Hemoxygenase-1 Promotes Head and Neck Cancer Cell Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioinformatic Analysis
2.2. Cell Culture
2.3. Human Tumor Tissue and Cell Culture
2.4. Pharmacological Modulation of HO-1
2.5. Stable Overexpression of HO-1 in Human HN13 Cell Line
2.6. Evaluation of Cell Viability and Proliferation
2.7. Immunofluorescence
2.8. Cell Cycle Analysis
2.9. Wound-Healing Assay
2.10. Immunoblotting
2.11. Immunohistochemical Staining
2.12. Statistical Analysis
3. Results
3.1. HO-1 mRNA Expression Is Associated with Patient Survival and HPV (-) Infection
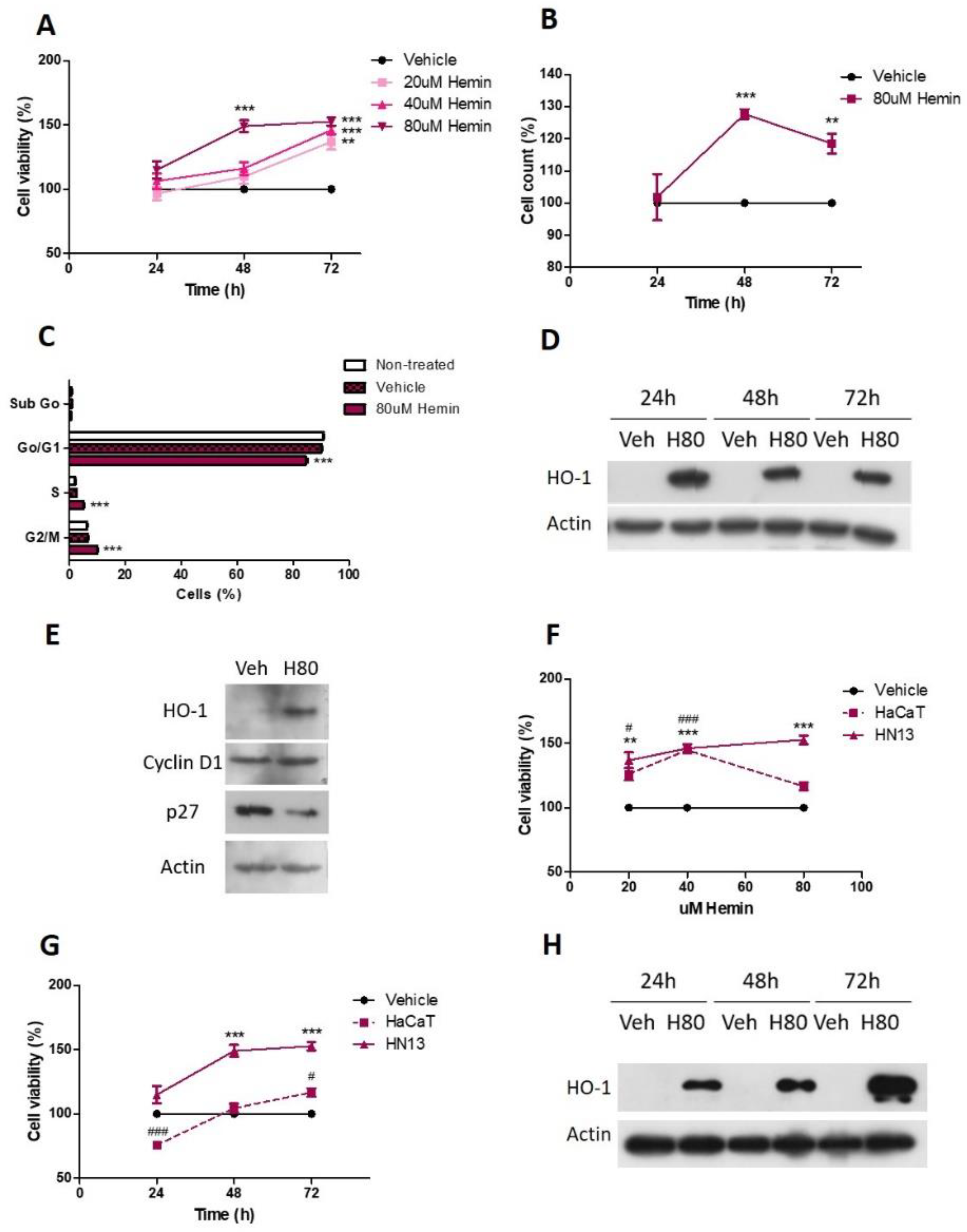
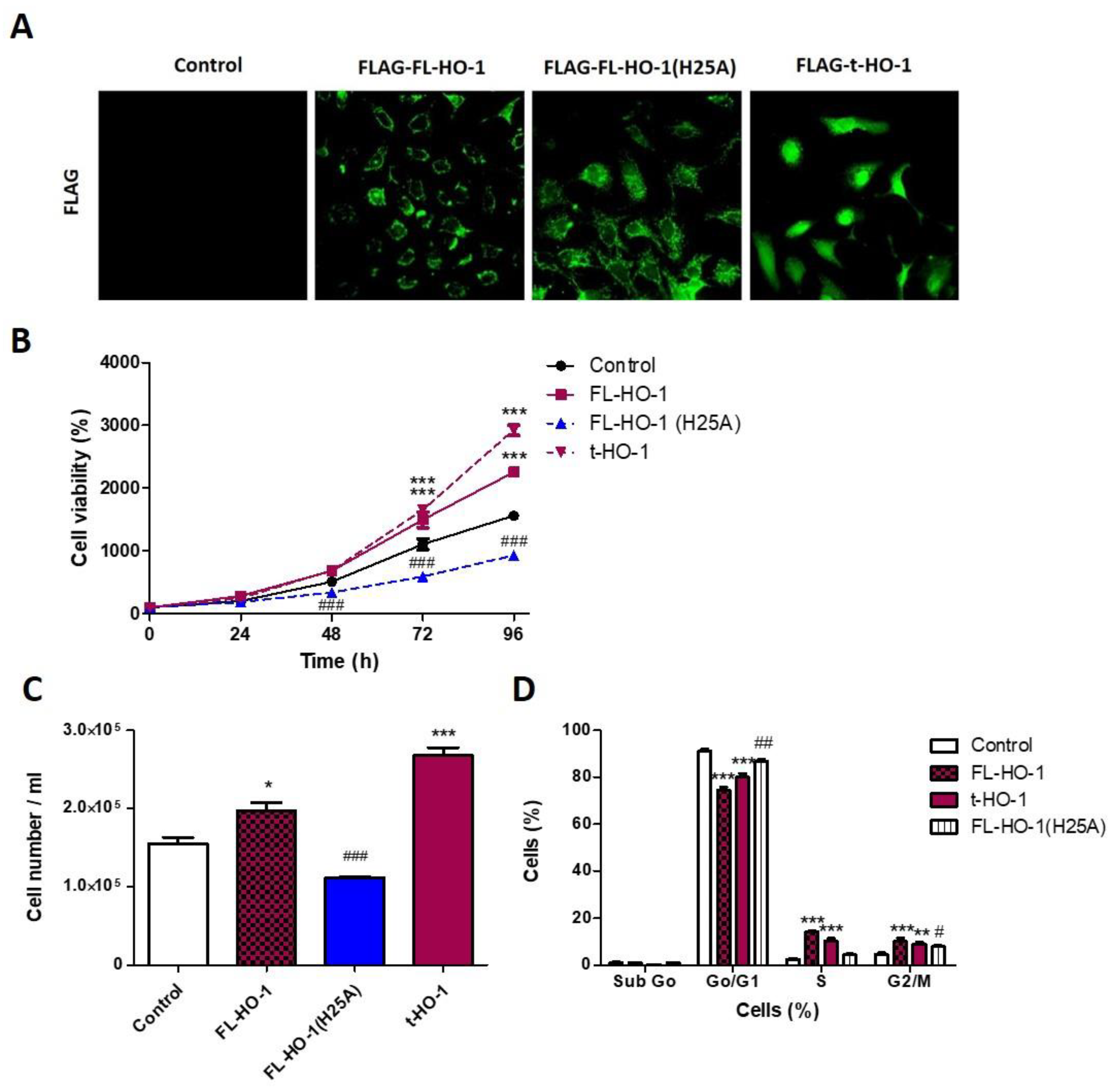
3.2. Pharmacological HO-1 Activation Increases Cell Viability and Cell Cycle Progression of HN13 Cells
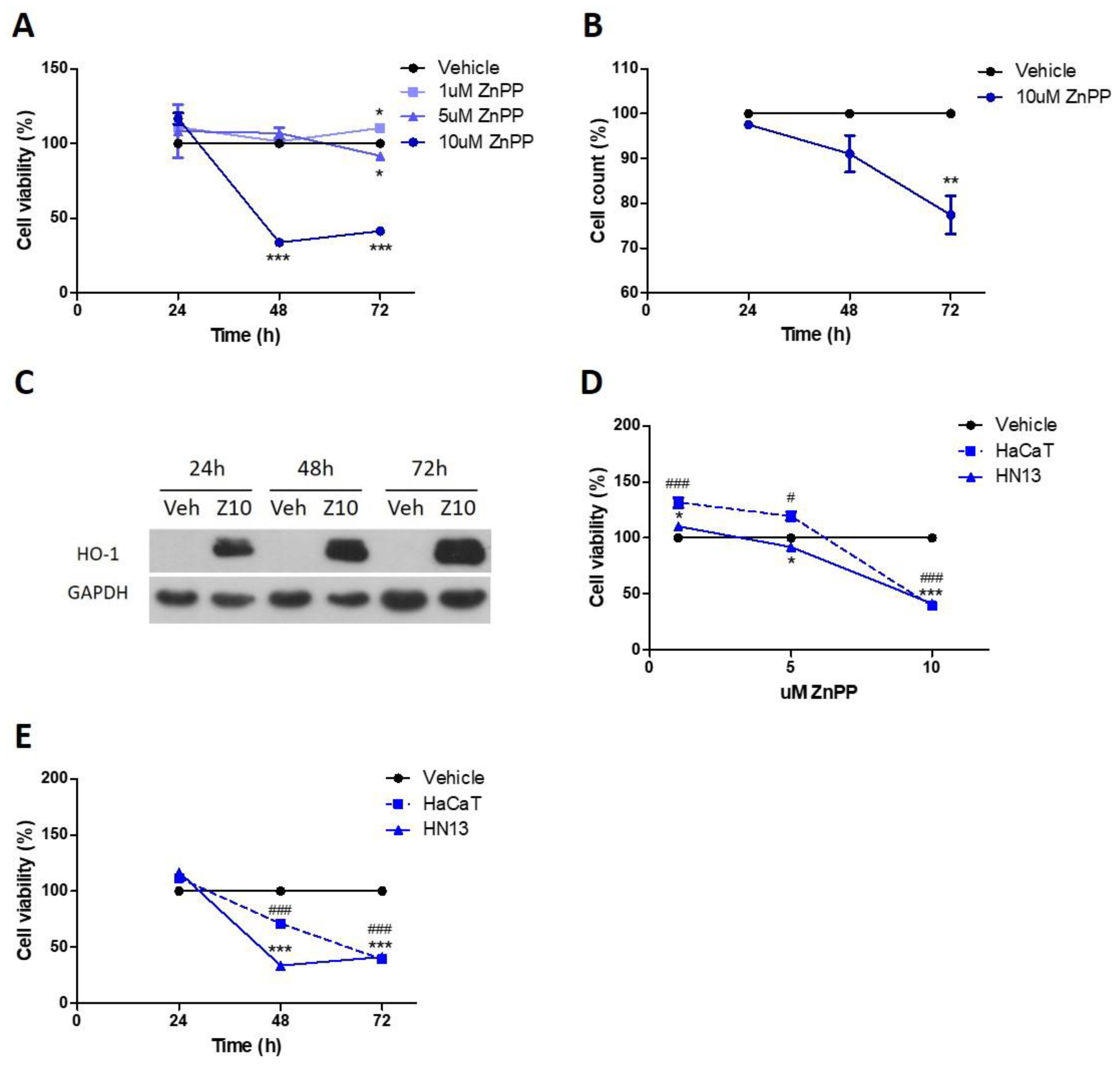
3.3. Pharmacological HO-1 Inhibition Decreases Cell Viability
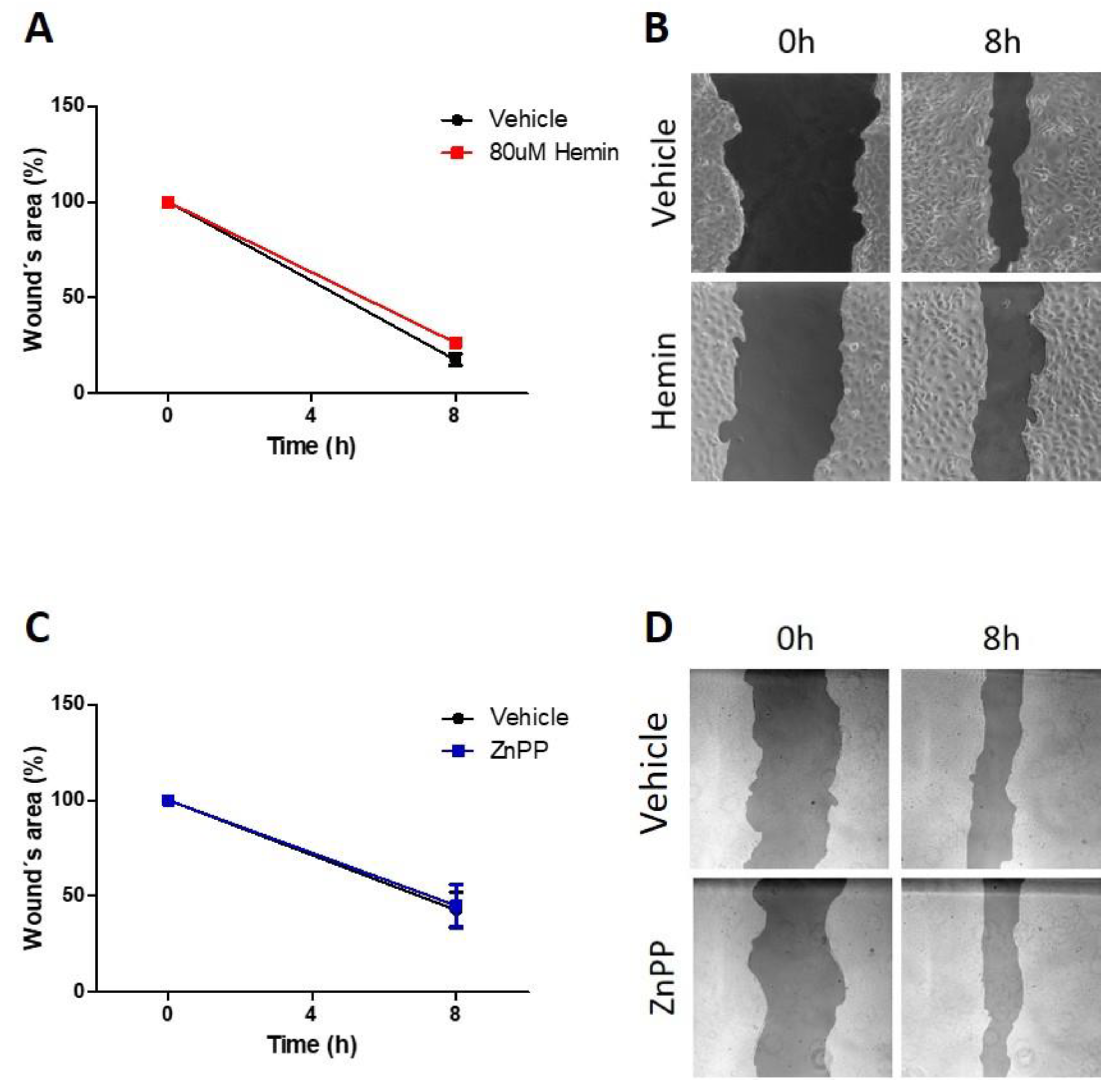
3.4. Pharmacological Modulation of HO-1 Does Not Alter Migratory Capacity
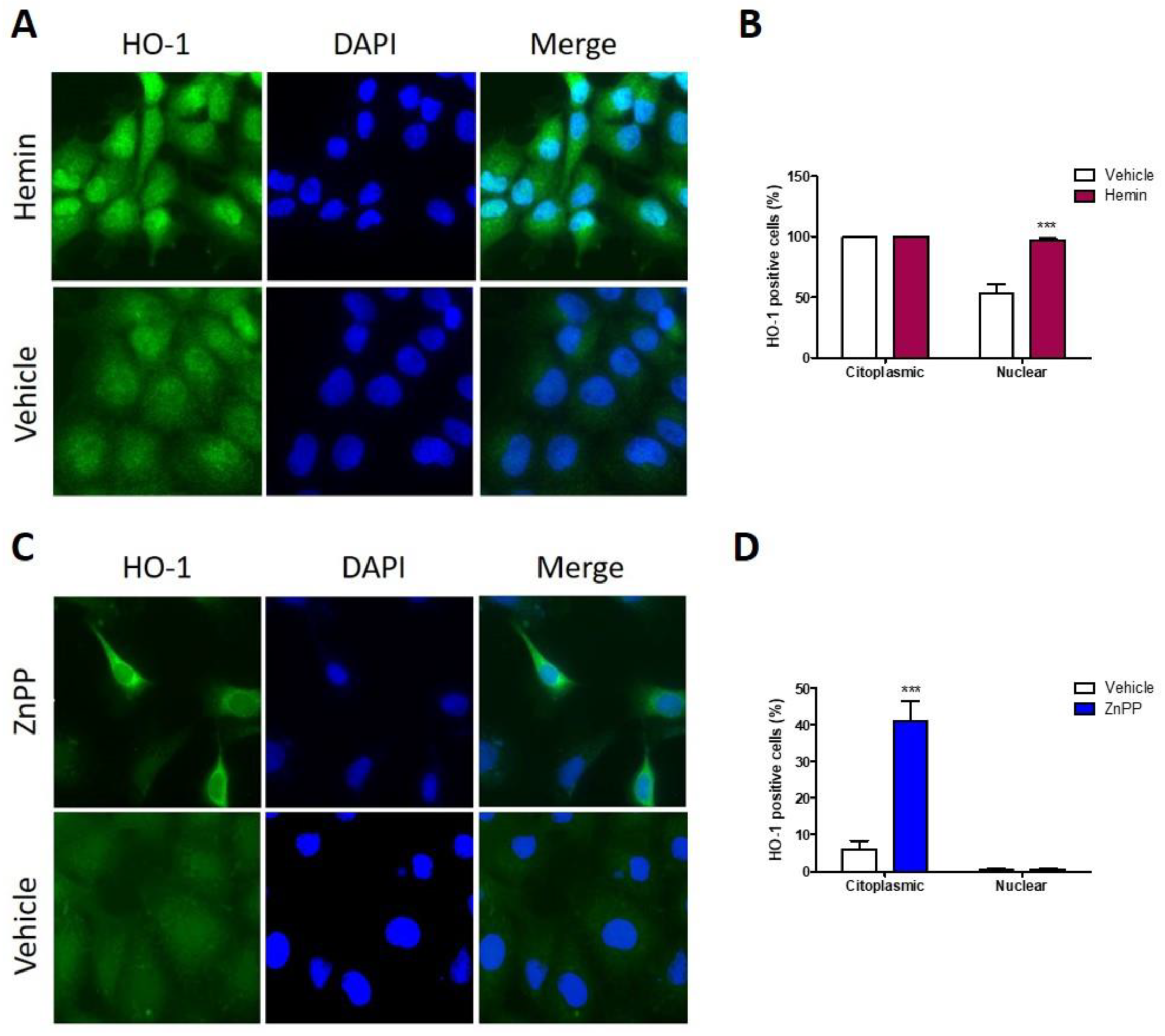
3.5. Nuclear HO-1 Expression Is Enhanced after Hemin Treatment, Whereas Strong Cytoplasmic HO-1 Is Found following ZnPP Treatment
3.6. Nuclear HO-1 Increases Cell Viability
3.7. Pharmacological Modulation of HO-1 Affects Squamous Cell Carcinoma Cells Viability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maines, M.D.; Gibbs, P.E.M. 30 some years of heme oxygenase: From a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem. Biophys. Res. Commun. 