Do Intestinal Unicellular Parasites Have a Role in the Inflammatory and Redox Status among the Severely Obese?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Study Population
2.3. Clinical Interview and Anthropometric Assessment
2.4. Stool Sampling and Molecular Parasitological Analysis
2.5. Laboratory Tests
2.6. Statistical Analysis
3. Results
3.1. Population Description
3.2. Inflammatory Biomarkers
3.3. Redox Biomarkers
3.4. Dietary Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, T.; Yang, W.; Chen, C.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, I.; Navarro, M.C.; Martín-Cordero, L.; Otero, E.; Hinchado, M.D.; Ortega, E. The Influence of Obesity and Weight Loss on the Bioregulation of Innate/Inflammatory Responses: Macrophages and Immunometabolism. Nutrients 2022, 14, 612. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of metabolic syndrome and dietary intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [Green Version]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. The Impact of Obesity, Adipose Tissue, and Tumor Microenvironment on Macrophage Polarization and Metastasis. Biology 2022, 11, 339. [Google Scholar] [CrossRef]
- Hertiš Petek, T.; Petek, T.; Močnik, M.; Marčun Varda, N. Systemic Inflammation, Oxidative Stress and Cardiovascular Health in Children and Adolescents: A Systematic Review. Antioxidants 2022, 11, 894. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Dent, M.S.; Hall, K. The insulin-like growth factor system in obesity, insulin resistance and type 2 diabetes mellitus. J. Clin. Med. 2014, 3, 1561–1574. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Koliaki, C.; Katsilambros, N. Repositioning the Role of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) on the TRAIL to the Development of Diabetes Mellitus: An Update of Experimental and Clinical Evidence. Int. J. Mol. Sci. 2022, 23, 3225. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef]
- Mastrocola, R.; Collotta, D.; Gaudioso, G.; Le Berre, M.; Cento, A.S.; Ferreira Alves, G.; Chiazza, F.; Verta, R.; Bertocchi, I.; Manig, F. Effects of exogenous dietary advanced glycation end products on the cross-talk mechanisms linking microbiota to metabolic inflammation. Nutrients 2020, 12, 2497. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Invest. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailloux, R.J. An update on mitochondrial reactive oxygen species production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Karaouzene, N.; Merzouk, H.; Aribi, M.; Merzouk, S.A.; Berrouiguet, A.Y.; Tessier, C.; Narce, M. Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: A comparison of older with young men. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 792–799. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.S.; Hua, N.W.; Reaven, G.M.; Stoohs, R.A. Hyperinsulinemia: The missing link among oxidative stress and age-related diseases? Free. Radic. Biol. Med. 2000, 29, 1302–1306. [Google Scholar] [CrossRef]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef]
