Targeting Thymidylate Synthase and tRNA-Derived Non-Coding RNAs Improves Therapeutic Sensitivity in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Measurement of Cell Viability and Proliferation
2.3. Annexin V and Propidium Iodide (PI) Staining
2.4. Cell Migration Assay
2.5. Cell Growth Observation
2.6. Protein Expression Analysis
2.7. Analysis of Mitochondrial Dysfunction
2.8. Intracellular ROS and Ca2+ Detection
2.9. tiRNA Detection
2.10. AOM/DSS CRC Model
2.11. Histological Analysis
2.12. Quantitative PCR
2.13. Immune Cell Analysis
2.14. Statistics
3. Results
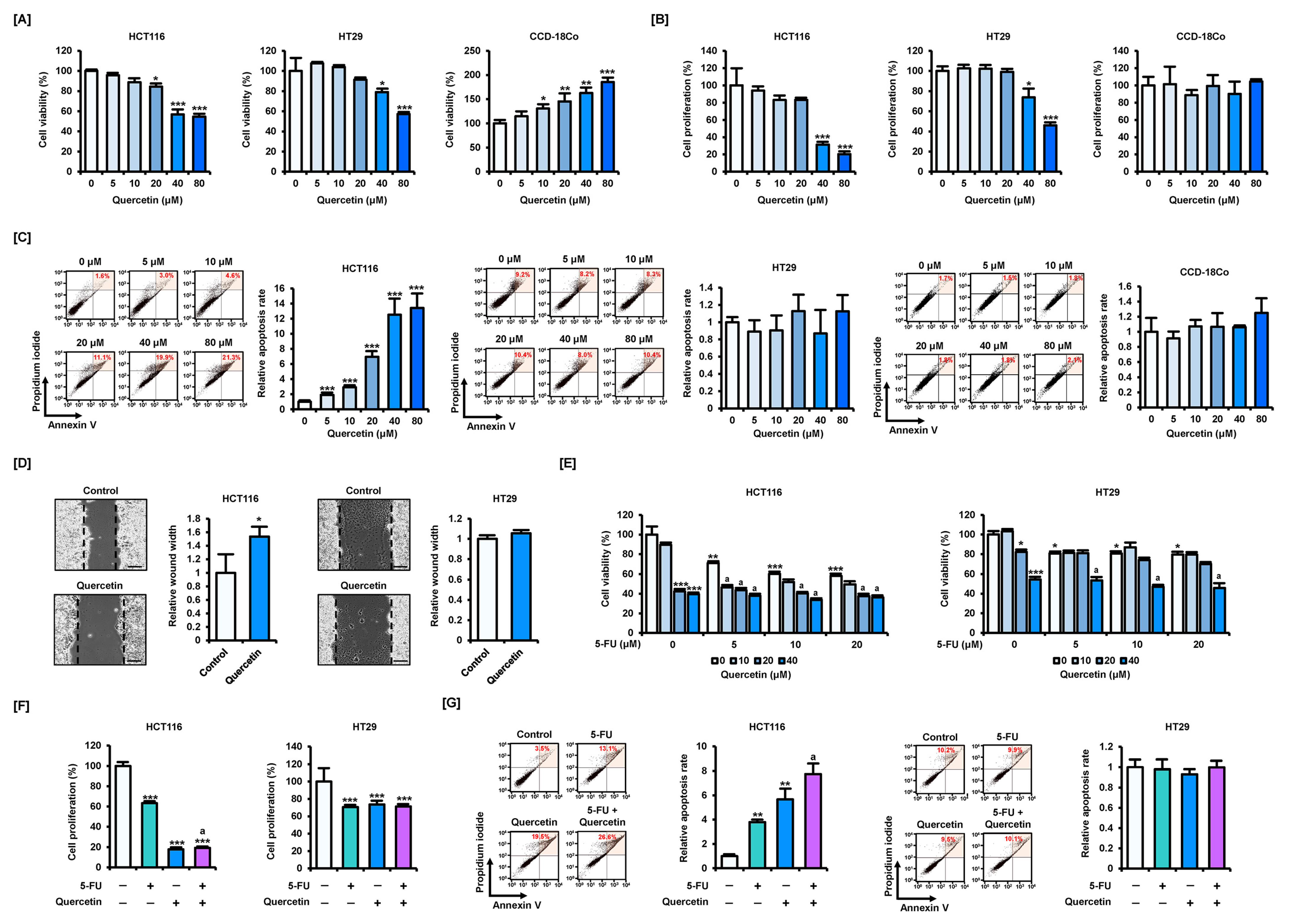
3.1. Quercetin Potentiates the Effects of 5-FU on Characteristics of CRC Cells
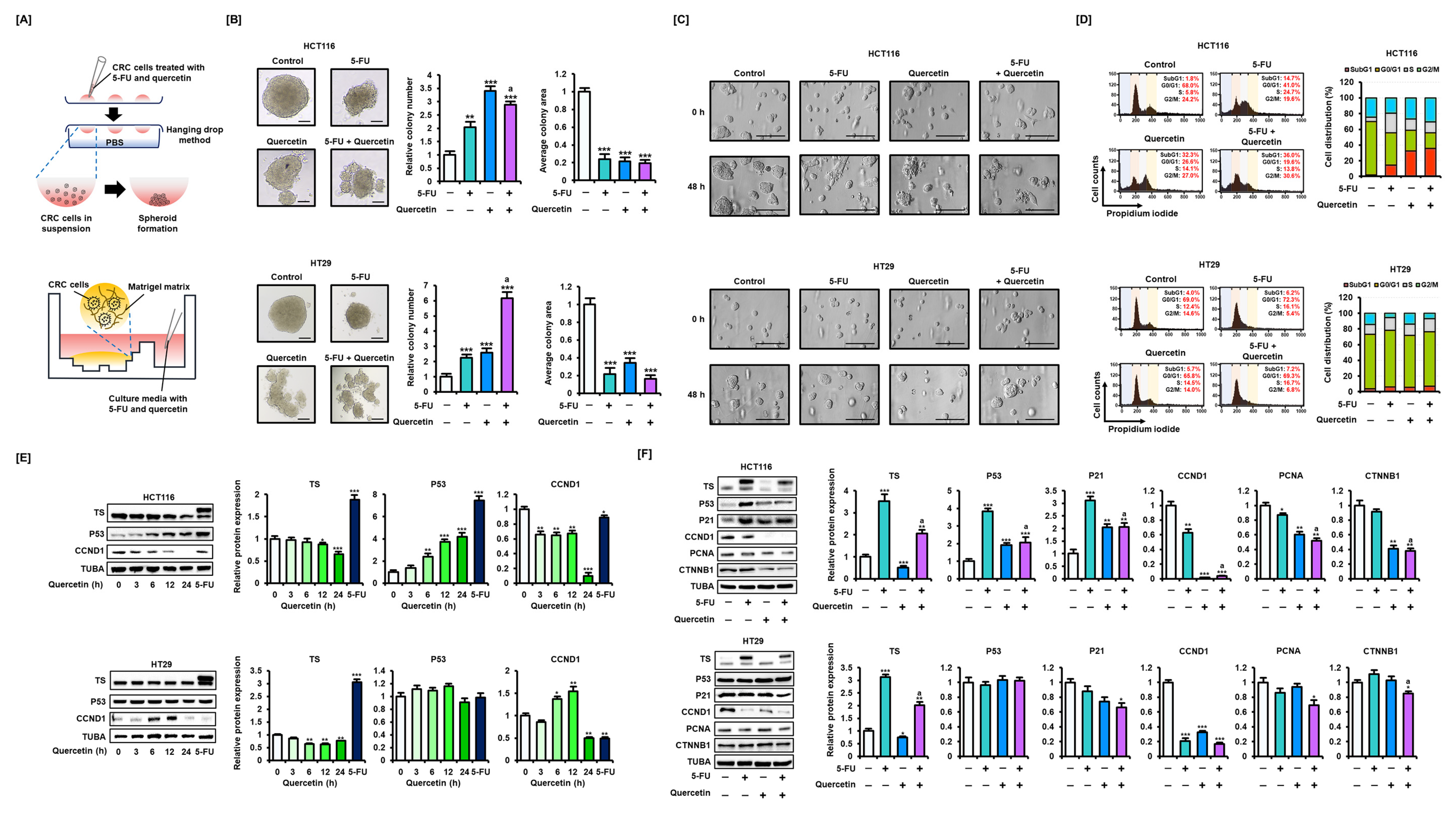
3.2. Quercetin Overcomes 5-FU Resistance by Regulating the Expression of TS and p53 in CRC Cells
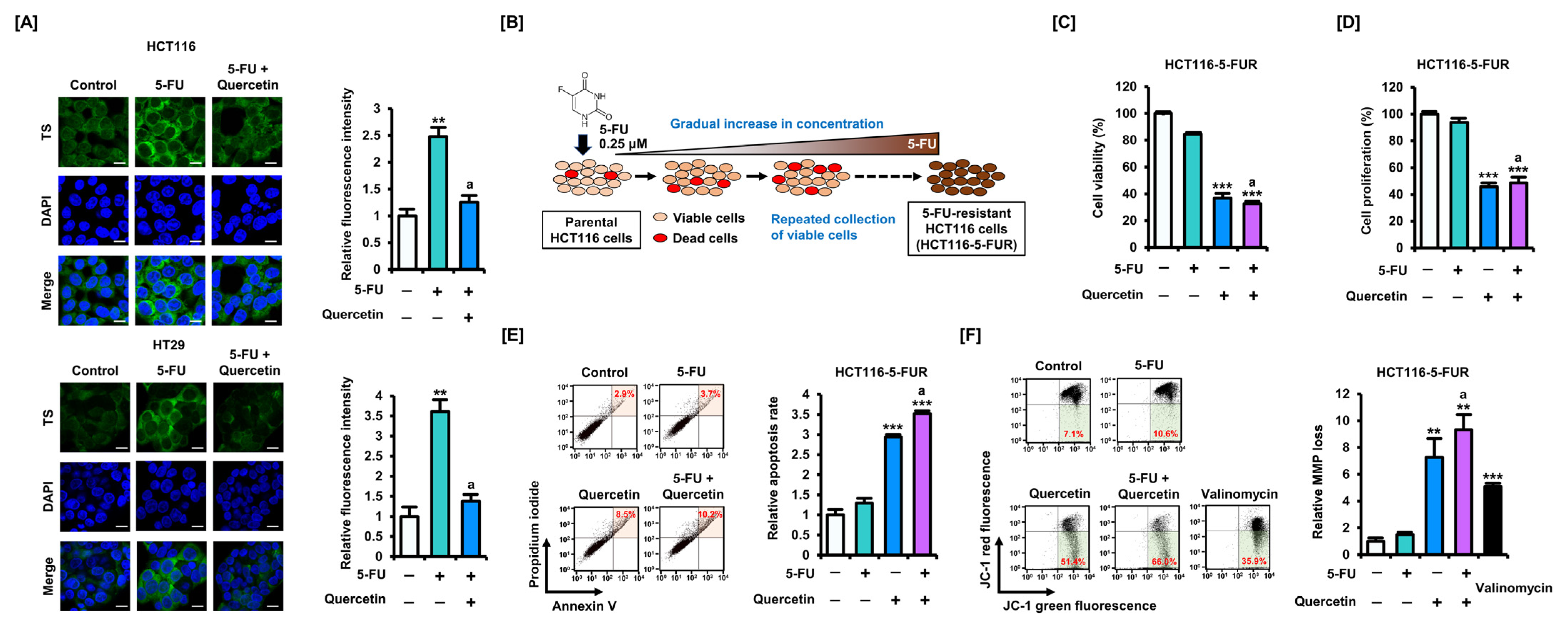
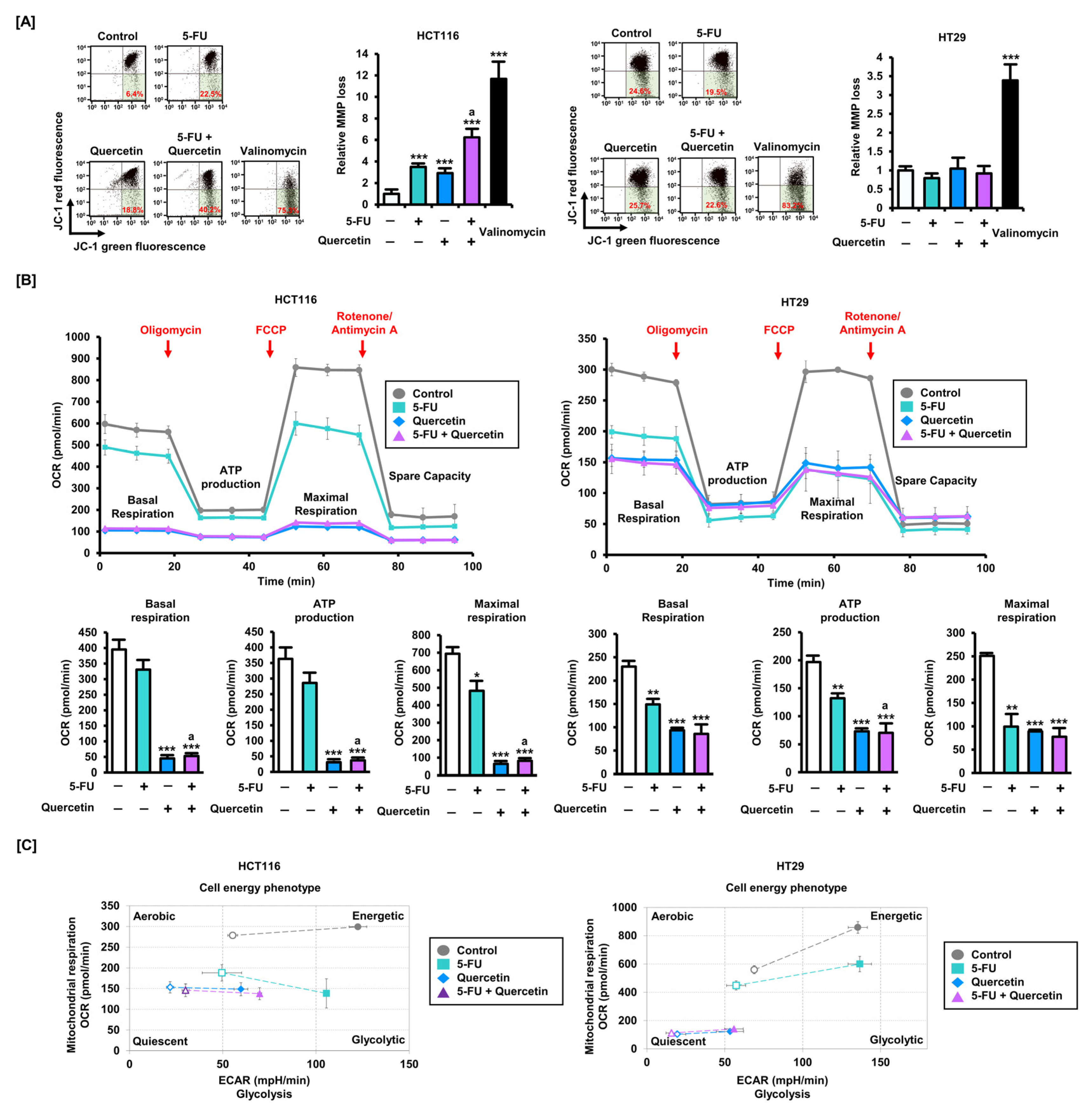
3.3. Quercetin Induces Mitochondrial Dysfunction in CRC Cells and Enhances Sensitivity to 5-FU
3.4. Quercetin Induces ROS Production and Abnormal Regulation of Ca2+ in CRC Cells
3.5. Quercetin Regulates the Expression Level of tiRNAs in CRC Cells and tiRNAHisGTG Potentiates the Apoptotic Effect of Quercetin
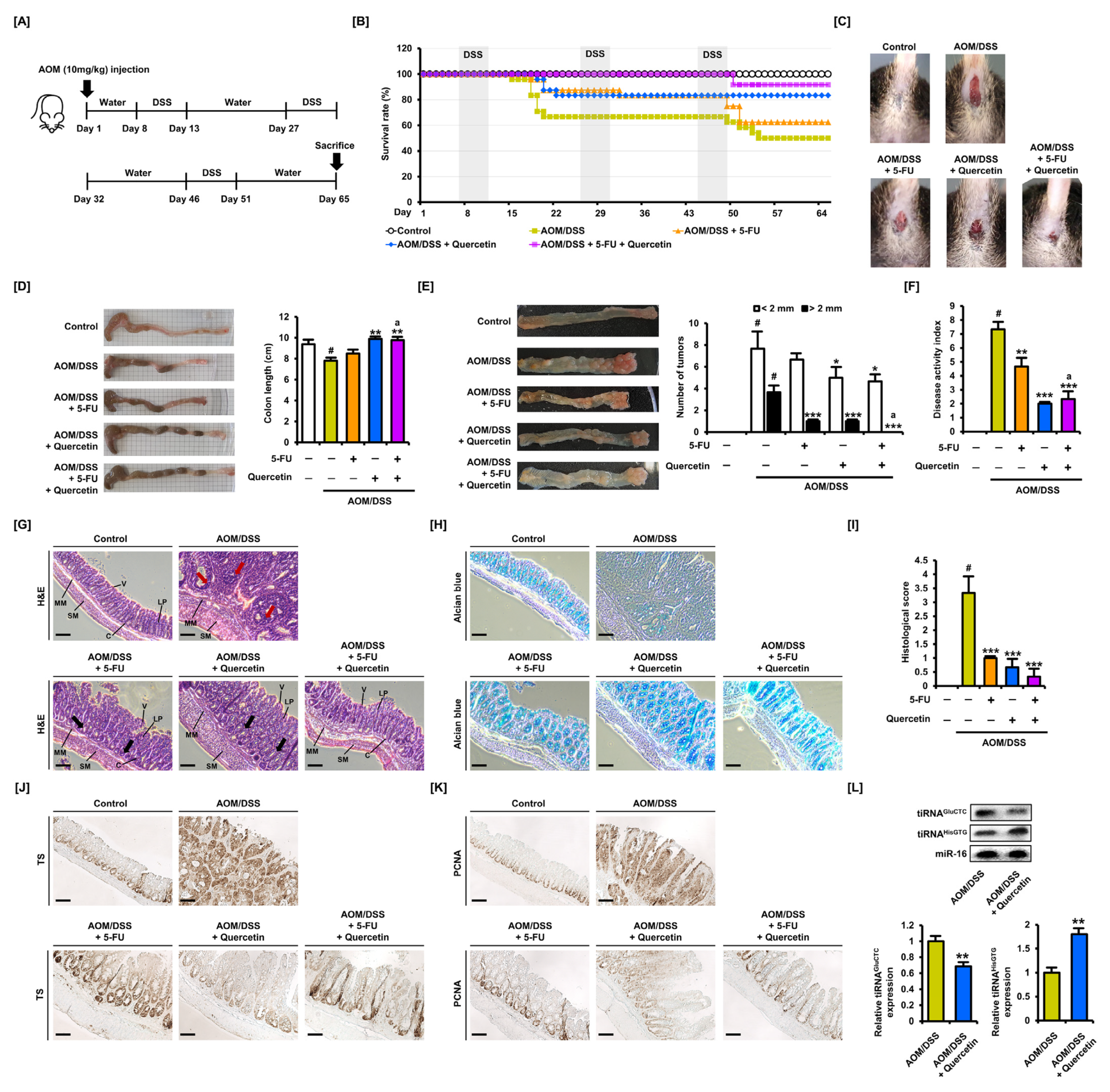
3.6. Oral Administration of Quercetin Induces an Inhibitory Effect on Tumor Growth in Colitis-Associated CRC Mice
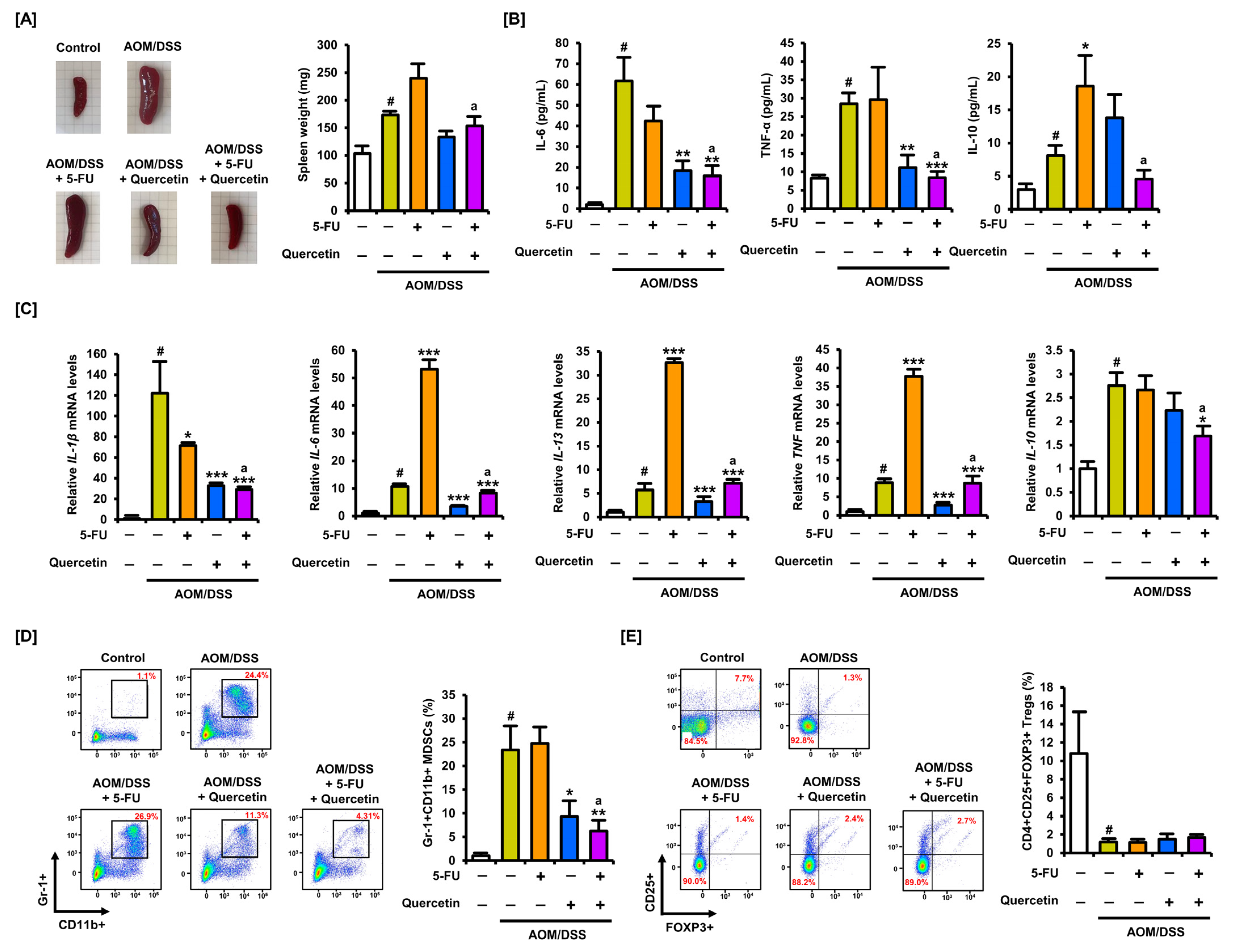
