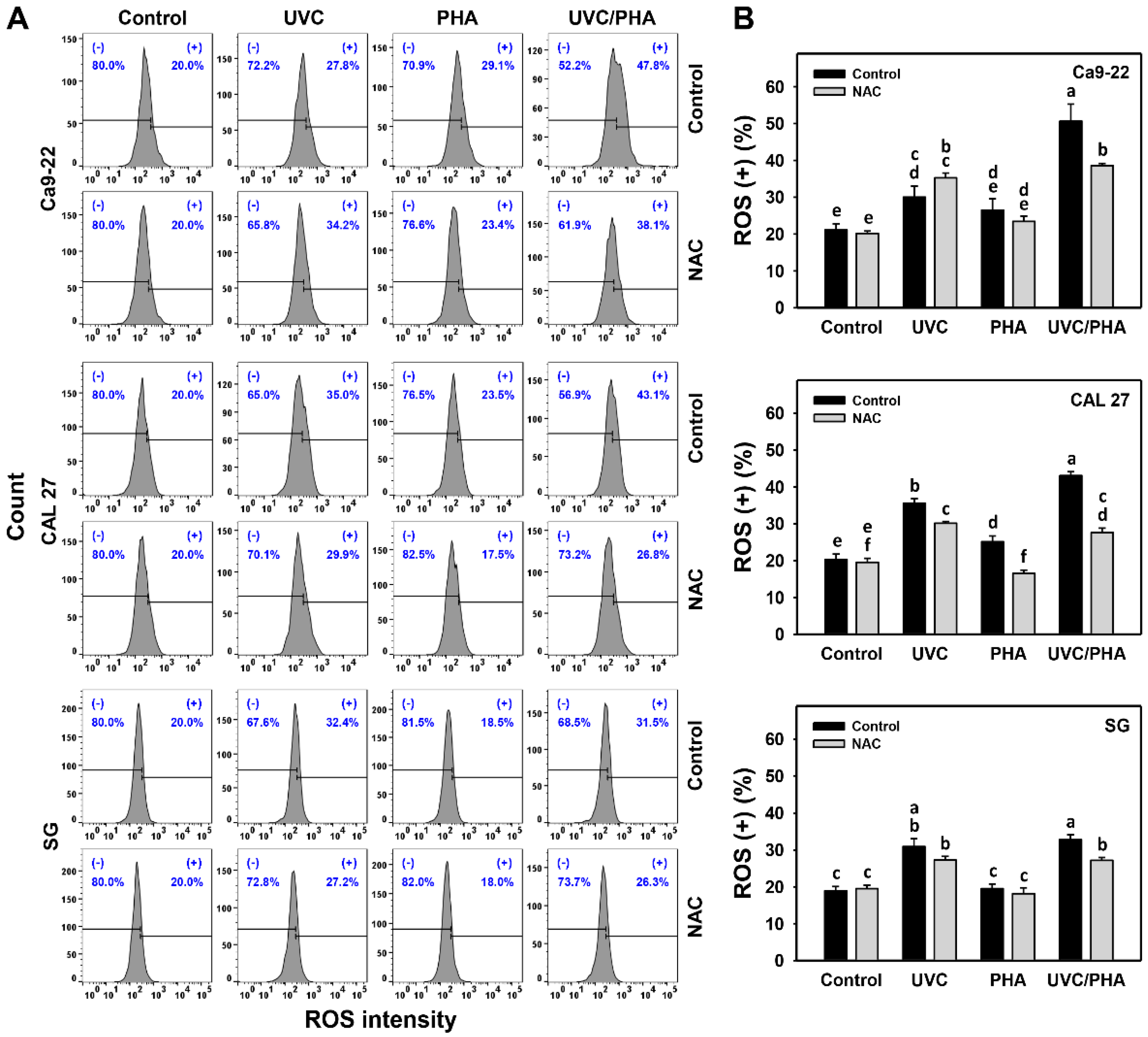
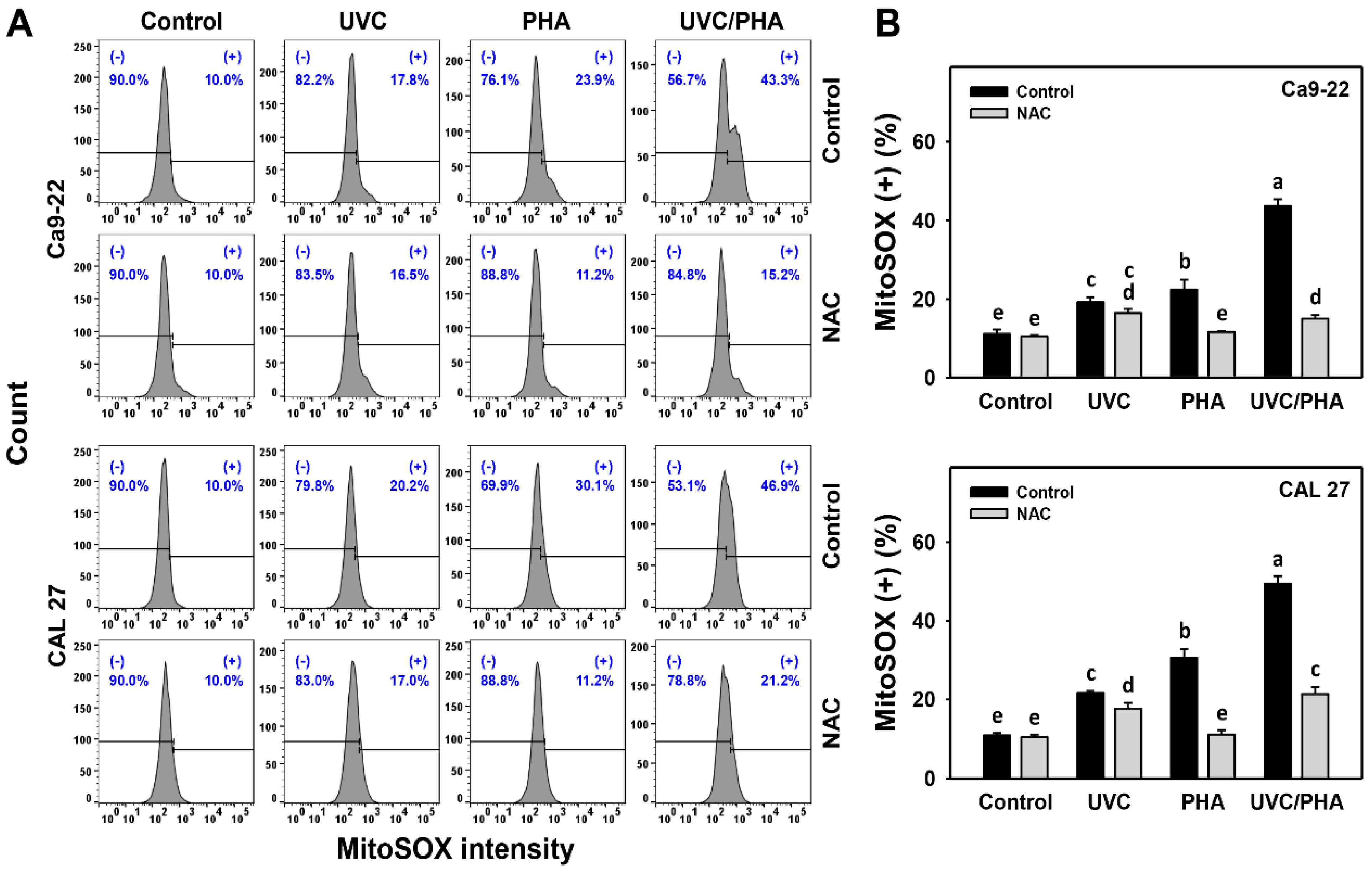
2.6. ROS and Mitochondrial Superoxide (MitoSOX) Assays
2.7. Mitochondrial Membrane Potential (MitoMP) Assays
2.8. γH2AX and 8-Hydroxyl-2’-Deoxyguanosine (8-OHdG) Assays
2.9. Statistics
3. Results
3.1. Proliferation-Modulating Effects of UVC/PHA
3.2. Cell-Cycle-Modulating Effects of UVC/PHA
3.3. Apoptosis-Modulating Effects of UVC/PHA
3.4. ROS-Modulating Effects of UVC/PHA
3.5. MitoSOX-Modulating Effects of UVC/PHA
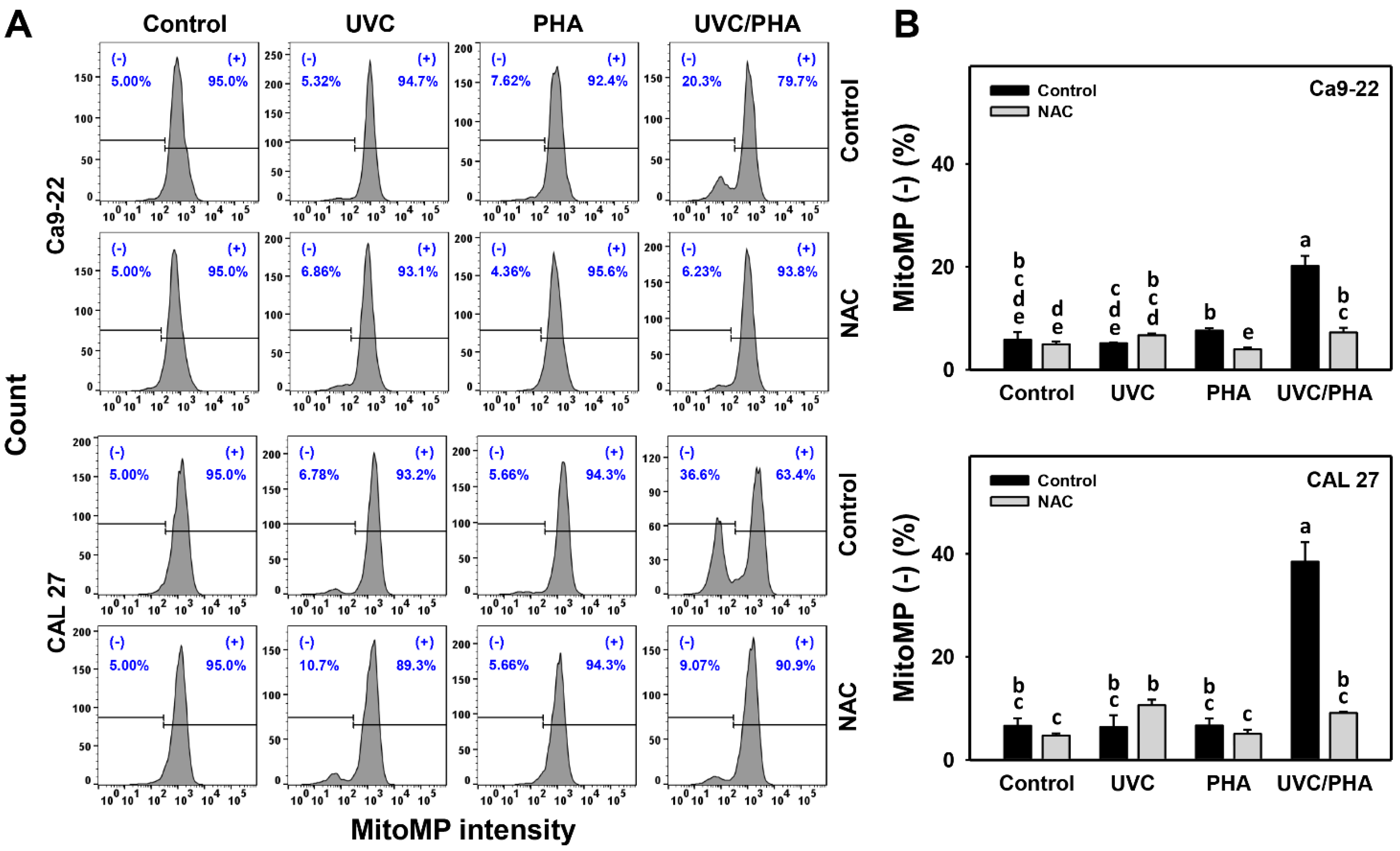
3.6. MitoMP-Modulating Effects of UVC/PHA
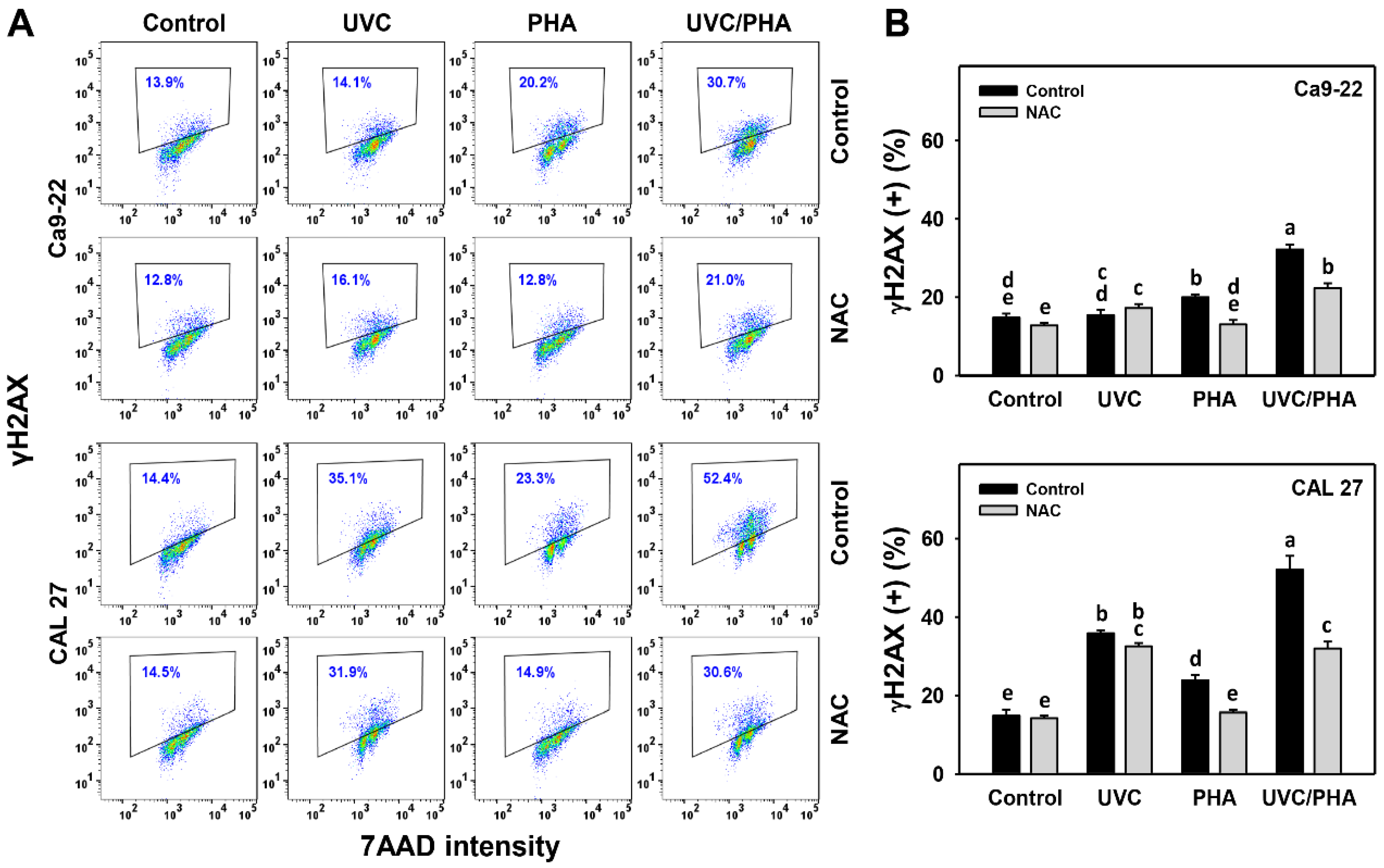
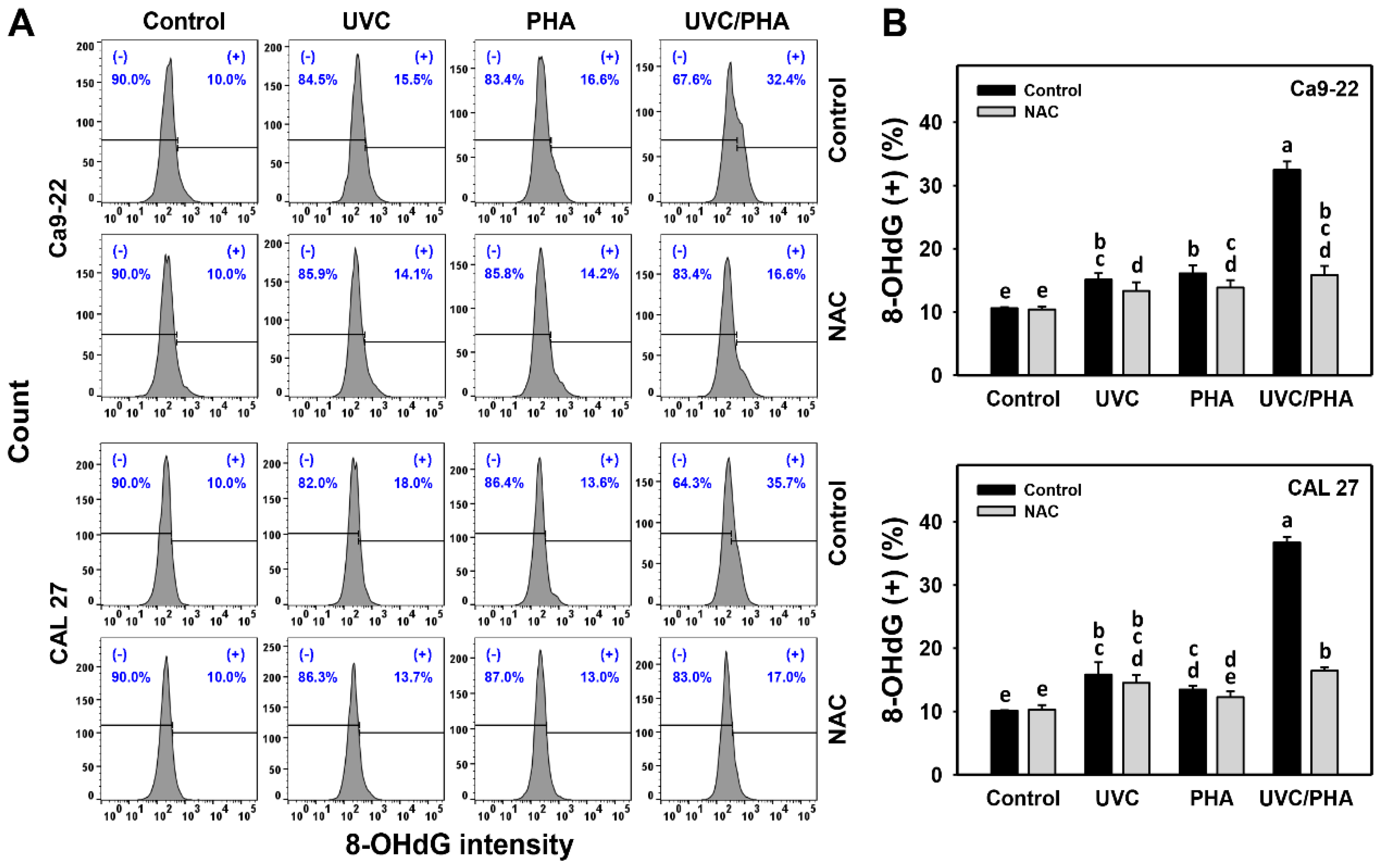
3.7. DNA-Damage-Modulating Effects of UVC/PHA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Galvis, M.; Loveless, R.; Kowalski, L.P.; Teng, Y. Impacts of environmental factors on head and neck cancer pathogenesis and progression. Cells 2021, 10, 389. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e233–e240. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Richardson, R.A.; Dewhirst, M.W. Hypoxia and radiotherapy: Opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007, 26, 241–248. [Google Scholar] [CrossRef]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Nisar, S.; Masoodi, T.; Prabhu, K.S.; Kuttikrishnan, S.; Zarif, L.; Khatoon, S.; Ali, S.; Uddin, S.; Akil, A.A.; Singh, M.; et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomed. Pharmacother. 2022, 154, 113610. [Google Scholar] [CrossRef]