- Kumar Rajendran, N.; George, B.P.; Chandran, R.; Tynga, I.M.; Houreld, N.; Abrahamse, H. The influence of light on reactive oxygen species and NF-κB in disease progression. Antioxidants 2019, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albano, E.; Mottaran, E.; Occhino, G.; Reale, E.; Vidali, M. role of oxidative stress in the progression of non-alcoholic steatosis. Aliment. Pharmacol. Ther. 2005, 22, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Gokulakrishnan, K.; Sampathkumar, R.; Farooq, S.; Ravikumar, R.; Mohan, V.; Balasubramanyam, M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin. Biochem. 2010, 43, 815–821. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Bryan, S.; Baregzay, B.; Spicer, D.; Singal, P.K.; Khaper, N. Redox-inflammatory synergy in the metabolic syndrome. Can. J. Physiol. Pharmacol. 2013, 91, 22–30. [Google Scholar] [CrossRef]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Wiria, A.E.; Djuardi, Y.; Supali, T.; Sartono, E.; Yazdanbakhsh, M. Helminth infection in populations undergoing epidemiological transition: A friend or foe? In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 34, pp. 889–901. [Google Scholar]
- Berbudi, A.; Ajendra, J.; Wardani, A.P.; Hoerauf, A.; Hübner, M.P. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes. Metab. Res. 2016, 32, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.; Lumb, F.E.; Harnett, M.M.; Harnett, W. Parasite excretory-secretory products and their effects on metabolic syndrome. Parasite Immunol. 2017, 39, e12410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, H.; Wu, Z.; Teo, J.D.; Tan, K.S. Statin pleiotropy prevents rho kinase-mediated intestinal epithelial barrier compromise induced by B lastocystis cysteine proteases. Cell. Microbiol. 2012, 14, 1474–1484. [Google Scholar] [CrossRef]
- Poirier, P.; Wawrzyniak, I.; Vivarès, C.P.; Delbac, F.; El Alaoui, H. New insights into Blastocystis spp.: A potential link with irritable bowel syndrome. PLoS Pathog. 2012, 8, e1002545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, H.; Handschack, A.; König, W.; Ambrosch, A. Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol. Res. 2001, 87, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.X.; Png, C.W.; Tay, C.Y.B.; Teo, J.D.W.; Jiao, H.; Lehming, N.; Tan, K.S.W.; Zhang, Y. Differential regulation of proinflammatory cytokine expression by mitogen-activated protein kinases in macrophages in response to intestinal parasite infection. Infect. Immun. 2014, 82, 4789–4801. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, V.S.; Chen, W.; Shu, D.; Paulus, B.; Bethwaite, P.; Tie, A.; Wilson, I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002, 122, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Mitchell, K.; Muench, D.G.; Scott, K. Giardia lamblia disrupts tight junctional ZO-1 and increases permeability in non-transformed human small intestinal epithelial monolayers: Effects of epidermal growth factor. Parasitology 2002, 125, 11–19. [Google Scholar] [CrossRef]
- Cotton, J.; Amat, C.; Buret, A. Disruptions of Host Immunity and Inflammation by Giardia Duodenalis: Potential Consequences for Co-Infections in the Gastro-Intestinal Tract. Pathogens 2015, 4, 764–792. [Google Scholar] [CrossRef] [Green Version]
- Ozbilge, H.; Aksoy, N.; Kilic, E.; Saraymen, R.; Yazar, S.; Vural, H. Evaluation of oxidative stress in cutaneous leishmaniasis. J. Dermatol. 2005, 32, 7–11. [Google Scholar] [CrossRef]
- Demirci, M.; Delibas, N.; Altuntas, I.; Oktem, F.; Yönden, Z. Serum iron, zinc and copper levels and lipid peroxidation in children with chronic giardiasis. J. Health Popul. Nutr. 2003, 21, 72–75. [Google Scholar] [PubMed]
- Bosch, S.S.; Kronenberger, T.; Meissner, K.A.; Zimbres, F.M.; Stegehake, D.; Izui, N.M.; Schettert, I.; Liebau, E.; Wrenger, C. Oxidative stress control by apicomplexan parasites. BioMed Res. Int. 2015, 2015, 351289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, E.; Yazar, S.; Saraymen, R.; Ozbilge, H. Serum malondialdehyde level in patients infected with Ascaris lumbricoides. World J. Gastroenterol. 2003, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Caudet, J.; Trelis, M.; Cifre, S.; Soriano, J.M.; Merino-Torres, J.F. Presence and significance of intestinal unicellular parasites in a morbidly obese population. Int. J. Obes. 2021, 46, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Caudet, J.; Trelis, M.; Cifre, S.; Soriano, J.M.; Rico, H.; Merino-Torres, J.F. Interplay between Intestinal Bacterial Communities and Unicellular Parasites in a Morbidly Obese Population: A Neglected Trinomial. Nutrients 2022, 14, 3211. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Bouzakri, K.; Zierath, J.R. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-α-induced insulin resistance. J. Biol. Chem. 2007, 282, 7783–7789. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.B.; Butterfield, D.A. Measurement of oxidized/reduced glutathione ratio. In Protein Misfolding and Cellular Stress in Disease and Aging; Springer: Berlin/Heidelberg, Germany, 2010; pp. 269–277. [Google Scholar]
- Stuehr, D.J. Mammalian nitric oxide synthases. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Hasan, S.; Maachee, M.; Córdova, O.M.; de la Guardia, R.D.; Martins, M.; Osuna, A. Human secretory immune response to fatty acid-binding protein fraction from Giardia lamblia. Infect. Immun. 2002, 70, 2226–2229. [Google Scholar] [CrossRef]