3.7. Quercetin Alleviates the Inflammatory Response Induced by 5-FU in Colitis-Associated CRC Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bathe, O.F.; Franceschi, D.; Livingstone, A.S.; Moffat, F.L.; Tian, E.; Ardalan, B. Increased thymidylate synthase gene expression in liver metastases from colorectal carcinoma: Implications for chemotherapeutic options and survival. Cancer J. Sci. Am. 1999, 5, 34–40. [Google Scholar] [PubMed]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Bae, H.; Lee, W.; Song, J.; Hong, T.; Kim, M.H.; Ham, J.; Song, G.; Lim, W. Polydatin Counteracts 5-Fluorouracil Resistance by Enhancing Apoptosis via Calcium Influx in Colon Cancer. Antioxidants 2021, 10, 1477. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Park, J.-H.; Jung, Y.; Kim, J.-H.; Jong, H.-S.; Kim, T.-Y.; Bang, Y.-J. Histone deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by down-regulating thymidylate synthase in human cancer cells. Mol. Cancer Ther. 2006, 5, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Bae, H.; Song, G.; Lim, W. Quercetin Affects Spermatogenesis-Related Genes of Mouse Exposed to High-Cholesterol Diet. J. Anim. Reprod. Biotechnol. 2020, 35, 73–85. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef]
- Ju, J.; Pedersen-Lane, J.; Maley, F.; Chu, E. Regulation of p53 expression by thymidylate synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 3769–3774. [Google Scholar] [CrossRef]
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 2021, 47, 102144. [Google Scholar] [CrossRef]
