Monofunctional Heme-Catalases
Abstract
:1. Introduction
2. Catalase Expression in Different Organisms
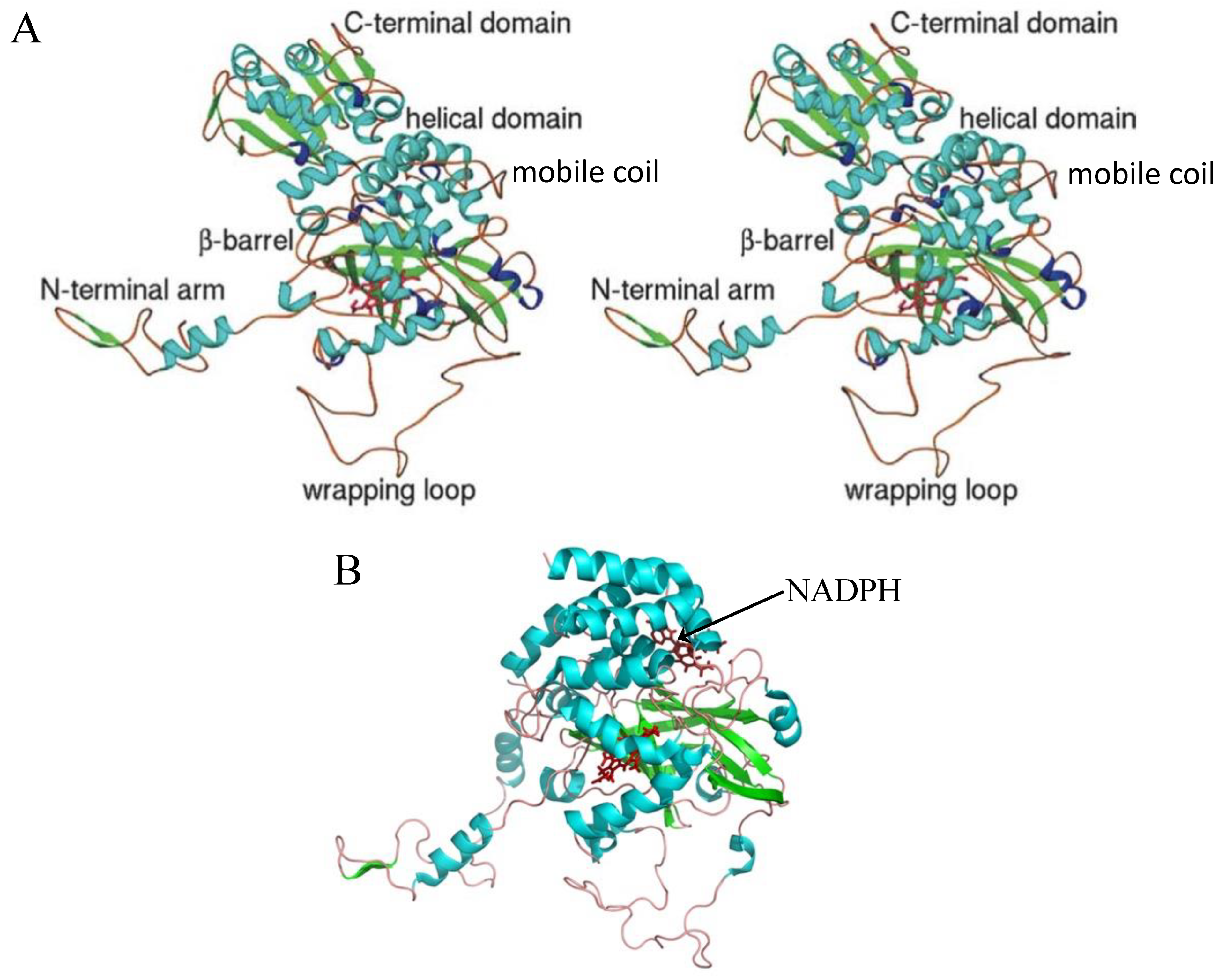
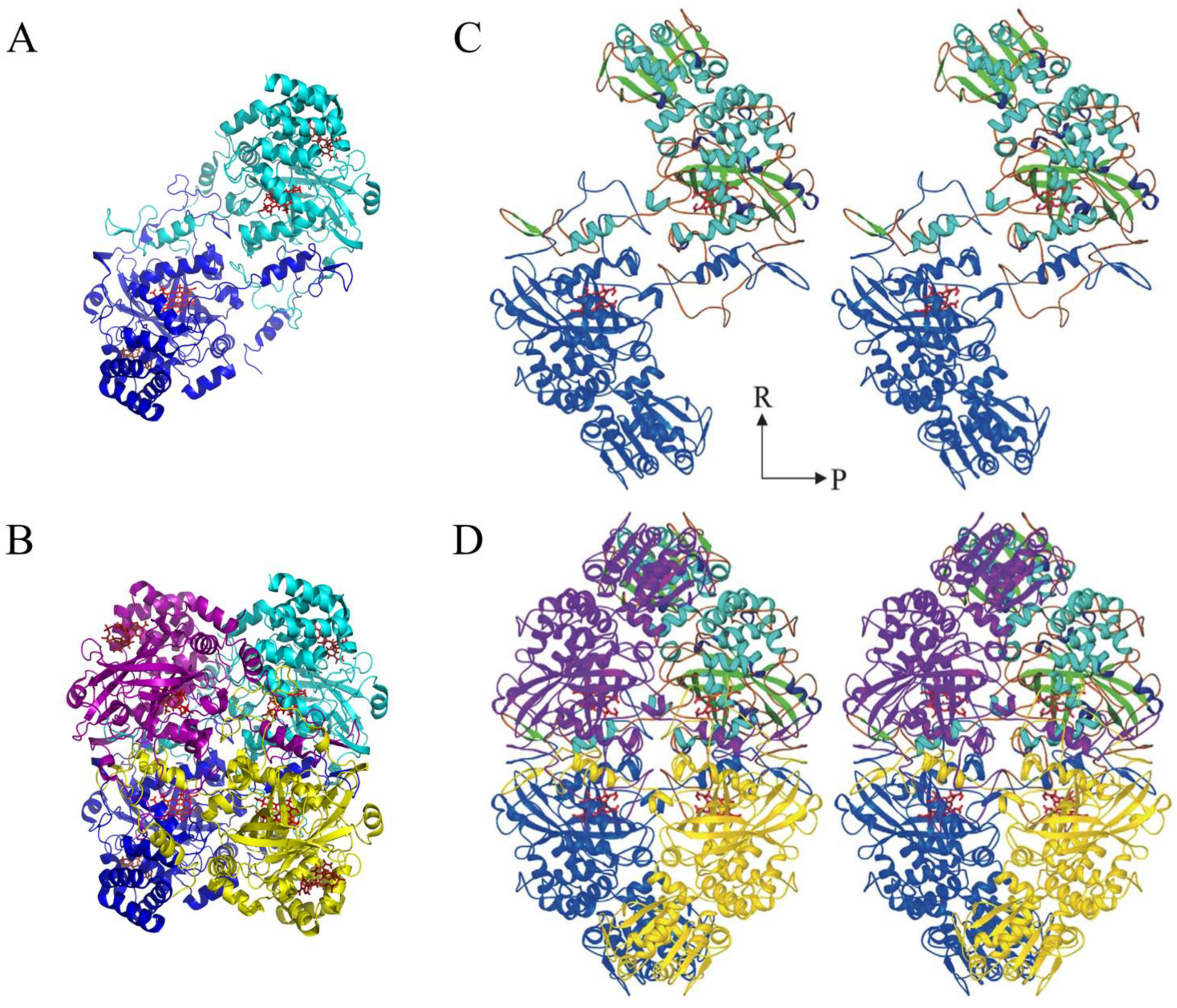
3. Catalase Structures and Their Active Site
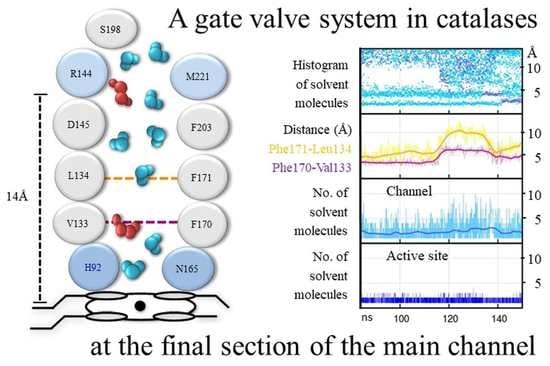
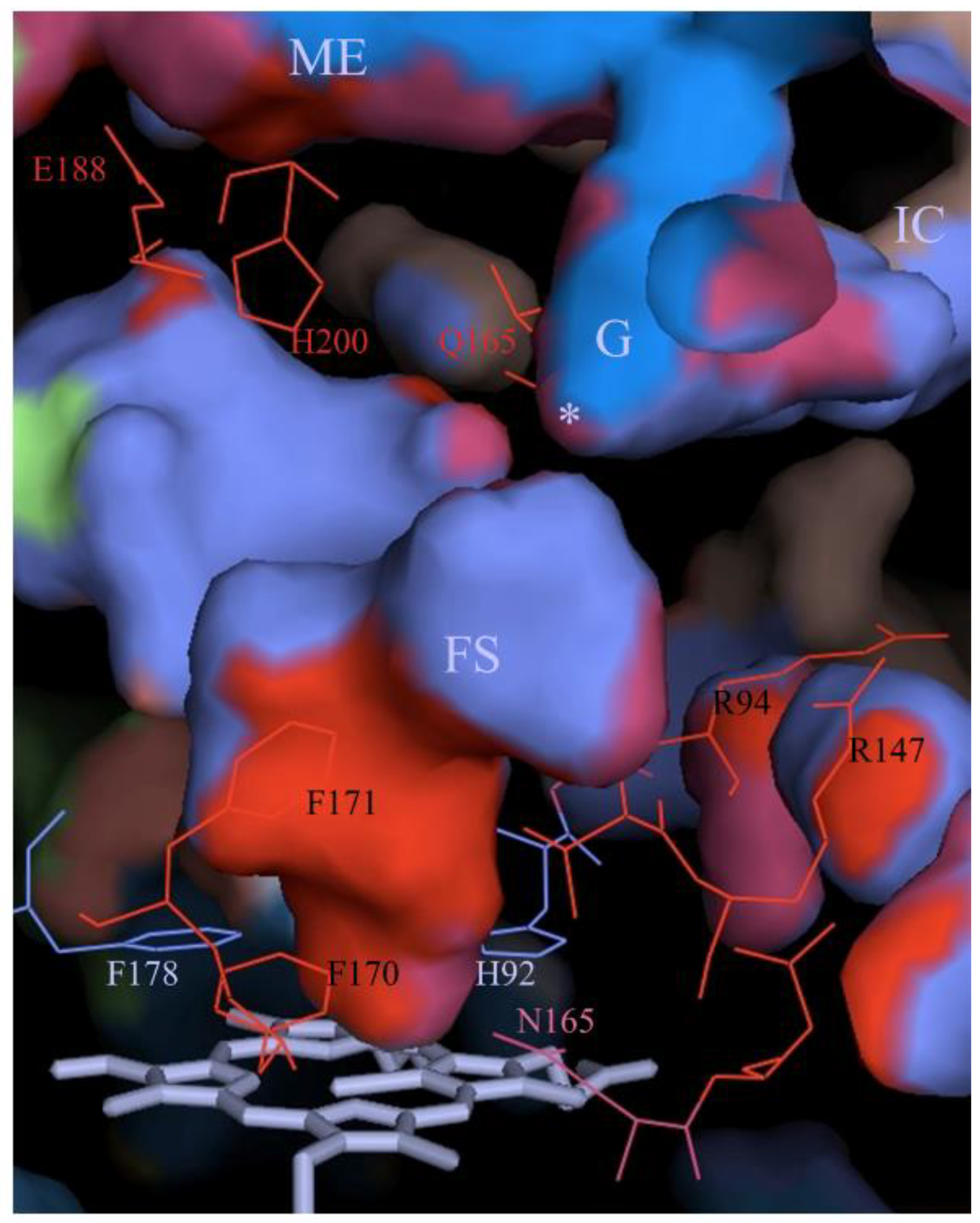
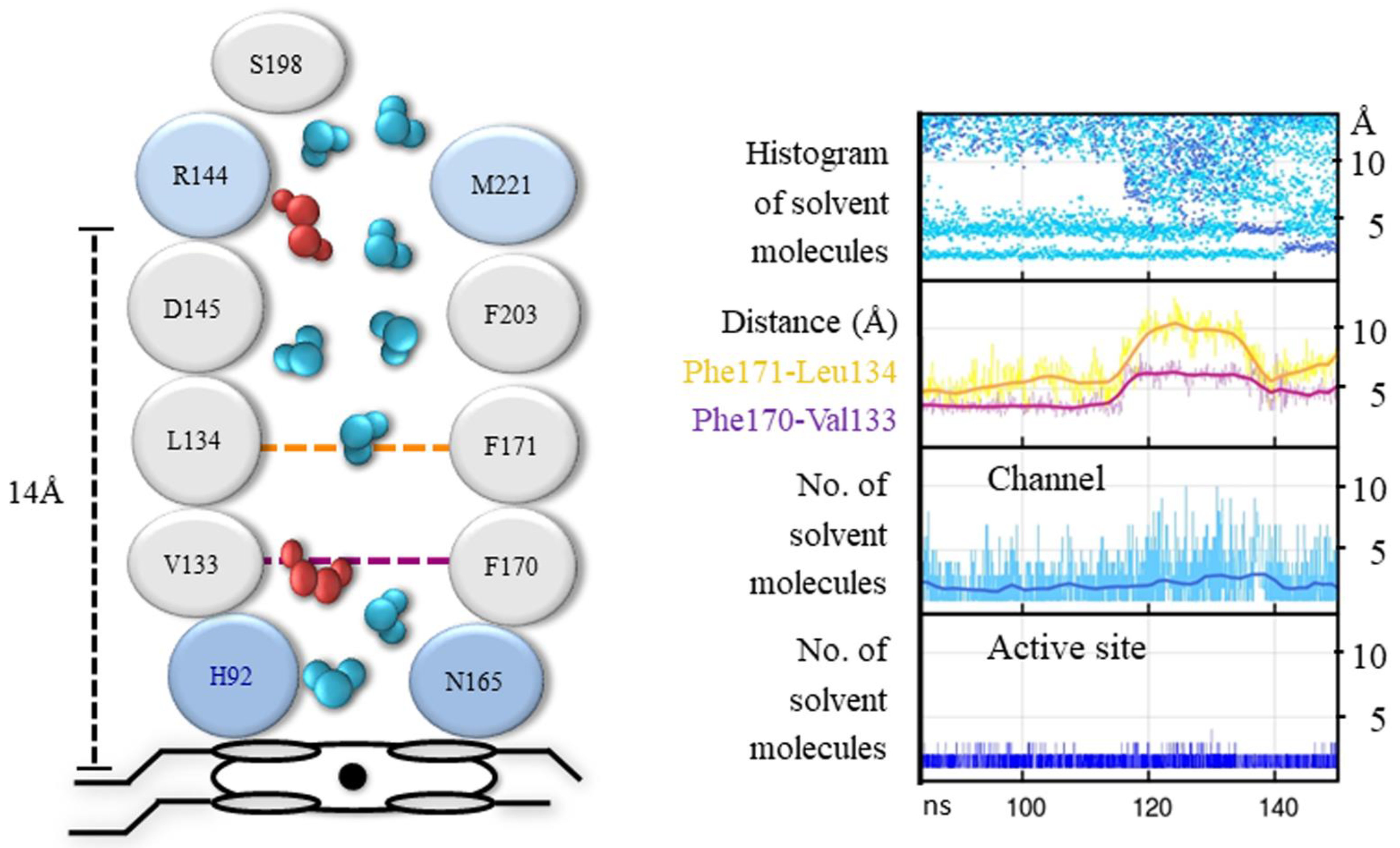
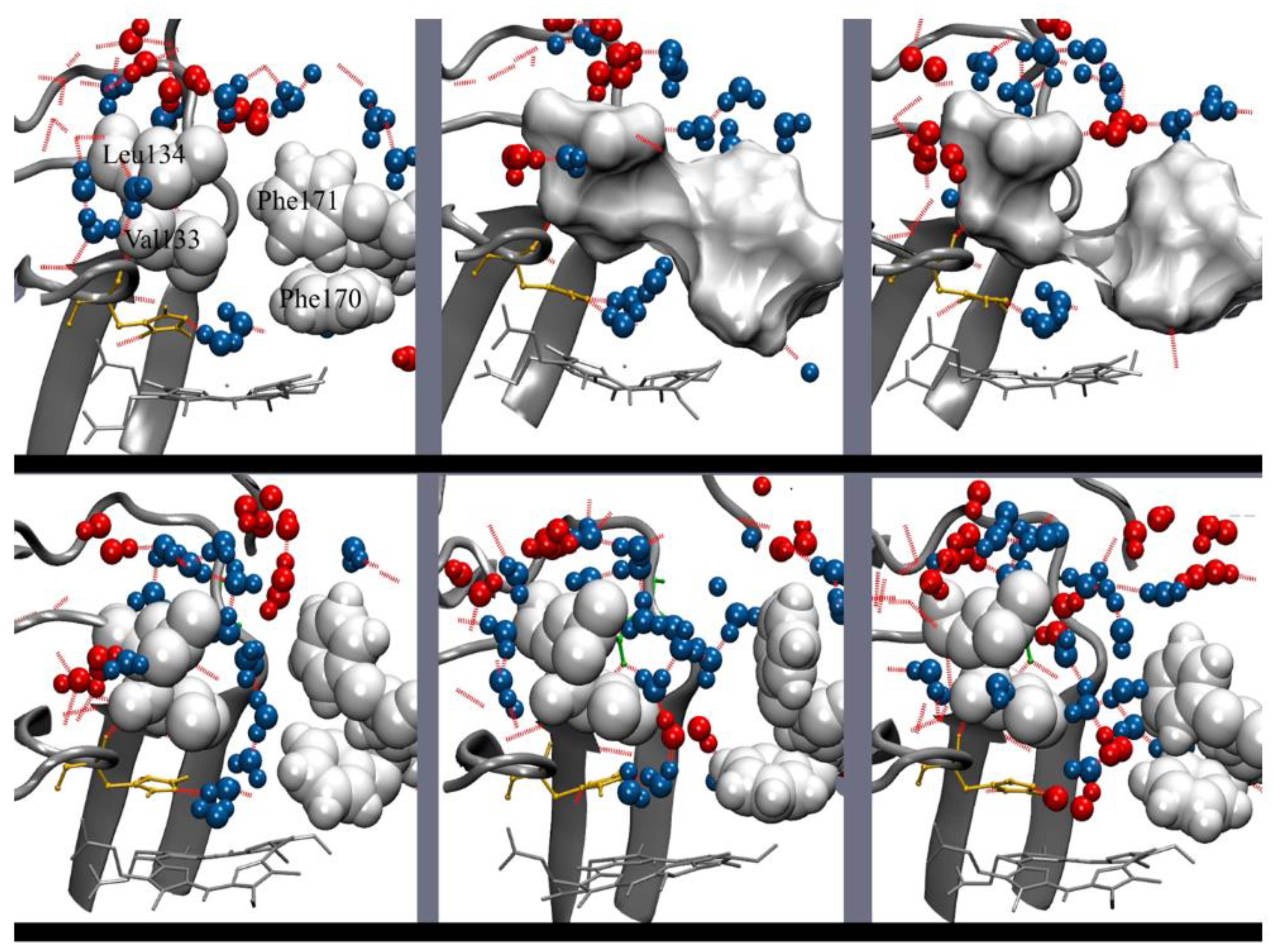
3.1. The Main Channel and the Other Conserved Channels
3.2. The Size of the Main Channel in Different Catalases
3.3. Rules for Prediction of NADPH Binding and Heme Orientation
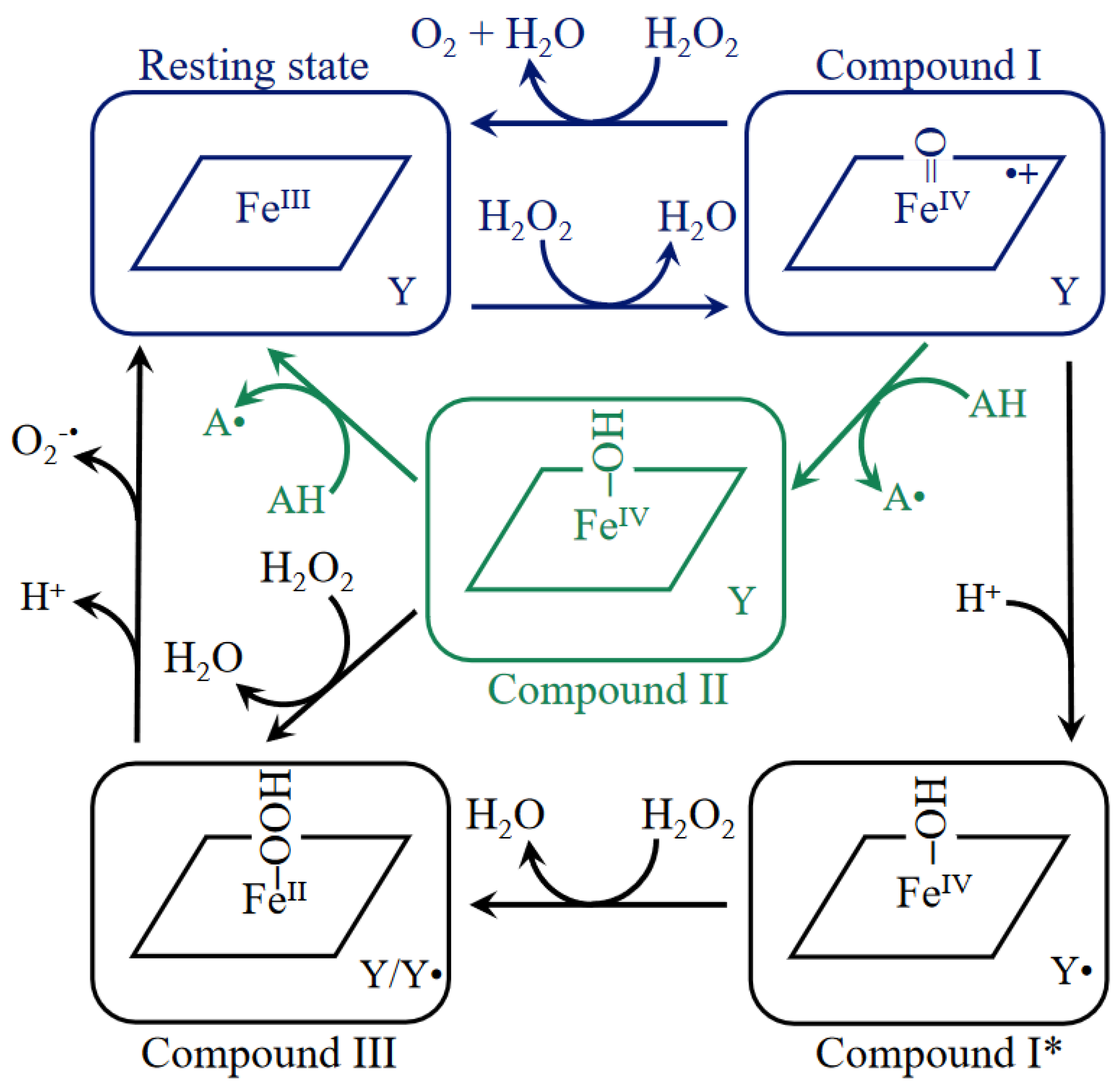
4. Catalase Reaction Mechanism and Kinetics
5. How Do Catalases Select H2O2 in a Sea of Water?
6. How Do Catalases Deal with an Unproductive Reactive Cpd I Intermediate?
Other Reactions of Catalases
7. What Is the Origin of the Heme b to Heme d Modification?
Modification of the Tyr That Ligated the Fe of the Heme at the Proximal Side
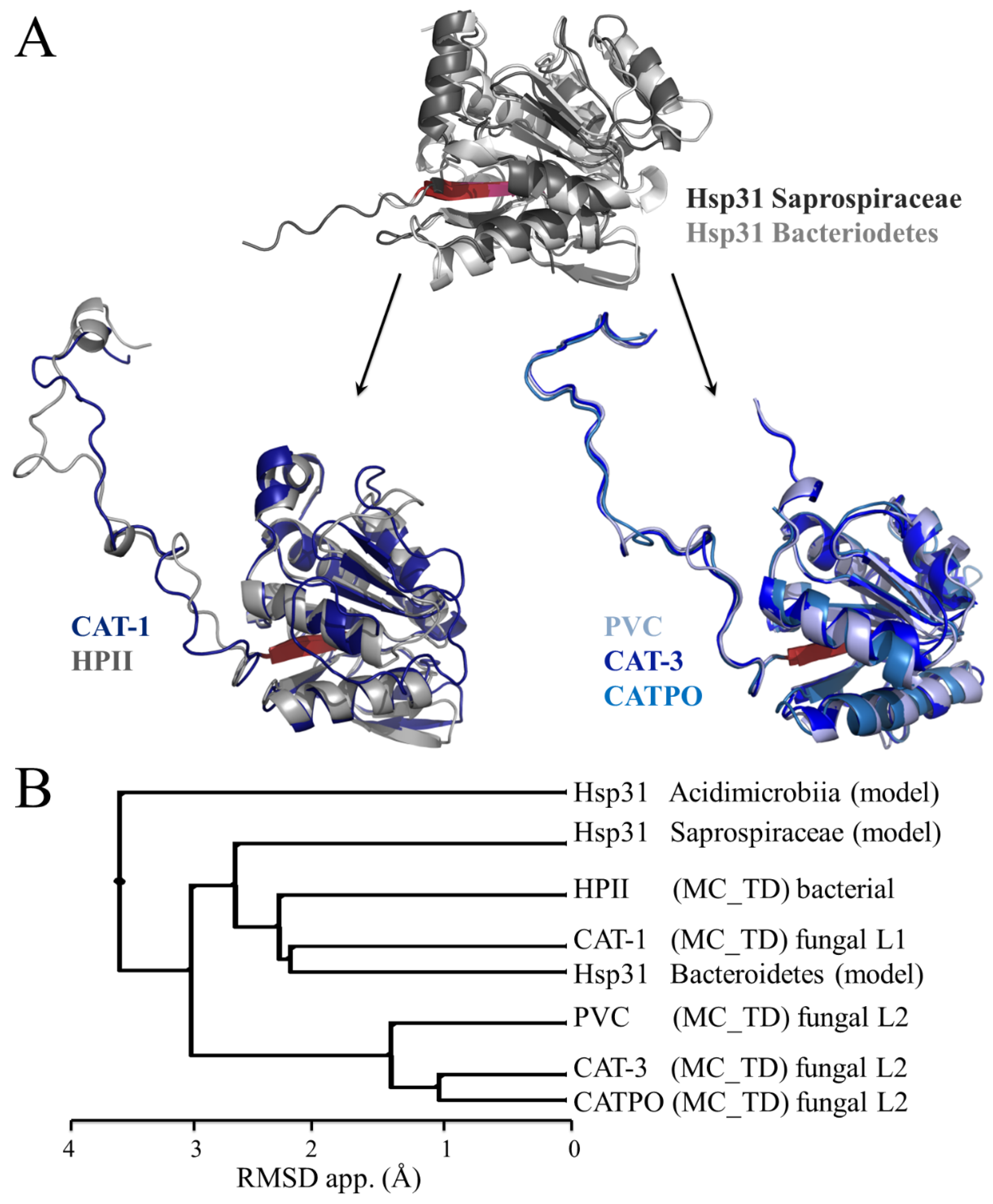
8. What Is the Origin and Function of the TD in LSCs?
9. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- De Cubas, L.; Pak, V.V.; Belousov, V.V.; Ayte, J.; Hidalgo, E. The Mitochondria-to-Cytosol H2O2 Gradient Is Caused by Peroxiredoxin-Dependent Cytosolic Scavenging. Antioxidants 2021, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Domenech, A.; Ayte, J.; Antunes, F.; Hidalgo, E. Using in vivo oxidation status of one- and two-component redox relays to determine H2O2 levels linked to signaling and toxicity. BMC Biol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Singh, R.; Wiseman, B.; Deemagarn, T.; Jha, V.; Switala, J.; Loewen, P.C. Comparative study of catalase-peroxidases (KatGs). Arch. Biochem. Biophys. 2008, 471, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Zamocky, M.; Droghetti, E.; Bellei, M.; Gasselhuber, B.; Pabst, M.; Furtmuller, P.G.; Battistuzzi, G.; Smulevich, G.; Obinger, C. Eukaryotic extracellular catalase-peroxidase from Magnaporthe grisea—Biophysical/chemical characterization of the first representative from a novel phytopathogenic KatG group. Biochimie 2012, 94, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Ndontsa, E.N.; Moore, R.L.; Goodwin, D.C. Stimulation of KatG catalase activity by peroxidatic electron donors. Arch. Biochem. Biophys. 2012, 525, 215–222. [Google Scholar] [CrossRef]
- Vega-García, V.; Díaz-Vilchis, A.; Saucedo-Vázquez, J.P.; Solano-Peralta, A.; Rudiño-Piñera, E.; Hansberg, W. Structure, kinetics, molecular and redox properties of a cytosolic and developmentally regulated fungal catalase-peroxidase. Arch. Biochem. Biophys. 2018, 640, 17–26. [Google Scholar] [CrossRef]
- Switala, J.; Loewen, P.C. Diversity of properties among catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Díaz, A.; Muñoz-Clares, R.A.; Rangel, P.; Valdés, V.J.; Hansberg, W. Functional and structural analysis of catalase oxidized by singlet oxygen. Biochimie 2005, 87, 205–214. [Google Scholar] [CrossRef]
- Díaz, A.; Valdés, V.J.; Rudiño-Piñera, E.; Horjales, E.; Hansberg, W. Structure-function relationships in fungal large-subunit catalases. J. Mol. Biol. 2009, 386, 218–232. [Google Scholar] [CrossRef]
- Kim, K.M.; Qin, T.; Jiang, Y.Y.; Chen, L.L.; Xiong, M.; Caetano-Anolles, D.; Zhang, H.Y.; Caetano-Anolles, G. Protein domain structure uncovers the origin of aerobic metabolism and the rise of planetary oxygen. Structure 2012, 20, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, J.W. Non-heme manganese catalase--the ‘other’ catalase. Arch. Biochem. Biophys. 2012, 525, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. Adv. Inorg. Chem. 2000, 51, 51–106. [Google Scholar] [CrossRef]