- Rosales-Borjas, D.M.; Díaz-Rivadeneyra, J.; Doña-Leyva, A.; Zambrano-Villa, S.A.; Mascaró, C.; Osuna, A.; Ortiz-Ortiz, L. Secretory immune response to membrane antigens during Giardia lamblia infection in humans. Infect. Immun. 1998, 66, 756–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, R.G. IgE, allergies and helminth parasites: A new perspective on an old conundrum. Immunol. Cell Biol. 1996, 74, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, M.C.; Hagel, I.; Lynch, N.R.; Jimenez, J.C.; Rojas, R.; Gil, M.; Mata, E. Association between giardiasis and allergy. Ann. Allergy Asthma Immunol. 1998, 81, 261–265. [Google Scholar] [CrossRef]
- Matowicka-Karna, J.; Dymicka-Piekarska, V.; Kemona, H. IFN-gamma, IL-5, IL-6 and IgE in patients infected with Giardia intestinalis. Folia Histochem. Cytobiol. 2009, 47, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Perez, O.; Lastre, M.; Bandera, F.; Díaz, M.; Domenech, I.; Fagundo, R.; Torres, D.; Finlay, C.; Campa, C.; Sierra, G. Evaluation of the immune response in symptomatic and asymptomatic human giardiasis. Arch. Med. Res. 1994, 25, 171–177. [Google Scholar]
- Gray, T.J.; Kwan, Y.L.; Phan, T.; Robertson, G.; Cheong, E.Y.; Gottlieb, T. Dientamoeba fragilis: A family cluster of disease associated with marked peripheral eosinophilia. Clin. Infect. Dis. 2013, 57, 845–848. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.I.D.; Vituri, C.D.L. Some hematimetric findings in human Giardia lambia infection. Rev. Inst. Med. Trop. São Paulo 1996, 38, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Lepczyńska, M.; Chen, W.; Dzika, E. Mysterious chronic urticaria caused by Blastocystis spp.? Int. J. Dermatol. 2016, 55, 259–266. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 2016, 22 (Suppl. S3), 1–203. [Google Scholar]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [Green Version]
- Ortega, R.M.; López-Sobaler, A.M.; Andrés, P.; Requejo, A.M.; Aparicio, A.; Molinero, L.M. DIAL Software for Assessing Diets and Food Calculations (for Windows, Version 3.0.0.5). 2013. Available online: http://www.alceingenieria.net/nutricion.htm (accessed on 1 September 2020).
- Lupton, J.R.; Brooks, J.; Butte, N.F.; Caballero, B.; Flatt, J.P.; Fried, S.K. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academy Press: Washington, DC, USA, 2002; Volume 5, pp. 589–768. [Google Scholar]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, S.L.; Faria, O.P.; Cardeal, M.D.A.; Ito, M.K. Validation Study of Multi-Frequency Bioelectrical Impedance with Dual-Energy X-ray Absorptiometry Among Obese Patients. Obes. Surg. 2014, 24, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arbelaez, D.; Bellido, D.; Castro, A.I.; Ordoñez-Mayan, L.; Carreira, J.; Galban, C.; Martinez-Olmos, M.A.; Crujeiras, A.B.; Sajoux, I.; Casanueva, F.F. Body Composition Changes After Very-Low-Calorie Ketogenic Diet in Obesity Evaluated by 3 Standardized Methods. J. Clin. Endocrinol. Metab. 