- Zhu, L.; Ge, J.; Li, T.; Shen, Y.; Guo, J. tRNA-derived fragments and tRNA halves: The new players in cancers. Cancer Lett. 2019, 452, 31–37. [Google Scholar] [CrossRef]
- Li, S.; Shi, X.; Chen, M.; Xu, N.; Sun, D.; Bai, R.; Chen, H.; Ding, K.; Sheng, J.; Xu, Z. Angiogenin promotes colorectal cancer metastasis via tiRNA production. Int. J. Cancer 2019, 145, 1395–1407. [Google Scholar] [CrossRef]
- Tsiakanikas, P.; Adamopoulos, P.G.; Tsirba, D.; Artemaki, P.I.; Papadopoulos, I.N.; Kontos, C.K.; Scorilas, A. High Expression of a tRNAPro Derivative Associates with Poor Survival and Independently Predicts Colorectal Cancer Recurrence. Biomedicines 2022, 10, 1120. [Google Scholar] [CrossRef]
- Luan, N.; Mu, Y.; Mu, J.; Chen, Y.; Ye, X.; Zhou, Q.; Xu, M.; Deng, Q.; Hu, Y.; Tang, Z.; et al. Dicer1 Promotes Colon Cancer Cell Invasion and Migration Through Modulation of tRF-20-MEJB5Y13 Expression Under Hypoxia. Front. Genet. 2021, 12, 638244. [Google Scholar] [CrossRef]
- Luan, N.; Chen, Y.; Li, Q.; Mu, Y.; Zhou, Q.; Ye, X.; Deng, Q.; Ling, L.; Wang, J.; Wang, J. TRF-20-M0NK5Y93 suppresses the metastasis of colon cancer cells by impairing the epithelial-to-mesenchymal transition through targeting Claudin-1. Am. J. Transl. Res. 2021, 13, 124–142. [Google Scholar]
- Lee, J.-Y.; Bae, H.; Yang, C.; Park, S.; Youn, B.-S.; Kim, H.-S.; Song, G.; Lim, W. Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer. Cancers 2020, 12, 1459. [Google Scholar] [CrossRef]