- Horakova, E.; Faktorova, D.; Kraeva, N.; Kaur, B.; Van Den Abbeele, J.; Yurchenko, V.; Lukes, J. Catalase compromises the development of the insect and mammalian stages of Trypanosoma brucei. FEBS J. 2020, 287, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Klotz, M.G.; Loewen, P.C. The molecular evolution of catalatic hydroperoxidases: Evidence for multiple lateral transfer of genes between prokaryota and from bacteria into eukaryota. Mol. Biol. Evol. 2003, 20, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Gasselhuber, B.; Furtmuller, P.G.; Obinger, C. Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 2012, 525, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Hansberg, W.; Salas-Lizana, R.; Domínguez, L. Fungal catalases: Function, phylogenetic origin and structure. Arch. Biochem. Biophys. 2012, 525, 170–180. [Google Scholar] [CrossRef]
- Yuan, F.; Yin, S.; Xu, Y.; Xiang, L.; Wang, H.; Li, Z.; Fan, K.; Pan, G. The Richness and Diversity of Catalases in Bacteria. Front. Microbiol. 2021, 12, 645477. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Catalase: A tetrameric enzyme with four tightly bound molecules of NADPH. Proc. Natl. Acad. Sci. USA 1984, 81, 4343–4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Mateos, R.M.; Lopez-Jaramillo, J.; Rodriguez-Ruiz, M.; Gonzalez-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.M.; Schellhorn, H.E. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch. Biochem. Biophys. 2012, 525, 161–169. [Google Scholar] [CrossRef]
- Bagyan, I.; Casillas-Martinez, L.; Setlow, P. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by sigmaF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J. Bacteriol. 1998, 180, 2057–2062. [Google Scholar] [CrossRef] [Green Version]
- Checinska, A.; Burbank, M.; Paszczynski, A.J. Protection of Bacillus pumilus spores by catalases. Appl. Environ. Microbiol. 2012, 78, 6413–6422. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, L.; Wysong, D.; Diamond, R.; Aguirre, J. Two divergent catalase genes are differentially regulated during Aspergillus nidulans development and oxidative stress. J. Bacteriol. 1997, 179, 3284–3292. [Google Scholar] [CrossRef] [Green Version]
- Michán, S.; Lledías, F.; Baldwin, J.D.; Natvig, D.O.; Hansberg, W. Regulation and oxidation of two large monofunctional catalases. Free Radic. Biol. Med. 2002, 33, 521–532. [Google Scholar] [CrossRef]
- Lledías, F.; Rangel, P.; Hansberg, W. Singlet oxygen is part of a hyperoxidant state generated during spore germination. Free Radic. Biol. Med. 1999, 26, 1396–1404. [Google Scholar] [CrossRef]
- Toledo, I.; Aguirre, J.; Hansberg, W. Aerial growth in Neurospora crassa: Characterization of an experimental model system. Exp. Mycol. 1986, 10, 114–125. [Google Scholar] [CrossRef]
- Hansberg, W.; Aguirre, J. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J. Theor. Biol. 1990, 142, 201–221. [Google Scholar] [CrossRef]
- Hansberg, W.; Aguirre, J.; Rios-Momberg, M.; Rangel, P.; Peraza, L.; Montes de Oca, Y.; Cano-Domínguez, N. Cell differentiation as a response to oxidative stress. In Stress in Yeasts and Filamentous Fungi; Academic Press: London, UK, 2008. [Google Scholar]
- Aguirre, J.; Rios-Momberg, M.; Hewitt, D.; Hansberg, W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005, 13, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Michán, S.; Lledías, F.; Hansberg, W. Asexual development is increased in Neurospora crassa cat-3-null mutant strains. Eukaryot. Cell 2003, 2, 798–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- .Petriv, O.I.; Rachubinski, R.A. Lack of peroxisomal catalase causes a progeric phenotype in Caenorhabditis elegans. J. Biol. Chem. 2004, 279, 19996–20001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkut, C.; Vasilj, A.; Boland, S.; Habermann, B.; Shevchenko, A.; Kurzchalia, T.V. Molecular strategies of the Caenorhabditis elegans dauer larva to survive extreme desiccation. PLoS ONE 2013, 8, e82473. [Google Scholar] [CrossRef] [Green Version]
- Fujiki, Y.; Bassik, M.C. A New Paradigm in Catalase Research. Trends Cell Biol. 2021, 31, 148–151. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Bohm, B.; Heinzelmann, S.; Motz, M.; Bauer, G. Extracellular localization of catalase is associated with the transformed state of malignant cells. Biol. Chem. 2015, 396, 1339–1356. [Google Scholar] [CrossRef] [Green Version]