- Akter, R.; Najda, A.; Rahman, M.H.; Shah, M.; Wesolowska, S.; Hassan, S.S.U.; Mubin, S.; Bibi, P.; Saeeda, S. Potential role of natural products to combat radiotherapy and their future perspectives. Molecules 2021, 26, 5997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- He, M.; Wang, N.; Zheng, W.; Cai, X.; Qi, D.; Zhang, Y.; Han, C. Ameliorative effects of ginsenosides on myelosuppression induced by chemotherapy or radiotherapy. J. Ethnopharmacol. 2021, 268, 113581. [Google Scholar] [CrossRef]
- Yamauchi, T.; Adachi, S.; Yasuda, I.; Nakashima, M.; Kawaguchi, J.; Yoshioka, T.; Hirose, Y.; Kozawa, O.; Moriwaki, H. Ultra-violet irradiation induces apoptosis via mitochondrial pathway in pancreatic cancer cells. Int. J. Oncol. 2011, 39, 1375–1380. [Google Scholar]
- Adachi, S.; Yasuda, I.; Nakashima, M.; Yamauchi, T.; Kawaguchi, J.; Shimizu, M.; Itani, M.; Nakamura, M.; Nishii, Y.; Yoshioka, T.; et al. Ultraviolet irradiation can induce evasion of colon cancer cells from stimulation of epidermal growth factor. J. Biol. Chem. 2011, 286, 26178–26187. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Tang, J.Y.; Li, R.N.; Huang, H.W.; Wu, C.Y.; Chiu, C.C.; Chang, F.R.; Zhang, H.W.; Lee, Y.J.; Sheu, J.H.; et al. Oxidative stress-dependent synergistic antiproliferation, apoptosis, and DNA damage of ultraviolet-C and coral-derived sinularin combined treatment for oral cancer cells. Cancers 2021, 13, 2450. [Google Scholar] [CrossRef] [PubMed]