- Lee, W.; Song, G.; Bae, H. Laminarin Attenuates ROS-Mediated Cell Migration and Invasiveness through Mitochondrial Dysfunction in Pancreatic Cancer Cells. Antioxidants 2022, 11, 1714. [Google Scholar] [CrossRef]
- Lim, W.; Yang, C.; Bazer, F.W.; Song, G. Chrysophanol Induces Apoptosis of Choriocarcinoma Through Regulation of ROS and the AKT and ERK1/2 Pathways. J. Cell. Physiol. 2017, 232, 331–339. [Google Scholar] [CrossRef]
- Pliatsika, V.; Loher, P.; Magee, R.; Telonis, A.; Londin, E.; Shigematsu, M.; Kirino, Y.; Rigoutsos, I. MINTbase v2.0: A comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all The Cancer Genome Atlas projects. Nucleic Acids Res. 2018, 46, D152–D159. [Google Scholar] [CrossRef]
- Yang, C.; Park, S.; Song, G.; Lim, W. Inhibition of the cleaved half of tRNAGly enhances palmitic acid-induced apoptosis in human trophoblasts. J. Nutr. Biochem. 2022, 99, 108866. [Google Scholar] [CrossRef]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol. Biol. 2016, 1422, 297–307. [Google Scholar] [CrossRef]
- Shang, J.; Li, L.; Wang, X.; Pan, H.; Liu, S.; He, R.; Li, J.; Zhao, Q. Disruption of Tumor Necrosis Factor Receptor-Associated Factor 5 Exacerbates Murine Experimental Colitis via Regulating T Helper Cell-Mediated Inflammation. Mediat. Inflamm. 2016, 2016, 9453745. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lee, M.; Song, G.; Lim, W. tRNALys-Derived Fragment Alleviates Cisplatin-Induced Apoptosis in Prostate Cancer Cells. Pharmaceutics 2021, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, K.; Okamoto, I.; Tsukioka, S.; Uchida, J.; Kiniwa, M.; Fukuoka, M.; Nakagawa, K. Identification of thymidylate synthase as a potential therapeutic target for lung cancer. Br. J. Cancer 2010, 103, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Walle, T. Quercetin Induces Necrosis and Apoptosis in SCC-9 Oral Cancer Cells. Nutr. Cancer 2005, 53, 220–231. [Google Scholar] [CrossRef]
- Varghese, V.; Magnani, L.; Harada-Shoji, N.; Mauri, F.; Szydlo, R.M.; Yao, S.; Lam, E.W.-F.; Kenny, L.M. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci. Rep. 2019, 9, 1505. [Google Scholar] [CrossRef]
- Kramer, H.B.; Lai, C.-F.; Patel, H.; Periyasamy, M.; Lin, M.-L.; Feller, S.M.; Fuller-Pace, F.V.; Meek, D.W.; Ali, S.; Buluwela, L. LRH-1 drives colon cancer cell growth by repressing the expression of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res. 2016, 44, 582–594. [Google Scholar] [CrossRef]
- Samuel, T.; Fadlalla, K.; Mosley, L.; Katkoori, V.; Turner, T.; Manne, U. Dual-mode interaction between quercetin and DNA-damaging drugs in cancer cells. Anticancer Res. 2012, 32, 61–71. [Google Scholar]
- Can, G.; Akpinar, B.; Baran, Y.; Zhivotovsky, B.; Olsson, M. 5-Fluorouracil signaling through a calcium–calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene 2013, 32, 4529–4538. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Lee, M.; Yang, C.; Song, G.; Lim, W. Eupatilin Impacts on the Progression of Colon Cancer by Mitochondria Dysfunction and Oxidative Stress. Antioxidants 2021, 10, 957. [Google Scholar] [CrossRef]