2005, 338, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Mascaró, M.; Alonso, E.N.; Alonso, E.G.; Lacunza, E.; Curino, A.C.; Facchinetti, M.M. Nuclear Localization of Heme Oxygenase-1 in Pathophysiological Conditions: Does It Explain the Dual Role in Cancer? Antioxidants 2021, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Andrés, N.C.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Ferro, A.; Donna, L.G.; Curino, A.C.; Facchinetti, M.M. Heme oxygenase-1 has antitumoral effects in colorectal cancer: Involvement of p53. Exp. Mol. Pathol. 2014, 97, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zou, C.; Cheng, W.; Li, Q.; Han, Z.; Wang, X.; Jin, J.; Zou, J.; Liu, Z.; Zhou, Z.; et al. Heme oxygenase-1 retards hepatocellular carcinoma progression through the microRNA pathway. Oncol. Rep. 2016, 36, 2715–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueron, G.; Giudice, J.; Valacco, P.; Paez, A.; Elguero, B.; Toscani, M.; Jaworski, F.; Leskow, F.C.; Cotignola, J.; Marti, M.; et al. Heme-oxygenase-1 implications in cell morphology and the adhesive behavior of prostate cancer cells. Oncotarget 2014, 5, 4087–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagawa, T.; Omura, K.; Harada, H.; Nakaso, K.; Iwasa, S.; Koyama, Y.; Onizawa, K.; Yusa, H.; Yoshida, H. Heme oxygenase-1 expression predicts cervical lymph node metastasis of tongue squamous cell carcinomas. Oral Oncol. 2004, 40, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Gandini, N.A.; Alonso, E.N.; Fermento, M.E.; Mascaró, M.; Abba, M.C.; Coló, G.P.; Arévalo, J.; Ferronato, M.J.; Guevara, J.A.; Núñez, M.; et al. Heme Oxygenase-1 Has an Antitumor Role in Breast Cancer. Antioxid. Redox Signal. 2019, 30, 2030–2049. [Google Scholar] [CrossRef]