- Pronin, S.; Koh, C.H.; Hughes, M. Cytotoxicity of ultraviolet-C radiation on a heterogeneous population of human glioblastoma multiforme cells: Meta-analysis. Photodiagnosis Photodyn. Ther. 2018, 24, 158–163. [Google Scholar] [CrossRef]
- Kawaguchi, J.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Nakashima, M.; Ohno, T.; Shimizu, M.; Yoshioka, T.; Itani, M.; Kozawa, O.; et al. Cisplatin and ultra-violet-C synergistically down-regulate receptor tyrosine kinases in human colorectal cancer cells. Mol. Cancer 2012, 11, 45. [Google Scholar] [CrossRef]
- Murray, D.; Mirzayans, R. Cellular responses to platinum-based anticancer drugs and UVC: Role of p53 and implications for cancer therapy. Int. J. Mol. Sci. 2020, 21, 5766. [Google Scholar] [CrossRef]
- Chuang, Y.T.; Shiau, J.P.; Yen, C.Y.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Fucoidan/UVC combined treatment exerts preferential antiproliferation in oral cancer cells but not normal cells. Antioxidants 2022, 11, 1797. [Google Scholar] [CrossRef]
- Xu, Y.M.; Wijeratne, E.M.K.; Babyak, A.L.; Marks, H.R.; Brooks, A.D.; Tewary, P.; Xuan, L.J.; Wang, W.Q.; Sayers, T.J.; Gunatilaka, A.A.L. Withanolides from aeroponically grown Physalis peruviana and their selective cytotoxicity to prostate cancer and renal carcinoma cells. J. Nat. Prod. 2017, 80, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Widodo, N.; Priyandoko, D.; Shah, N.