2017, 102, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Autier, B.; Gangneux, J.; Robert-Gangneux, F. Evaluation of the AllplexTM Gastrointestinal Panel—Parasite Assay for Protozoa Detection in Stool Samples: A Retrospective and Prospective Study. Microorganisms 2020, 8, 569. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, H.S.; Kim, J.J.; Park, Y.J.; Kim, D.; Yong, D. Detection of Intestinal Protozoa in Korean Patients Using BD MAX Enteric Parasite Panel and Seegene Allplex Gastrointestinal Parasite Assay. Lab. Med. Online 2020, 10, 221–226. [Google Scholar] [CrossRef]
- Ascaso, J.F.; Real, J.T.; Priego, A.; Carmena, R.; Romero, P.; Valdecabres, C. Cuantificación de insulinorresistencia con los valores de insulina basal e índice HOMA en una población no diabética. Med. Clínica 2001, 117, 530–533. [Google Scholar] [CrossRef]
- Carretero, A.; León, Z.; García-Cañaveras, J.C.; Zaragoza, Á.; Gómez-Lechón, M.J.; Donato, M.T.; Lahoz, A. In vitro/in vivo screening of oxidative homeostasis and damage to DNA, protein, and lipids using UPLC/MS-MS. Anal. Bioanal. Chem. 2014, 406, 5465–5476. [Google Scholar] [CrossRef]
- Verdon, C.P.; Burton, B.A.; Prior, R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP when the Griess reaction is used to assay for nitrite. Anal. Biochem. 1995, 224, 502–508. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- National Heart, Lung and Blood Institute in cooperation with the National Institute of Diabetes and Digestive and Kidney Diseases (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report; National Heart, Lung, and Blood Institute: Rockville, MD, USA, 1998. [Google Scholar]
- Reeves, G.M.; Postolache, T.T.; Mazaheri, S.; Snitker, S.; Langenberg, P.; Giegling, I.; Hartmann, A.; Konte, B.; Friedl, M.; Okusaga, O. A positive association between T. gondii seropositivity and obesity. Front. Public Health 2013, 1, 73. [Google Scholar] [CrossRef] [Green Version]
- Salem, D.A.; Salem, N.A.; Hendawy, S.R. Association between Toxoplasma gondii infection and metabolic syndrome in obese adolescents: A possible immune-metabolic link. Parasitol. Int. 2021, 83, 102343. [Google Scholar] [CrossRef]