- Melik-Adamyan, W.R.; Barynin, V.V.; Vagin, A.A.; Borisov, V.V.; Vainshtein, B.K.; Fita, I.; Murthy, M.R.; Rossmann, M.G. Comparison of beef liver and Penicillium vitale catalases. J. Mol. Biol. 1986, 188, 63–72. [Google Scholar] [CrossRef]
- Díaz, A.; Horjales, E.; Rudiño-Piñera, E.; Arreola, R.; Hansberg, W. Unusual Cys-Tyr covalent bond in a large catalase. J. Mol. Biol. 2004, 342, 971–985. [Google Scholar] [CrossRef]
- Kalko, S.G.; Gelpi, J.L.; Fita, I.; Orozco, M. Theoretical study of the mechanisms of substrate recognition by catalase. J. Am. Chem. Soc. 2001, 123, 9665–9672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez, L.; Sosa-Peinado, A.; Hansberg, W. Catalase evolved to concentrate H2O2 at its active site. Arch. Biochem. Biophys. 2010, 500, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Mate, M.J.; Zamocky, M.; Nykyri, L.M.; Herzog, C.; Alzari, P.M.; Betzel, C.; Koller, F.; Fita, I. Structure of catalase-A from Saccharomyces cerevisiae. J. Mol. Biol. 1999, 286, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Melik-Adamyan, W.; Bravo, J.; Carpena, X.; Switala, J.; Mate, M.J.; Fita, I.; Loewen, P.C. Substrate flow in catalases deduced from the crystal structures of active site variants of HPII from Escherichia coli. Proteins 2001, 44, 270–281. [Google Scholar] [CrossRef] [Green Version]
- Maj, M.; Nicholls, P.; Obinger, C.; Hillar, A.; Loewen, P.C. Reaction of E. coli catalase HPII with cyanide as ligand and as inhibitor. Biochim. Biophys. Acta 1996, 1298, 241–249. [Google Scholar] [CrossRef]
- Putnam, C.D.; Arvai, A.S.; Bourne, Y.; Tainer, J.A. Active and inhibited human catalase structures: Ligand and NADPH binding and catalytic mechanism. J. Mol. Biol. 2000, 296, 295–309. [Google Scholar] [CrossRef]
- Margoliash, E.; Novogrodsky, A.; Schejter, A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem. J. 1960, 74, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Borovik, A.A.; Grebenko, A.I.; Melik-Adamyan, V.R. X-ray Diffraction Study of Penicillium vitale Catalase in the Complex with Aminotriazole. Crystallogr. Rep. 2011, 56, 590–595. [Google Scholar] [CrossRef]
- Yuzugullu Karakus, Y.; Goc, G.; Balci, S.; Yorke, B.A.; Trinh, C.H.; McPherson, M.J.; Pearson, A.R. Identification of the site of oxidase substrate binding in Scytalidium thermophilum catalase. Acta Cryst. D Struct. Biol. 2018, 74, 979–985. [Google Scholar] [CrossRef] [Green Version]
- Hara, I.; Ichise, N.; Kojima, K.; Kondo, H.; Ohgiya, S.; Matsuyama, H.; Yumoto, I. Relationship between the size of the bottleneck 15 A from iron in the main channel and the reactivity of catalase corresponding to the molecular size of substrates. Biochemistry 2007, 46, 11–22. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Grebenko, A.I.; Brannigan, J.A.; Antson, A.A.; Barynin, V.V.; Dodson, G.G.; Dauter, Z.; Wilson, K.S.; Melik-Adamyan, W.R. The structures of Micrococcus lysodeikticus catalase, its ferryl intermediate (compound II) and NADPH complex. Acta Cryst. D Biol. Cryst. 2002, 58, 1972–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zárate-Romero, A.; Stojanoff, V.; Rojas-Trejo, S.P.; Hansberg, W.; Rudiño-Piñera, E. Conformational stability and crystal packing: Polymorphism in Neurospora crassa CAT-3. Acta Cryst. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 753–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarate-Romero, A.; Stojanoff, V.; Cohen, A.E.; Hansberg, W.; Rudino-Pinera, E. X-ray driven reduction of Cpd I of Catalase-3 from N. crassa reveals differential sensitivity of active sites and formation of ferrous state. Arch. Biochem. Biophys. 2019, 666, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Alfonso-Prieto, M.; Borovik, A.; Carpena, X.; Murshudov, G.; Melik-Adamyan, W.; Fita, I.; Rovira, C.; Loewen, P.C. The structures and electronic configuration of compound I intermediates of Helicobacter pylori and Penicillium vitale catalases determined by X-ray crystallography and QM/MM density functional theory calculations. J. Am. Chem. Soc. 2007, 129, 4193–4205. [Google Scholar] [CrossRef] [Green Version]