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by Ashwagandha leaf extract and its component Withanone involves ROS signaling. PLoS ONE 2010, 5, e13536. [Google Scholar] [CrossRef] [PubMed]
- Royston, K.J.; Paul, B.; Nozell, S.; Rajbhandari, R.; Tollefsbol, T.O. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp. Cell Res. 2018, 368, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; You, B.J.; Lee, C.L.; Wu, Y.C.; Lin, W.H.; Lu, T.L.; Chang, F.C.; Lee, H.Z. The roles of 4beta-hydroxywithanolide E from Physalis peruviana on the Nrf2-anti-oxidant system and the cell cycle in breast cancer cells. Am. J. Chin. Med. 2016, 44, 617–636. [Google Scholar] [CrossRef] [PubMed]
- Machin, R.P.; Veleiro, A.S.; Nicotra, V.E.; Oberti, J.C.; Padrón, J.M. Antiproliferative activity of withanolides against human breast cancer cell lines. J. Nat. Prod. 2010, 73, 966–968. [Google Scholar] [CrossRef]
- Yu, T.J.; Yen, C.Y.; Cheng, Y.B.; Yen, C.H.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Physapruin A enhances DNA damage and inhibits DNA repair to suppress oral cancer cell proliferation. Int. J. Mol. Sci. 2022, 23, 8839. [Google Scholar] [CrossRef]