- Li, T.; Shi, H.; Zhao, Y. Acetaldehyde induces tau phosphorylation via activation of p38 MAPK/JNK and ROS production. Mol. Cell. Toxicol. 2022, 18, 311–320. [Google Scholar] [CrossRef]
- Choi, J.; Yang, C.; Lim, W.; Song, G.; Choi, H. Antioxidant and apoptotic activity of cocoa bean husk extract on prostate cancer cells. Mol. Cell. Toxicol. 2022, 18, 193–203. [Google Scholar] [CrossRef]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, Y.; Yin, B.; Hao, W.; Zhao, L.; Ju, W.; Bai, C. 5-Fluorouracil and oxaliplatin modify the expression profiles of microRNAs in human colon cancer cells in vitro. Oncol. Rep. 2010, 23, 121–128. [Google Scholar] [CrossRef]
- Matuszyk, J. MALAT1-miRNAs network regulate thymidylate synthase and affect 5FU-based chemotherapy. Mol. Med. 2022, 28, 89. [Google Scholar] [CrossRef]
- Xu, F.; Ye, M.L.; Zhang, Y.P.; Li, W.J.; Li, M.T.; Wang, H.Z.; Qiu, X.; Xu, Y.; Yin, J.W.; Hu, Q.; et al. MicroRNA-375-3p enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Cancer Sci. 2020, 111, 1528–1541. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, N.; Han, Q.; Zhao, L.; Bai, C.; Chen, Y.; Zhou, J.; Zhao, R.C. MicroRNA-197 influences 5-fluorouracil resistance via thymidylate synthase in colorectal cancer. Clin. Transl. Oncol. 