- Andreoletti, P.; Pernoud, A.; Sainz, G.; Gouet, P.; Jouve, H.M. Structural studies of Proteus mirabilis catalase in its ground state, oxidized state and in complex with formic acid. Acta Cryst. D Biol. Cryst. 2003, 59, 2163–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fita, I.; Rossmann, M.G. The NADPH binding site on beef liver catalase. Proc. Natl. Acad. Sci. USA 1985, 82, 1604–1608. [Google Scholar] [CrossRef] [Green Version]
- Loewen, P.C.; Carpena, X.; Rovira, C.; Ivancich, A.; Perez-Luque, R.; Haas, R.; Odenbreit, S.; Nicholls, P.; Fita, I. Structure of Helicobacter pylori catalase, with and without formic acid bound, at 1.6 A resolution. Biochemistry 2004, 43, 3089–3103. [Google Scholar] [CrossRef]
- Jha, V.; Louis, S.; Chelikani, P.; Carpena, X.; Donald, L.J.; Fita, I.; Loewen, P.C. Modulation of heme orientation and binding by a single residue in catalase HPII of Escherichia coli. Biochemistry 2011, 50, 2101–2110. [Google Scholar] [CrossRef]
- Goc, G.; Balci, S.; Yorke, B.A.; Pearson, A.R.; Yuzugullu Karakus, Y. Probing the role of Val228 on the catalytic activity of Scytalidium catalase. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140662. [Google Scholar] [CrossRef]
- Chance, B. An intermediate compound in the catalase-hydrogen peroxide reaction. Acta Chem. Scand. 1947, 1, 236–267. [Google Scholar] [CrossRef]
- Ivancich, A.; Jouve, H.M.; Gaillard, J. EPR Evidence for a Tyrosyl Radical Intermediate in Bovine Liver Catalase. J. Am. Chem. Soc. 1996, 118, 12852–12853. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Oberhofer, H.; Klein, M.L.; Rovira, C.; Blumberger, J. Proton transfer drives protein radical formation in Helicobacter pylori catalase but not in Penicillium vitale catalase. J. Am. Chem. Soc. 2011, 133, 4285–4298. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Mestdagh, M.M.; Rouxhet, P.G. Reversible inhibition and irreversible inactivation of catalase in presence of hydrogen peroxide. Biochim. Biophys. Acta 1996, 1295, 222–238. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnes, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, L.; Sosa-Peinado, A.; Hansberg, W. How catalase recognizes H(2)O(2) in a sea of water. Proteins 2014, 82, 45–56. [Google Scholar] [CrossRef]
- Zamocky, M.; Koller, F. Understanding the structure and function of catalases: Clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 1999, 72, 19–66. [Google Scholar] [CrossRef]
- Amara, P.; Andreoletti, P.; Jouve, H.M.; Field, M.J. Ligand diffusion in the catalase from Proteus mirabilis: A molecular dynamics study. Protein Sci. 2001, 10, 1927–1935. [Google Scholar] [CrossRef] [Green Version]
- Chelikani, P.; Carpena, X.; Fita, I.; Loewen, P.C. An electrical potential in the access channel of catalases enhances catalysis. J. Biol. Chem. 2003, 278, 31290–31296. [Google Scholar] [CrossRef] [Green Version]
- Kremer, M.L. Peroxidatic activity of catalase. Biochim. Biophys. Acta 1970, 198, 199–209. [Google Scholar] [CrossRef]
- Krych-Madej, J.; Gebicka, L. Interactions of nitrite with catalase: Enzyme activity and reaction kinetics studies. J. Inorg. Biochem. 2017, 171, 10–17. [Google Scholar] [CrossRef]
- Obinger, C.; Maj, M.; Nicholls, P.; Loewen, P. Activity, peroxide compound formation, and heme d synthesis in Escherichia coli HPII catalase. Arch. Biochem. Biophys. 1997, 342, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, Y.; Fridovich, I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982, 257, 5751–5754. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Rouxhet, P.G. Characterization of hydrogen peroxide and superoxide degrading pathways of Aspergillus niger catalase: A steady-state analysis. Free Radic. Res. 1994, 20, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Reversible binding and inhibition of catalase by nitric oxide. Eur. J. Biochem. 1995, 232, 188–191. [Google Scholar] [CrossRef]