- Yu, T.J.; Cheng, Y.B.; Lin, L.C.; Tsai, Y.H.; Yao, B.Y.; Tang, J.Y.; Chang, F.R.; Yen, C.H.; Ou-Yang, F.; Chang, H.W. Physalis peruviana-derived physapruin A (PHA) inhibits breast cancer cell proliferation and induces oxidative-stress-mediated apoptosis and DNA damage. Antioxidants 2021, 10, 393. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar]
- Wang, S.C.; Wang, Y.Y.; Lin, L.C.; Chang, M.Y.; Yuan, S.F.; Tang, J.Y.; Chang, H.W. Combined treatment of sulfonyl chromen-4-ones (CHW09) and ultraviolet-C (UVC) enhances proliferation inhibition, apoptosis, oxidative stress, and DNA damage against oral cancer cells. Int. J. Mol. Sci. 2020, 21, 6443. [Google Scholar] [CrossRef]
- Kasten, F.H.; Pineda, L.F.; Schneider, P.E.; Rawls, H.R.; Foster, T.A. Biocompatibility testing of an experimental fluoride releasing resin using human gingival epithelial cells in vitro. In Vitro Cell Dev. Biol. 1989, 25, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Soileau, K.; Meffert, R.M. Quantitative evaluation of human gingival epithelial cell attachment to implant surfaces in vitro. Int. J. Periodontics Restor. Dent. 1990, 10, 68–79. [Google Scholar]
- Hsieh, P.L.; Liao, Y.W.; Hsieh, C.W.; Chen, P.N.; Yu, C.C. Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating miR-34a/RTCB axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chang, Y.L.; Liu, S.T.; Chen, G.S.; Lee, S.P.; Huang, S.M. Differential cytotoxicity mechanisms of copper complexed with disulfiram in oral cancer cells. Int. J. Mol. Sci. 2021, 22, 3711. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H.; Shiao, N.H.; Lu, P.Z. CdSe quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial-dependent pathways and inhibition of survival signals. Toxicol. Lett. 2006, 167, 191–200. [Google Scholar] [CrossRef]