- Skrzypek, K.; Tertil, M.; Golda, S.; Ciesla, M.; Weglarczyk, K.; Collet, G.; Guichard, A.; Kozakowska, M.; Boczkowski, J.; Was, H.; et al. Interplay Between Heme Oxygenase-1 and miR-378 Affects Non-Small Cell Lung Carcinoma Growth, Vascularization, and Metastasis. Antioxid. Redox Signal. 2013, 19, 644–660. [Google Scholar] [CrossRef] [Green Version]
- Gandini, N.A.; Fermento, M.E.; Salomón, D.G.; Blasco, J.; Patel, V.; Gutkind, J.S.; Molinolo, A.A.; Facchinetti, M.M.; Curino, A.C. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp. Mol. Pathol. 2012, 93, 237–245. [Google Scholar] [CrossRef]
- Degese, M.S.; Mendizabal, J.E.; Gandini, N.A.; Gutkind, J.S.; Molinolo, A.; Hewitt, S.M.; Curino, A.C.; Coso, O.A.; Facchinetti, M.M. Expression of heme oxygenase-1 in non-small cell lung cancer (NSCLC) and its correlation with clinical data. Lung Cancer 2012, 77, 168–175. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Getz, G.; Ally, A.; Balasundaram, M.; Birol, I.; et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Podkalicka, P.; Mieczkowski, M.; Kachamakova-Trojanowska, N.; Dulak, J.; Łoboda, A.; Stępniewski, J.; Mucha, O.; Józkowicz, A.; Biela, A.; Czarnek, M. Pharmacological versus genetic inhibition of heme oxygenase-1—The comparison of metalloporphyrins, shRNA and CRISPR/Cas9 system. Acta Biochim. Pol. 2018, 65, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Hsu, F.