- Brooks, G.C.; Blaha, M.J.; Blumenthal, R.S. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol. 2010, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’Agostino, R., Jr.; Williams, K.; Karter, A.J.; Mayer-Davis, E.J.; Tracy, R.P.; Haffner, S.M. The relation of body fat mass and distribution to markers of chronic inflammation. Int. J. Obes. 2001, 25, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, V.; Schmitt, V.H.; Zeller, T.; Panova-Noeva, M.; Schulz, A.; Laubert-Reh, D.; Juenger, C.; Schnabel, R.B.; Abt, T.G.J.; Laskowski, R.; et al. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care 2015, 38, 1356–1364. [Google Scholar] [CrossRef] [Green Version]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandramathi, S.; Suresh, K.; Shuba, S.; Mahmood, A.; Kuppusamy, U.R. High levels of oxidative stress in rats infected with Blastocystis hominis. Parasitology 2010, 137, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, M.; Lee, S.; Davis, C.G.; Koo, S.I.; Chun, O.K. Dietary total antioxidant capacity is associated with diet and plasma antioxidant status in healthy young adults. J. Acad. Nutr. Diet. 2012, 112, 1626–1635. [Google Scholar] [CrossRef]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and Systemic Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Olusi, S.O. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. 2002, 26, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Ozata, M.; Mergen, M.; Oktenli, C.; Aydin, A.; Sanisoglu, S.Y.; Bolu, E.; Yilmaz, M.I.; Sayal, A.; Isimer, A.; Ozdemir, I.C. Increased oxidative stress and hypozincemia in male obesity. Clin. Biochem. 2002, 35, 627–631. [Google Scholar] [CrossRef]
- Hermsdorff, H.H.M.; Puchau, B.; Volp, A.C.P.; Barbosa, K.B.; Bressan, J.; Zulet, M.; Martínez, J.A. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr. Metab. 2011, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Ciotola, M.; Schisano, B.; Misso, L.; Giannetti, G.; Ceriello, A.; Giugliano, D. Oxidative stress in the metabolic syndrome. J. Endocrinol. Invest. 2006, 29, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Tinahones, F.J.; Murri-Pierri, M.; Garrido-Sánchez, L.; García-Almeida, J.M.; García-Serrano, S.; García-Arnés, J.; García-Fuentes, E. Oxidative stress in severely obese persons is greater in those with insulin resistance. Obesity 2009, 17, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Maschirow, L.; Khalaf, K.; Al-Aubaidy, H.A.; Jelinek, H.F. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes—Biomarkers as a possible tool for early disease detection for rural screening. Clin. Biochem. 2015, 48, 581–585. [Google Scholar] [CrossRef] [PubMed]
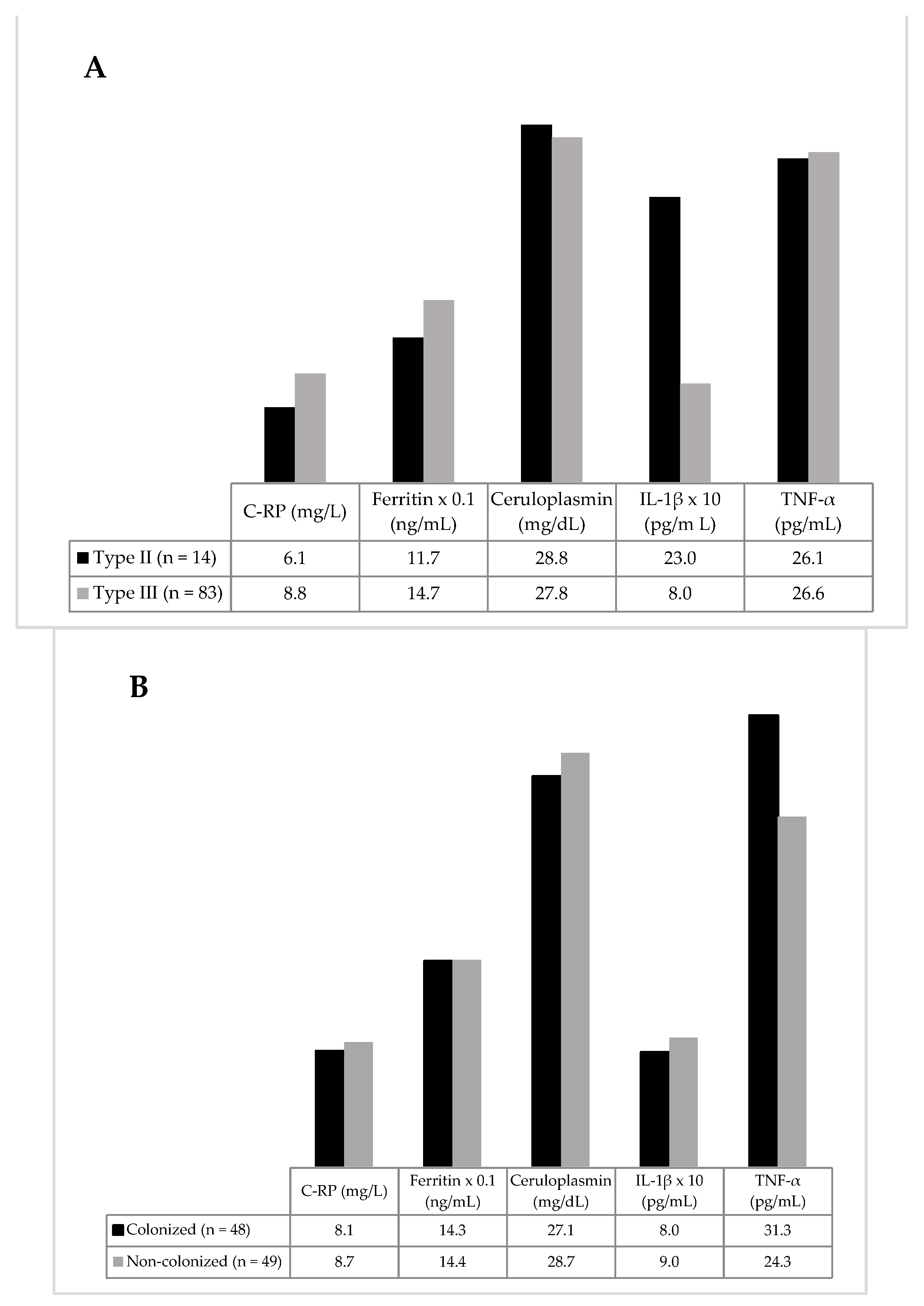
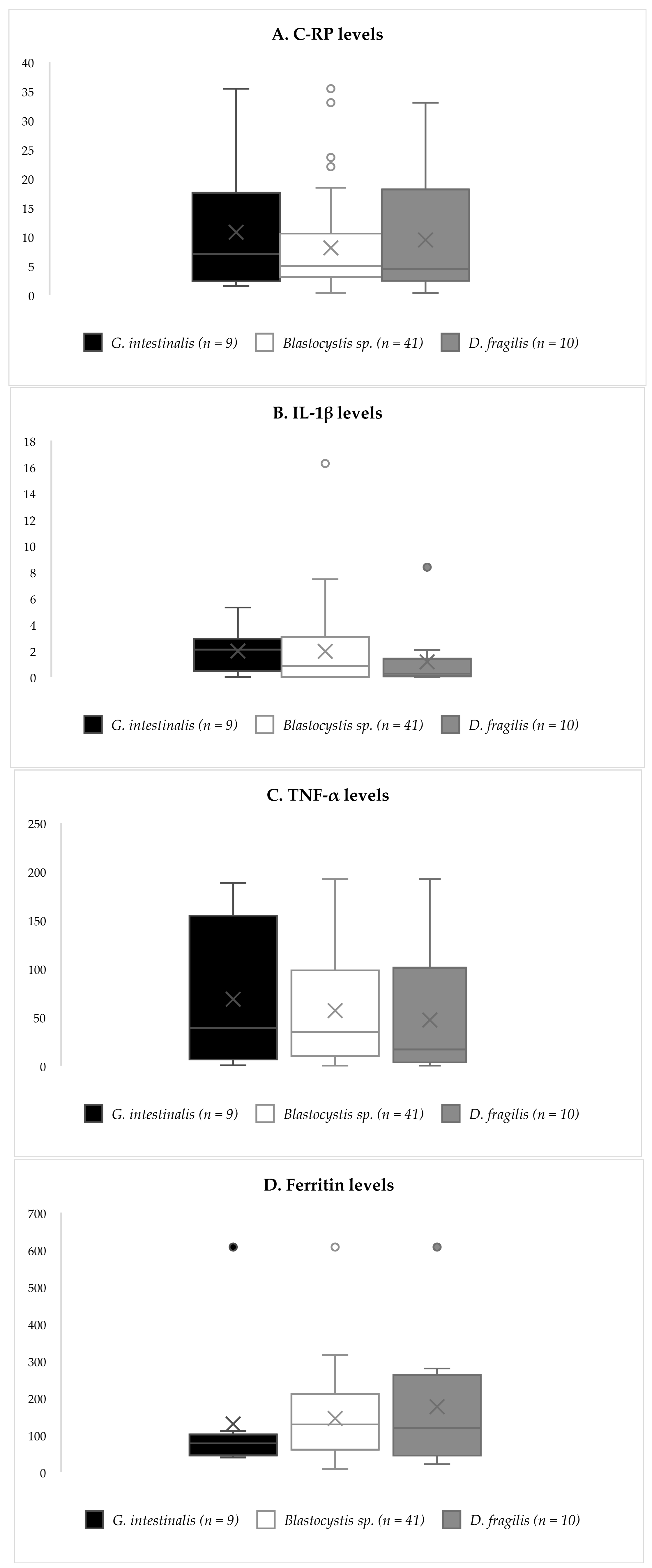

| Colonized | Non-Colonized | Chi 2 Test | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Women | 27 | 56.3 | 31 | 63.3 | Ns |
| Type II obesity | 6 | 12.5 | 8 | 16.3 | Ns |
| Smokers | 12 | 25 | 9 | 18.4 | Ns |
| Hypertension | 25 | 52.1 | 29 | 59.2 | Ns |
| Dyslipidaemia | 38 | 79.2 | 35 | 71.4 | Ns |
| Type 2 Diabetes | 11 | 22.9 * | 20 | 40.8 * | p = 0.047 |
| Hepatic steatosis | 31+ | 33.7 | 38+ | 41.3 | Ns |