- Hung, J.H.; Chen, C.Y.; Omar, H.A.; Huang, K.Y.; Tsao, C.C.; Chiu, C.C.; Chen, Y.L.; Chen, P.H.; Teng, Y.N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2016, 31, 1888–1898. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Yu, T.J.; Tang, J.Y.; Shiau, J.P.; Hou, M.F.; Yen, C.H.; Ou-Yang, F.; Chen, C.Y.; Chang, H.W. Gingerenone A induces antiproliferation and senescence of breast cancer cells. Antioxidants 2022, 11, 587. [Google Scholar] [CrossRef]
- Peng, S.Y.; Wang, Y.Y.; Lan, T.H.; Lin, L.C.; Yuan, S.F.; Tang, J.Y.; Chang, H.W. Low dose combined treatment with ultraviolet-C and withaferin a enhances selective killing of oral cancer cells. Antioxidants 2020, 9, 1120. [Google Scholar] [CrossRef]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.C.; Hsieh, Y.C.; Li, L.H.; Chang, C.C.; Janouskova, K.; Ramani, M.V.; Subbaraju, G.V.; Cheng, K.T.; Chang, C.C. Dehydroxyhispolon methyl ether, a hispolon derivative, inhibits WNT/beta-catenin signaling to elicit human colorectal carcinoma cell apoptosis. Int. J. Mol. Sci. 2020, 21, 8839. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Lee, M.G.; El-Shazly, M.; Lai, K.H.; Ke, S.C.; Su, C.W.; Shih, S.P.; Sung, P.J.; Hong, M.C.; Wen, Z.H.; et al. Isoaaptamine induces T-47D cells apoptosis and autophagy via oxidative stress. Mar. Drugs 2018, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.P.; Lu, M.C.; El-Shazly, M.; Lin, Y.H.; Chen, C.L.; Yu, S.S.F.; Liu, Y.C. The antileukemic and anti-prostatic effect of aeroplysinin-1 is mediated through ROS-induced apoptosis via NOX activation and inhibition of HIF-1a activity. Life 2022, 12, 687. [Google Scholar] [CrossRef]
- Peng, S.Y.; Lin, L.C.; Yang, Z.W.; Chang, F.R.; Cheng, Y.B.; Tang, J.Y.; Chang, H.W. Combined treatment with low cytotoxic ethyl acetate Nepenthes extract and ultraviolet-C improves antiproliferation to oral cancer cells via oxidative stress. Antioxidants 2020, 9, 873. [Google Scholar] [CrossRef]
- Nasihun, T.; Widayati, E. Administration of purwoceng (Pimpinella alpina Molk) improves oxidative stress biomarker following UVC irradiation in spargue-dawley male rats. J. Nat. Remedies 2016, 16, 115–124. [Google Scholar] [CrossRef]
- Chan, W.H.; Yu, J.S. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J. Cell Biochem. 2000, 78, 73–84. [Google Scholar] [CrossRef]
- Chan, W.H.; Wu, C.C.; Yu, J.S. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J. Cell Biochem. 2003, 90, 327–338. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Garg, V.; Tuli, H.S.; Kumar, G.; Kumar, M.; Mukherjee, T. Reactive oxygen species (ROS): An activator of apoptosis and autophagy in cancer. J. Biol. Chem. Sci. 2016, 3, 256–264. [Google Scholar]