- Brunelli, L.; Yermilov, V.; Beckman, J.S. Modulation of catalase peroxidatic and catalatic activity by nitric oxide. Free Radic. Biol. Med. 2001, 30, 709–714. [Google Scholar] [CrossRef]
- Purwar, N.; McGarry, J.M.; Kostera, J.; Pacheco, A.A.; Schmidt, M. Interaction of nitric oxide with catalase: Structural and kinetic analysis. Biochemistry 2011, 50, 4491–4503. [Google Scholar] [CrossRef]
- Candelaresi, M.; Gumiero, A.; Adamczyk, K.; Robb, K.; Bellota-Anton, C.; Sangal, V.; Munnoch, J.; Greetham, G.M.; Towrie, M.; Hoskisson, P.A.; et al. A structural and dynamic investigation of the inhibition of catalase by nitric oxide. Org. Biomol. Chem. 2013, 11, 7778–7788. [Google Scholar] [CrossRef]
- Korth, H.G.; Meier, A.C.; Auferkamp, O.; Sicking, W.; De Groot, H.; Sustmann, R.; Kirsch, M. Ascorbic acid reduction of compound I of mammalian catalases proceeds via specific binding to the NADPH binding pocket. Biochemistry 2012, 51, 4693–4703. [Google Scholar] [CrossRef]
- Gaetani, G.F.; Ferraris, A.M.; Sanna, P.; Kirkman, H.N. A novel NADPH:(bound) NADP+ reductase and NADH:(bound) NADP+ transhydrogenase function in bovine liver catalase. Biochem. J. 2005, 385, 763–768. [Google Scholar] [CrossRef]
- Gouet, P.; Jouve, H.M.; Dideberg, O. Crystal structure of Proteus mirabilis PR catalase with and without bound NADPH. J. Mol. Biol. 1995, 249, 933–954. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Galiano, S.; Gaetani, G.F. The function of catalase-bound NADPH. J. Biol. Chem. 1987, 262, 660–666. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Rolfo, M.; Ferraris, A.M.; Gaetani, G.F. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J. Biol. Chem. 1999, 274, 13908–13914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillar, A.; Nicholls, P.; Switala, J.; Loewen, P.C. NADPH binding and control of catalase compound II formation: Comparison of bovine, yeast, and Escherichia coli enzymes. Biochem. J. 1994, 300 Pt 2, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, L.P.; Bruice, T.C. Electron tunneling and ab initio calculations related to the one-electron oxidation of NAD(P)H bound to catalase. Biochemistry 1995, 34, 7335–7347. [Google Scholar] [CrossRef]
- Andreoletti, P.; Gambarelli, S.; Sainz, G.; Stojanoff, V.; White, C.; Desfonds, G.; Gagnon, J.; Gaillard, J.; Jouve, H.M. Formation of a tyrosyl radical intermediate in Proteus mirabilis catalase by directed mutagenesis and consequences for nucleotide reactivity. Biochemistry 2001, 40, 13734–13743. [Google Scholar] [CrossRef]
- Sicking, W.; Korth, H.G.; De Groot, H.; Sustmann, R. On the functional role of a water molecule in clade 3 catalases: A proposal for the mechanism by which NADPH prevents the formation of compound II. J. Am. Chem. Soc. 2008, 130, 7345–7356. [Google Scholar] [CrossRef]
- Schonbaum, G.R.; Chance, B. Catalase. In The Enzymes, 3rd ed.; Boyer, P.D., Ed.; Academic Press: Cambridge, MA, USA, 1976. [Google Scholar]
- De la Fuente, I.M.; Martinez, L.; Carrasco-Pujante, J.; Fedetz, M.; Lopez, J.I.; Malaina, I. Self-Organization and Information Processing: From Basic Enzymatic Activities to Complex Adaptive Cellular Behavior. Front. Genet. 2021, 12, 644615. [Google Scholar] [CrossRef]
- Moller, A.C.; Hauser, M.J.; Olsen, L.F. Oscillations in peroxidase-catalyzed reactions and their potential function in vivo. Biophys. Chem. 1998, 72, 63–72. [Google Scholar] [CrossRef]
- Holland, J.T.; Lau, C.; Brozik, S.; Atanassov, P.; Banta, S. Engineering of glucose oxidase for direct electron transfer via site-specific gold nanoparticle conjugation. J. Am. Chem. Soc. 2011, 133, 19262–19265. [Google Scholar] [CrossRef]