-F.; Yeh, C.-T.; Sun, Y.-J.; Chiang, M.-T.; Lan, W.-M.; Li, F.-A.; Lee, W.-H.; Chau, L.-Y. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene 2015, 34, 2360–2370. [Google Scholar] [CrossRef]
- Ferronato, M.J.; Nadal Serrano, M.; Arenas Lahuerta, E.J.; Bernadó Morales, C.; Paolillo, G.; Martinez-Sabadell Aliguer, A.; Santalla, H.; Mascaró, M.; Vitale, C.; Fall, Y.; et al. Vitamin D analogues exhibit antineoplastic activity in breast cancer patient-derived xenograft cells. J. Steroid Biochem. Mol. Biol. 2021, 208, 105735. [Google Scholar] [CrossRef]
- Mascaró, M.; Pibuel, M.A.; Lompardía, S.L.; Díaz, M.; Zotta, E.; Bianconi, M.I.; Lago, N.; Otero, S.; Jankilevich, G.; Alvarez, E.; et al. Low molecular weight hyaluronan induces migration of human choriocarcinoma JEG-3 cells mediated by RHAMM as well as by PI3K and MAPK pathways. Histochem. Cell Biol. 2017, 148, 173–187. [Google Scholar] [CrossRef]
- Gebäck, T.; Schulz, M.M.P.; Koumoutsakos, P.; Detmar, M. TScratch: A novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques 2009, 46, 265–274. [Google Scholar] [CrossRef]
- Wang, S.; Avery, J.E.; Hannafon, B.N.; Lind, S.E.; Ding, W.Q. Zinc protoporphyrin suppresses cancer cell viability through a heme oxygenase-1-independent mechanism: The involvement of the Wnt/β-catenin signaling pathway. Biochem. Pharmacol. 2013, 85, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Min, S.-K.; Lee, S.-K.; Pae, H.-O.; Chung, H.-T.; Kim, H.-R.; Guo, H.-Y.; Hong, S.-H.; Lee, H.