2015, 17, 876–883. [Google Scholar] [CrossRef]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-Induced tRNA Fragments Inhibit Translation Initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, J. Mucosal immunity and tRNA, tRF, and tiRNA. J. Mol. Med. 2020, 99, 47–56. [Google Scholar] [CrossRef]
- Li, S.; Xu, Z.; Sheng, J. tRNA-Derived Small RNA: A Novel Regulatory Small Non-Coding RNA. Genes 2018, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.-W.; Wang, H.-L.; Cheng, W.Y.; Liu, Q.-Q.; Chen, Y.-X.; Gao, Q.-Y. A specific tRNA half, 5′tiRNA-His-GTG, responds to hypoxia via the HIF1α/ANG axis and promotes colorectal cancer progression by regulating LATS2. J. Exp. Clin. Cancer Res. 2021, 40, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Ghareeb, W.M.; Lin, S.; Lu, X.; Huang, Y.; Huang, S.; Xu, Z.; Chi, P. A Comprehensive Repertoire of Transfer RNA-Derived Fragments and Their Regulatory Networks in Colorectal Cancer. J. Comput. Biol. 2020, 27, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, X.; Cai, X.; Liu, Y.; Li, C.; Liu, Q.; Qin, J.; Li, Y. Identification of tRNA-derived fragments in colon cancer by comprehensive small RNA sequencing. Oncol. Rep. 2019, 42, 735–744. [Google Scholar] [CrossRef]
- Shohan, M.; Nashibi, R.; Mahmoudian-Sani, M.-R.; Abolnezhadian, F.; Ghafourian, M.; Alavi, S.M.; Sharhani, A.; Khodadadi, A. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur. J. Pharmacol. 2022, 914, 174615. [Google Scholar] [CrossRef]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef]