- De Groot, H.; Auferkamp, O.; Bramey, T.; De Groot, K.; Kirsch, M.; Korth, H.G.; Petrat, F.; Sustmann, R. Non-oxygen-forming pathways of hydrogen peroxide degradation by bovine liver catalase at low hydrogen peroxide fluxes. Free Radic. Res. 2006, 40, 67–74. [Google Scholar] [CrossRef]
- Krych, J.; Gebicki, J.L.; Gebicka, L. Flavonoid-induced conversion of catalase to its inactive form—Compound II. Free Radic. Res. 2014, 48, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Li, D.; Ji, J. Probing the binding of flavonoids to catalase by molecular spectroscopy. J. Mol. Struct. 2007, 843, 38–44. [Google Scholar] [CrossRef]
- Vetrano, A.M.; Heck, D.E.; Mariano, T.M.; Mishin, V.; Laskin, D.L.; Laskin, J.D. Characterization of the oxidase activity in mammalian catalase. J. Biol. Chem. 2005, 280, 35372–35381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Summers, F.A.; Mason, R.P. Photooxidation of Amplex Red to resorufin: Implications of exposing the Amplex Red assay to light. Free Radic. Biol. Med. 2012, 53, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Durner, J.; Klessig, D.F. Salicylic acid is a modulator of tobacco and mammalian catalases. J. Biol. Chem. 1996, 271, 28492–28501. [Google Scholar] [CrossRef] [Green Version]
- Koclar Avci, G.; Coruh, N.; Bolukbasi, U.; Ogel, Z.B. Oxidation of phenolic compounds by the bifunctional catalase-phenol oxidase (CATPO) from Scytalidium thermophilum. Appl. Microbiol. Biotechnol. 2013, 97, 661–672. [Google Scholar] [CrossRef]
- Schusler-van Hees, M.T.; Beijersbergen van Henegouwen, G.M.; Stoutenberg, P. Autoxidation of catechol(amine)s. Pharm. Weekbl. Sci. 1985, 7, 245–251. [Google Scholar] [CrossRef]
- Xu, Z. Mechanics of metal-catecholate complexes: The roles of coordination state and metal types. Sci. Rep. 2013, 3, 2914. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cohen Stuart, M.A.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Teng, X.L.; Chen, N.; Xiao, X.G. Identification of a Catalase-Phenol Oxidase in Betalain Biosynthesis in Red Amaranth (Amaranthus cruentus). Front. Plant. Sci. 2015, 6, 1228. [Google Scholar] [CrossRef]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, T.; Peiretti, F.; Defoort, C.; Borel, P.; Govers, R. 2′,7′-dichlorofluorescin-based analysis of Fenton chemistry reveals auto-amplification of probe fluorescence and albumin as catalyst for the detection of hydrogen peroxide. Biochem. J. 2020, 477, 4689–4710. [Google Scholar] [CrossRef] [PubMed]
- Grotjohann, N.; Janning, A.; Eising, R. In vitro photoinactivation of catalase isoforms from cotyledons of sunflower (Helianthus annuus L.). Arch. Biochem. Biophys. 1997, 346, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Aubailly, M.; Haigle, J.; Giordani, A.; Morliere, P.; Santus, R. UV photolysis of catalase revisited: A spectral study of photolytic intermediates. J. Photochem. Photobiol. B 2000, 56, 61–67. [Google Scholar] [CrossRef]
- Djiadeu, P.; Azzouz, D.; Khan, M.A.; Kotra, L.P.; Sweezey, N.; Palaniyar, N. Ultraviolet irradiation increases green fluorescence of dihydrorhodamine (DHR) 123: False-positive results for reactive oxygen species generation. Pharm. Res. Perspect. 2017, 5, e00303. [Google Scholar] [CrossRef] [Green Version]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Bravo, J.; Verdaguer, N.; Tormo, J.; Betzel, C.; Switala, J.; Loewen, P.C.; Fita, I. Crystal structure of catalase HPII from Escherichia coli. Structure 1995, 3, 491–502. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Grebenko, A.I.; Barynin, V.; Dauter, Z.; Wilson, K.S.; Vainshtein, B.K.; Melik-Adamyan, W.; Bravo, J.; Ferran, J.M.; Ferrer, J.C.; et al. Structure of the heme d of Penicillium vitale and Escherichia coli catalases. J. Biol. Chem. 1996, 271, 8863–8868. [Google Scholar] [CrossRef] [Green Version]
- Yuzugullu, Y.; Trinh, C.H.; Smith, M.A.; Pearson, A.R.; Phillips, S.E.; Sutay Kocabas, D.; Bakir, U.; Ogel, Z.B.; McPherson, M.J. Structure, recombinant expression and mutagenesis studies of the catalase with oxidase activity from Scytalidium thermophilum. Acta Cryst. D Biol. Cryst. 2013, 69, 398–408. [Google Scholar] [CrossRef] [Green Version]