| Hyperuricemia | 23 | 47.9 | 25 | 51 | Ns |
| Metabolic syndrome | 23 | 47.9 | 31 | 63.3 | Ns |
| Cardiovascular disease | 3 | 6.3 | 5 | 10.2 | Ns |
| Mean | SD | Mean | SD | t test | |
| HOMA-IR | 5.2 * | 2.3 | 7.1 * | 4.7 | p = 0.021 |
| Age (years) | 47.9 | 9.7 | 47.8 | 9.7 | Ns |
| Insulin-Sensitivity Status 1 | WT | Fatty Liver Disease Status 2 | WT | |||
|---|---|---|---|---|---|---|
| Resistant (n = 59) | Sensitive (n = 28) | NAFLD + (n = 69) | NAFLD - (n = 23) | |||
| C-RP (mg/L) | 9.8 * | 5.9 * | p = 0.025 | 8.5 | 7.9 | Ns |
| Ferritin (ng/mL) | 215.1 | 101.0 | Ns | 155.8 * | 87.6 * | p = 0.009 |
| Ceruloplasmin (mg/dL) | 25.5 | 26.0 | Ns | 26.0 | 28.0 | Ns |
| IL-1β (pg/mL) | 1.0 | 2.6 | Ns | 1.9 | 2.4 | Ns |
| TNF-α (pg/mL) | 12.0 | 49.9 | Ns | 16.9 | 33.4 | Ns |
| Non-Colonized (n = 49) | Colonized (n = 48) | t Test | Blastocystis sp. (n = 41) | t Test | G. Intestinalis (n = 9) | D. Fragilis (n = 10) | |
|---|---|---|---|---|---|---|---|
| Leucocytes (×103/µL) | 7.5 | 7.8 | Ns | 7.8 | Ns | 8.4 | 7.3 |
| Neutrophils (×103/µL) | 4.1 | 4.6 | Ns | 4.5 | Ns | 5.0 | 4.2 |
| Lymphocytes (×103/µL) | 3.17 | 2.28 | Ns | 2.6 | Ns | 2.3 | 2.3 |
| Eosinophils (×103/µL) | 0.16 * | 0.24 * | p = 0.018 | 0.24 * | p = 0.021 | 0.25 | 0.24 |
| Monocytes (×103/µL) | 0.46 * | 0.61 * | p = 0.002 | 0.60 * | p = 0.010 | 0.58 | 0.58 |
| WT | WT | ||||||
| IgA (mg/dL) | 289.2 | 265.0 | Ns | 273.4 | Ns | 218.3 | 249.1 |
| IgE (kUA/L) | 47.0 | 70.0 | Ns | 62.0 | Ns | 83.0 | 62.0 |
| Vitamin A (mcg/dL) | ||||
|---|---|---|---|---|
| Deficiency (<42) (n = 16) | Normal (42–68) (n = 55) | High (>68) (n = 26) | ANOVA Test | |
| MDA (ng/mL) | 421.9 | 410.67 | 424.88 | |
| GSH/GSSG | 0.38 | 0.36 | 0.37 | 0.95 |
| TAC (mM) | 2.680 | 3.449 | 3.178 | 0.13 |
| SOD-3 (U/mL) | 1.081 | 1.630 | 1.273 | 0.28 |
| NOX (µg/mL) | 26.8 | 33.0 | 34.5 | 0.11 |
| Vitamin E (mcg/mL) | ||||
| Deficiency (<8.6) (n = 1) | Normal (8.6–13) (n = 21) | High (>13) (n = 75) | ANOVA test | |
| MDA (ng/mL) | 442.0 | 379.6 | 426.3 | 0.16 |
| GSH/GSSG | 0.16 | 0.37 | 0.36 | 0.46 |
| TAC (mM) | 1.611 * | 2.949 * | 3.355 * | p = 0.02 |
| SOD-3 (U/mL) | 0 | 1.289 | 1.444 | 0.35 |
| NOX (µg/mL) | 26.9 | 31.3 | 32.8 | 0.83 |
| Colonized (n = 48) | Non-Colonized (n = 49) | t Test | B/DF * (n = 39) Mean | Gi (n = 9) Mean | t Test | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| MDA (ng/mL) | 408.5 | 72.4 | 424.0 | 103.4 | 0.53 | 402.1 | 436.2 | 0.46 |
| GSH/GSSG | 0.33 * | 0.2 | 0.40 * | 0.2 | p = 0.034 * | 0.33 | 0.33 | 0.53 |
| TAC (mM) | 3.57 * | 1.1 | 2.93 * | 1.1 | p = 0.009 * | 3.69 | 3.11 | 0.16 |
| SOD-3 (U/mL) | 1.65 | 1.2 | 1.24 | 1.1 | 0.10 | 1.81 | 0.97 | 0.05 |
| NOX (µg/mL) | 29.9 | 15.2 | 34.7 | 18.0 | 0.34 | 29.9 | 30.2 | 0.98 |
| VitA (µg/dL) | 55.1 | 12.1 | 60.3 | 23.9 | 0.65 | 56.5 | 49.3 | 0.17 |