- Li, Z.; Yang, J.; Huang, H. Oxidative stress induces H2AX phosphorylation in human spermatozoa. FEBS Lett. 2006, 580, 6161–6168. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.-Y.; Yen, C.-Y.; Lan, T.-H.; Jeng, J.-H.; Tang, J.-Y.; Chang, H.-W. Combined Treatment (Ultraviolet-C/Physapruin A) Enhances Antiproliferation and Oxidative-Stress-Associated Mechanism in Oral Cancer Cells. Antioxidants 2022, 11, 2227. https://doi.org/10.3390/antiox11112227
Peng S-Y, Yen C-Y, Lan T-H, Jeng J-H, Tang J-Y, Chang H-W. Combined Treatment (Ultraviolet-C/Physapruin A) Enhances Antiproliferation and Oxidative-Stress-Associated Mechanism in Oral Cancer Cells. Antioxidants. 2022; 11(11):2227. https://doi.org/10.3390/antiox11112227
Chicago/Turabian StylePeng, Sheng-Yao, Ching-Yu Yen, Ting-Hsun Lan, Jiiang-Huei Jeng, Jen-Yang Tang, and Hsueh-Wei Chang. 2022. "Combined Treatment (Ultraviolet-C/Physapruin A) Enhances Antiproliferation and Oxidative-Stress-Associated Mechanism in Oral Cancer Cells" Antioxidants 11, no. 11: 2227. https://doi.org/10.3390/antiox11112227
APA StylePeng, S.-Y., Yen, C.-Y., Lan, T.-H., Jeng, J.-H., Tang, J.-Y., & Chang, H.-W. (2022). Combined Treatment (Ultraviolet-C/Physapruin A) Enhances Antiproliferation and Oxidative-Stress-Associated Mechanism in Oral Cancer Cells. Antioxidants, 11(11), 2227. https://doi.org/10.3390/antiox11112227