-J.; Lee, S.-K.; Lee, J.; et al. Differential induction of heme oxygenase-1 against nicotine-induced cytotoxicity via the PI3K, MAPK, and NF-kappa B pathways in immortalized and malignant human oral keratinocytes. J. Oral Pathol. Med. 2010, 37, 278–286. [Google Scholar] [CrossRef]
- Chien, M.H.; Yang, W.E.; Yang, Y.C.; Ku, C.C.; Lee, W.J.; Tsai, M.Y.; Lin, C.W.; Yang, S.F. Dual targeting of the p38 MAPK-HO-1 axis and cIAP1/XIAP by demethoxycurcumin triggers caspase-mediated apoptotic cell death in oral squamous cell carcinoma cells. Cancers 2020, 12, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.J.; Kim, Y.M.; Chang, K.C. Hemin Reduces HMGB1 Release by UVB in an AMPK/HO-1-dependent Pathway in Human Keratinocytes HaCaT Cells. Arch. Med. Res. 2017, 48, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Lin, C.-W.; Su, C.-W.; Yang, W.-E.; Chuang, C.-Y.; Su, S.-C.; Hsieh, M.-J.; Yang, S.-F. Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways. Biomedicines 2021, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-Y.; Lin, C.-W.; Su, C.-W.; Chen, Y.-T.; Yang, W.-E.; Yang, S.-F.; Su, S.-C. Deoxyshikonin Mediates Heme Oxygenase-1 Induction and Apoptotic Response via p38 Signaling in Tongue Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 7115. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-Y.; Chen, P.-J.; Chuang, Y.-C.; Lo, Y.-S.; Lin, C.-C.; Hsieh, M.-J.; Chen, M.-K. Picrasidine I Triggers Heme Oxygenase-1-Induced Apoptosis in Nasopharyngeal Carcinoma Cells via ERK and Akt Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 6103. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Lin, C.-C.; Lo, Y.-S.; Chuang, Y.-C.; Ho, H.-Y.; Chen, M.-K. Chrysosplenol D Triggers Apoptosis through Heme Oxygenase-1 and Mitogen-Activated Protein Kinase Signaling in Oral Squamous Cell Carcinoma. Cancers 2021, 13, 4327. [Google Scholar] [CrossRef]
- Liu, R.; Peng, J.; Wang, H.; Li, L.; Wen, X.; Tan, Y.; Zhang, L.; Wan, H.; Chen, F.; Nie, X. Oxysophocarpine Retards the Growth and Metastasis of Oral Squamous Cell Carcinoma by Targeting the Nrf2/HO-1 Axis. Cell. Physiol. Biochem. 2018, 49, 1717–1733. [Google Scholar] [CrossRef]
- Su, C.-W.; Chuang, C.-Y.; Chen, Y.-T.; Yang, W.-E.; Pan, Y.-P.; Lin, C.-W.; Yang, S.-F. FLLL32 Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells by Regulating the p38 Pathway. Int. J. Mol. Sci. 2021, 22, 11860. [Google Scholar] [CrossRef]
- Yang, I.-H.; Ahn, C.-H.; Cho, N.-P.; Jin, B.; Lee, W.; Jung, Y.C.; Hong, S.D.; Shin, J.; Cho, S. Heme Oxygenase-1 is a Key Molecule Underlying Differential Response of TW-37-Induced Apoptosis in Human Mucoepidermoid Carcinoma Cells. Molecules 2019, 24, 1700. [Google Scholar] [CrossRef] [Green Version]
- So, K.-Y.; Kim, S.-H.; Jung, K.-T.; Lee, H.-Y.; Oh, S.-H. MAPK/JNK1 activation protects cells against cadmium-induced autophagic cell death via differential regulation of catalase and heme oxygenase-1 in oral cancer cells. Toxicol. Appl. Pharmacol. 2017, 332, 81–91. [Google Scholar] [CrossRef]