- Alfonso-Prieto, M.; Vidossich, P.; Rovira, C. The reaction mechanisms of heme catalases: An atomistic view by ab initio molecular dynamics. Arch. Biochem. Biophys. 2012, 525, 121–130. [Google Scholar] [CrossRef]
- Loewen, P.C.; Switala, J.; von Ossowski, I.; Hillar, A.; Christie, A.; Tattrie, B.; Nicholls, P. Catalase HPII of Escherichia coli catalyzes the conversion of protoheme to cis-heme d. Biochemistry 1993, 32, 10159–10164. [Google Scholar] [CrossRef] [PubMed]
- Jakopitsch, C.; Auer, M.; Regelsberger, G.; Jantschko, W.; Furtmuller, P.G.; Ruker, F.; Obinger, C. Distal site aspartate is essential in the catalase activity of catalase-peroxidases. Biochemistry 2003, 42, 5292–5300. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Rangel, P.; Montes de Oca, Y.; Lledías, F.; Hansberg, W. Molecular and kinetic study of catalase-1, a durable large catalase of Neurospora crassa. Free Radic. Biol. Med. 2001, 31, 1323–1333. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Vidossich, P.; Rodriguez-Fortea, A.; Carpena, X.; Fita, I.; Loewen, P.C.; Rovira, C. Electronic state of the molecular oxygen released by catalase. J. Phys. Chem. A 2008, 112, 12842–12848. [Google Scholar] [CrossRef] [Green Version]
- de Visser, S.P. What external perturbations influence the electronic properties of catalase compound I? Inorg. Chem. 2006, 45, 9551–9557. [Google Scholar] [CrossRef]
- Lai, W.; Chen, H.; Cho, K.B.; Shaik, S. External Electric Field Can Control the Catalytic Cycle of Cytochrome P450cam: A QM/MM Study. J. Phys. Chem. Lett. 2010, 1, 2082–2087. [Google Scholar] [CrossRef]
- Khan, A.U.; Gebauer, P.; Hager, L.P. Chloroperoxidase generation of singlet Delta molecular oxygen observed directly by spectroscopy in the 1- to 1.6-mum region. Proc. Natl. Acad. Sci. USA 1983, 80, 5195–5197. [Google Scholar] [CrossRef] [Green Version]
- Kanofsky, J.R.; Wright, J.; Miles-Richardson, G.E.; Tauber, A.I. Biochemical requirements for singlet oxygen production by purified human myeloperoxidase. J. Clin. Investig. 1984, 74, 1489–1495. [Google Scholar] [CrossRef] [Green Version]
- Ingenbosch, K.N.; Quint, S.; Dyllick-Brenzinger, M.; Wunschik, D.S.; Kiebist, J.; Suss, P.; Liebelt, U.; Zuhse, R.; Menyes, U.; Scheibner, K.; et al. Singlet-Oxygen Generation by Peroxidases and Peroxygenases for Chemoenzymatic Synthesis. ChemBioChem 2021, 22, 398–407. [Google Scholar] [CrossRef]
- Andreoletti, P.; Mouesca, J.M.; Gouet, P.; Jaquinod, M.; Capeillere-Blandin, C.; Jouve, H.M. Verdoheme formation in Proteus mirabilis catalase. Biochim. Biophys. Acta 2009, 1790, 741–753. [Google Scholar] [CrossRef]
- Jha, V.; Donald, L.J.; Loewen, P.C. Mutation of Phe413 to Tyr in catalase KatE from Escherichia coli leads to side chain damage and main chain cleavage. Arch. Biochem. Biophys. 2012, 525, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W. Structure and function of heme proteins regulated by diverse post-translational modifications. Arch. Biochem. Biophys. 2018, 641, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lledías, F.; Rangel, P.; Hansberg, W. Oxidation of catalase by singlet oxygen. J. Biol. Chem. 1998, 273, 10630–10637. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.; Fita, I.; Ferrer, J.C.; Ens, W.; Hillar, A.; Switala, J.; Loewen, P.C. Identification of a novel bond between a histidine and the essential tyrosine in catalase HPII of Escherichia coli. Protein Sci. 1997, 6, 1016–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, M.M.; Grishin, N.V. The C-terminal domain of HPII catalase is a member of the type I glutamine amidotransferase superfamily. Proteins 2001, 42, 230–236. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Cookson, M.R. Evolutionary and functional relationships within the DJ1 superfamily. BMC Evol. Biol. 2004, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Chelikani, P.; Donald, L.J.; Duckworth, H.W.; Loewen, P.C. Hydroperoxidase II of Escherichia coli exhibits enhanced resistance to proteolytic cleavage compared to other catalases. Biochemistry 2003, 42, 5729–5735. [Google Scholar] [CrossRef]