- Heinz, S.A.; Henson, D.A.; Austin, M.D.; Jin, F.; Nieman, D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 2010, 62, 237–242. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Song, J.; Park, S.; Ham, J.; Park, W.; Park, H.; An, G.; Hong, T.; Kim, H.S.; Song, G.; et al. Targeting Thymidylate Synthase and tRNA-Derived Non-Coding RNAs Improves Therapeutic Sensitivity in Colorectal Cancer. Antioxidants 2022, 11, 2158. https://doi.org/10.3390/antiox11112158
Yang C, Song J, Park S, Ham J, Park W, Park H, An G, Hong T, Kim HS, Song G, et al. Targeting Thymidylate Synthase and tRNA-Derived Non-Coding RNAs Improves Therapeutic Sensitivity in Colorectal Cancer. Antioxidants. 2022; 11(11):2158. https://doi.org/10.3390/antiox11112158
Chicago/Turabian StyleYang, Changwon, Jisoo Song, Sunwoo Park, Jiyeon Ham, Wonhyoung Park, Hahyun Park, Garam An, Taeyeon Hong, Hee Seung Kim, Gwonhwa Song, and et al. 2022. "Targeting Thymidylate Synthase and tRNA-Derived Non-Coding RNAs Improves Therapeutic Sensitivity in Colorectal Cancer" Antioxidants 11, no. 11: 2158. https://doi.org/10.3390/antiox11112158
APA StyleYang, C., Song, J., Park, S., Ham, J., Park, W., Park, H., An, G., Hong, T., Kim, H. S., Song, G., & Lim, W. (2022). Targeting Thymidylate Synthase and tRNA-Derived Non-Coding RNAs Improves Therapeutic Sensitivity in Colorectal Cancer. Antioxidants, 11(11), 2158. https://doi.org/10.3390/antiox11112158