- Shi, L.; Fang, J. Implication of heme oxygenase-1 in the sensitivity of nasopharyngeal carcinomas to radiotherapy. J. Exp. Clin. Cancer Res. 2008, 27, 13. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Song, D.; Niu, Y.; Wang, B. Inhibition of heme oxygenase-1 enhances the chemosensitivity of laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis 2016, 21, 489–501. [Google Scholar] [CrossRef]
- Yu, T.-J.; Tang, J.-Y.; Ou-Yang, F.; Wang, Y.-Y.; Yuan, S.-S.F.; Tseng, K.; Lin, L.-C.; Chang, H.-W. Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules 2020, 10, 777. [Google Scholar] [CrossRef]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; Vanella, L.; Conticello, C.; Romano, A.; Saccone, S.; Godos, J.; Di Raimondo, F.; Li Volti, G. Heme oxygenase-1 nuclear translocation regulates bortezomib-induced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget 2016, 7, 28868–28880. [Google Scholar] [CrossRef] [Green Version]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; La Cava, P.; Parrinello, N.; Vanella, L.; Stagno, F.; Palumbo, G.A.; Li Volti, G.; Di Raimondo, F. Nuclear Translocation of Heme Oxygenase-1 Confers Resistance to Imatinib in Chronic Myeloid Leukemia Cells. Curr. Pharm. Des. 2013, 19, 2765–2770. [Google Scholar] [CrossRef]
- Yang, W.E.; Ho, C.C.; Yang, S.F.; Lin, S.H.; Yeh, K.T.; Lin, C.W.; Chen, M.K. Cathepsin B expression and the correlation with clinical aspects of oral squamous cell carcinoma. PLoS ONE 2016, 11, e0152165. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Fang, J.; Liu, Y.; Song, J.J.; Wang, Y.Q.; Xia, J.; Cheng, B.; Wang, Z. High level of calpain1 promotes cancer cell invasion and migration in oral squamous cell carcinoma. Oncol. Lett. 2017, 13, 4017–4026. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascaró, M.; Alonso, E.G.; Schweitzer, K.; Rabassa, M.E.; Carballido, J.A.; Ibarra, A.; Alonso, E.N.; Bermúdez, V.; Fernández Chavez, L.; Coló, G.P.; et al. Hemoxygenase-1 Promotes Head and Neck Cancer Cell Viability. Antioxidants 2022, 11, 2077. https://doi.org/10.3390/antiox11102077
Mascaró M, Alonso EG, Schweitzer K, Rabassa ME, Carballido JA, Ibarra A, Alonso EN, Bermúdez V, Fernández Chavez L, Coló GP, et al. Hemoxygenase-1 Promotes Head and Neck Cancer Cell Viability. Antioxidants. 2022; 11(10):2077. https://doi.org/10.3390/antiox11102077
Chicago/Turabian StyleMascaró, Marilina, Exequiel G. Alonso, Karen Schweitzer, Martín E. Rabassa, Jessica A. Carballido, Agustina Ibarra, Eliana N. Alonso, Vicente Bermúdez, Lucía Fernández Chavez, Georgina P. Coló, and et al. 2022. "Hemoxygenase-1 Promotes Head and Neck Cancer Cell Viability" Antioxidants 11, no. 10: 2077. https://doi.org/10.3390/antiox11102077
APA StyleMascaró, M., Alonso, E. G., Schweitzer, K., Rabassa, M. E., Carballido, J. A., Ibarra, A., Alonso, E. N., Bermúdez, V., Fernández Chavez, L., Coló, G. P., Ferronato, M. J., Pichel, P., Recio, S., Clemente, V., Fermento, M. E., Facchinetti, M. M., & Curino, A. C. (2022). Hemoxygenase-1 Promotes Head and Neck Cancer Cell Viability. Antioxidants, 11(10), 2077. https://doi.org/10.3390/antiox11102077