- Sevinc, M.S.; Switala, J.; Bravo, J.; Fita, I.; Loewen, P.C. Truncation and heme pocket mutations reduce production of functional catalase HPII in Escherichia coli. Protein Eng. 1998, 11, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Teo, J.W.; Kum, S.; Jureen, R.; Lin, R.T. Molecular Characterization of a Catalase-Negative Staphylococcus aureus Blood Culture Isolate. J. Clin. Microbiol. 2015, 53, 3699–3701. [Google Scholar] [CrossRef] [Green Version]
- Hansberg, W.; Nava-Ramirez, T.; Rangel-Silva, P.; Diaz-Vilchis, A.; Mendoza-Oliva, A. Large-Size Subunit Catalases Are Chimeric Proteins: A H2O2 Selecting Domain with Catalase Activity Fused to a Hsp31-Derived Domain Conferring Protein Stability and Chaperone Activity. Antioxidants 2022, 11, 979. [Google Scholar] [CrossRef]
- Nava-Ramírez, T.; Hansberg, W. Chaperone activity of large-size subunit catalases. Free Radic. Biol. Med. 2020, 156, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Loewen, P.C.; Fita, I.; Carpena, X. Thirty years of heme catalases structural biology. Arch. Biochem. Biophys. 2012, 525, 102–110. [Google Scholar] [CrossRef] [PubMed]



| Name | Organism | Type | Clade | Heme Type | Heme Orient. | NADPH Binding | PDB | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 217 | 203 | 305 | ||||||||
| katA * | Corynebacterium glutamicum | SSC | 3 | b | III | S | + | R | Q | 4B7G |
| MLC | Micrococcus lysodeikticus (luteus) | SSC | 3 | b | III | S | + | R | Q | 1HBZ, 1GWF |
| KLC | Kluyveromyces lactis | SSC | 3 | b | III | G | + | R | H | 6RJR |
| SCC-A | Saccharomyces cerevisiae | SSC | 3 | b | III | G | + | R | Q | 1A4E |
| KPC | Komagataella (Pichia) pastoris | SSC | 3 | b | III | G | + | R | H | 6RJN |
| PAC | Hansenula polymorpha (Pichia angusta) | SSC | 3 | b | III | G | + | R | H | 2XQ1 |
| HEC | Homo sapiens (erythrocyte) | SSC | 3 | b | III | S | + | R | H | 1F4J |
| BLC | Bos taurus (liver) | SSC | 3 | b | III | S | + | R | H | 3RE8 |
| EFC | Enterococcus faecalis | SSC | 3 | b | III | S | + | R | Q | 1SI8 |
| HPC | Helicobacter pylori | SSC | 3 | b | III | S | - | R | L | 1QWL, 2A9E |
| VSC | Vibrio salmonicida | SSC | 3 | b | III | S | + | R | H | 2ISA |
| KatA | Pseudomonas aeruginosa | SSC | 3 | b | III | S | + | R | H | 4E37 |
| PMC | Proteus mirabilis | SSC | 3 | b | III | S | + | R | H | 1M85, 1MQF, 1H7K |
| Cat-F | Pseudomonas syringae | SSC | 1 | b | IV | V | - | E | -- | 1M7S |
| DR1998 | Deinococcus radiodurans | SSC | 1 | b | IV | V | - | W | R | 4CAB |
| BPC | Bacillus pumilus | SSC | 1 | b | IV | V | - | E | E | 4QOQ |
| EKTA | Exiguobacterium oxidotolerans | SSC | 1 | b | IV | V | - | E | E | 2J2M |
| HPII | Escherichia coli | LSC | 2 | d | IV | I | - | R | E | 1IPH, 1GGF, 1YE9 |
| CAT-1 | Neurospora crassa | L1-LSC | 2 | d | IV | V | - | R | E | 3EJ6 |
| CAT-3 | Neurospora crassa | L2-LSC | 2 | b | IV | I | - | N | E | 1SY7, 4AJ9 |
| PVC | Penicillium janthinellum (vitale) | L2-LSC | 2 | d | IV | V | - | H | E | 4CAT, 2IUF, 2XF2 |
| CATPO | Mycothermus thermophilus (Scytalidium thermophilum) | L2-LSC | 2 | d | IV | V | - | H | E | 5ZZ1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansberg, W. Monofunctional Heme-Catalases. Antioxidants 2022, 11, 2173. https://doi.org/10.3390/antiox11112173
Hansberg W. Monofunctional Heme-Catalases. Antioxidants. 2022; 11(11):2173. https://doi.org/10.3390/antiox11112173
Chicago/Turabian StyleHansberg, Wilhelm. 2022. "Monofunctional Heme-Catalases" Antioxidants 11, no. 11: 2173. https://doi.org/10.3390/antiox11112173
APA StyleHansberg, W. (2022). Monofunctional Heme-Catalases. Antioxidants, 11(11), 2173. https://doi.org/10.3390/antiox11112173