| VitE (µg/mL) | 15.8 | 3.8 | 17.1 | 5.3 | 0.34 | 15.9 | 15.4 | 0.75 |
| Insulin-Sensitivity Status 1 | t Test | Fatty Liver Disease Status 2 | ||||
|---|---|---|---|---|---|---|
| Resistant (n = 59) | Sensitive (n = 28) | NAFLD + (n = 69) | NAFLD - (n = 23) | t Test | ||
| MDA (ng/mL) | 411.1 | 407.9 | 0.82 | 412.2 | 420.3 | 0.25 |
| GSH/GSSG | 0.388 * | 0.297 * | p = 0.011 * | 0.383 * | 0.303 * | p = 0.045 * |
| TAC (mM) | 3.29 | 3.16 | 0.59 | 3.28 | 3.15 | 0.60 |
| SOD-3 (U/mL) | 1.66 * | 1.04 * | p = 0.025 * | 1.47 | 1.35 | 0.61 |
| NOX (µg/mL) | 36.7 * | 25.3 * | p = 0.01 * | 34.9 * | 24.5 * | p = 0.002 * |
| Insulin-Resistant | Insulin-Sensitive | |||||
|---|---|---|---|---|---|---|
| Colonized (n = 30) | Non-Colonized (n = 29) | T Test | Colonized (n = 15) | Non-Colonized (n = 11) | T Test | |
| MDA (ng/mL) | 414.5 | 407.7 | 0.78 | 396.6 | 426.1 | 0.22 |
| GSH/GSSG | 0.33 * | 0.45 * | p = 0.007 * | 0.33 | 0.26 | p = 0.024 * |
| TAC (mM) | 3.59 * | 2.97 * | p = 0.022 * | 3.64 * | 2.54 * | p = 0.04 * |
| SOD-3 (U/mL) | 1.84 | 1.48 | 0.25 | 1.12 | 0.82 | 0.43 |
| NOX (µg/mL) | 33.3 | 39.8 | 0.19 | 25.4 | 24.8 | 0.89 |
| Correlation | Estimate | Confidence Interval | p-Value |
|---|---|---|---|
| MDA and HOMA-IR | 10.08 | [3.5, 16.67] | 0.003 |
| MDA and plasmatic leptin | −0.92 | [−1.70, −0.15] | 0.020 |
| MDA and plasmatic Vitamin A | −1.7 | [−2.92, −0.48] | 0.007 |
| NOX and plasmatic ferritin | 0.043 | [0.005, 0.081] | 0.028 |
| SOD-3 and plasmatic C-RP levels | 0.05 | [0.007, 0.096] | 0.025 |
| TAC and % fat mass | 0.123 | [−0.247, 0.001] | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caudet, J.; Trelis, M.; Cifre, S.; Tapia, G.; Soriano, J.M.; Rodrigo, R.; Merino-Torres, J.F. Do Intestinal Unicellular Parasites Have a Role in the Inflammatory and Redox Status among the Severely Obese? Antioxidants 2022, 11, 2090. https://doi.org/10.3390/antiox11112090
Caudet J, Trelis M, Cifre S, Tapia G, Soriano JM, Rodrigo R, Merino-Torres JF. Do Intestinal Unicellular Parasites Have a Role in the Inflammatory and Redox Status among the Severely Obese? Antioxidants. 2022; 11(11):2090. https://doi.org/10.3390/antiox11112090
Chicago/Turabian StyleCaudet, Jana, María Trelis, Susana Cifre, Gabriela Tapia, José M. Soriano, Regina Rodrigo, and Juan F. Merino-Torres. 2022. "Do Intestinal Unicellular Parasites Have a Role in the Inflammatory and Redox Status among the Severely Obese?" Antioxidants 11, no. 11: 2090. https://doi.org/10.3390/antiox11112090
APA StyleCaudet, J., Trelis, M., Cifre, S., Tapia, G., Soriano, J. M., Rodrigo, R., & Merino-Torres, J. F. (2022). Do Intestinal Unicellular Parasites Have a Role in the Inflammatory and Redox Status among the Severely Obese? Antioxidants, 11(11), 2090. https://doi.org/10.3390/antiox11112090











