Lyso-DGTS Lipid Derivatives Enhance PON1 Activities and Prevent Oxidation of LDL: A Structure–Activity Relationship Study
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Isolation of Lyso-DGTS
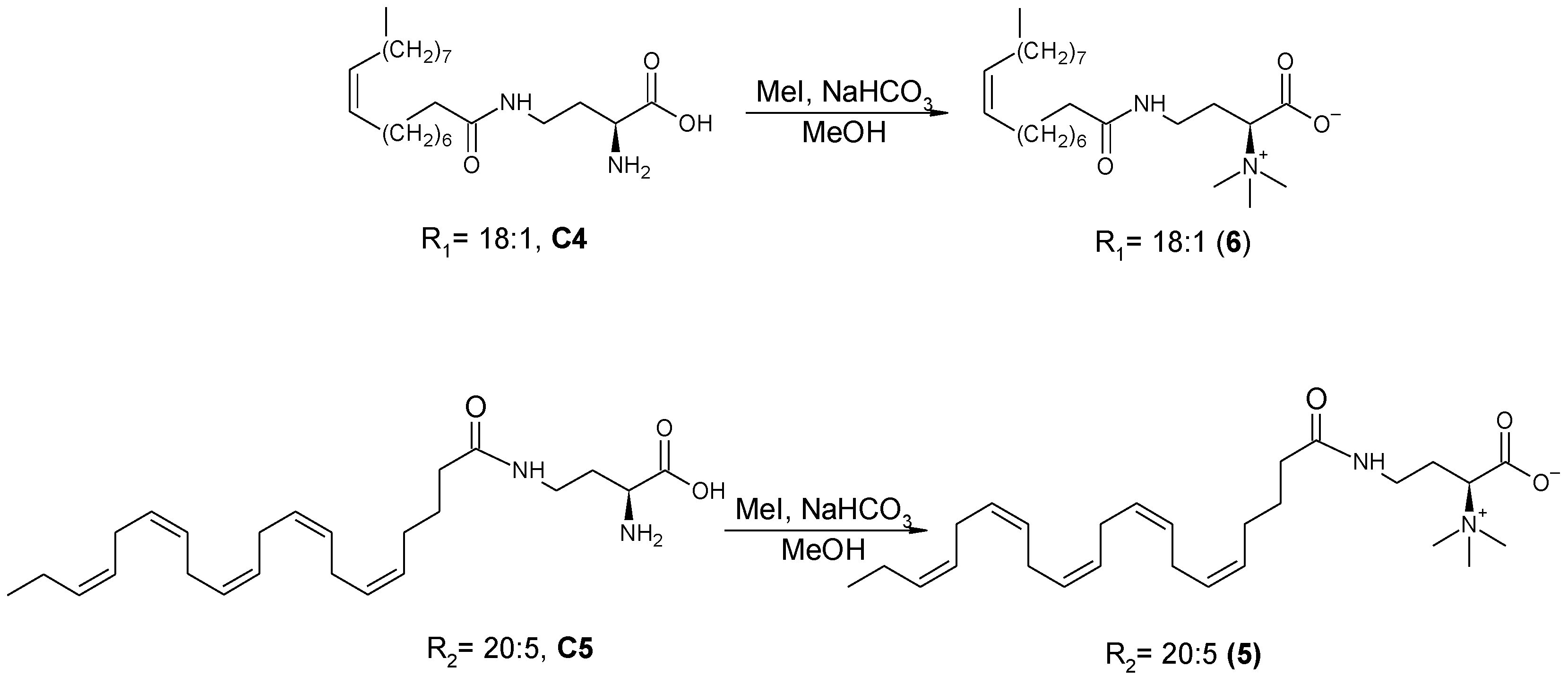
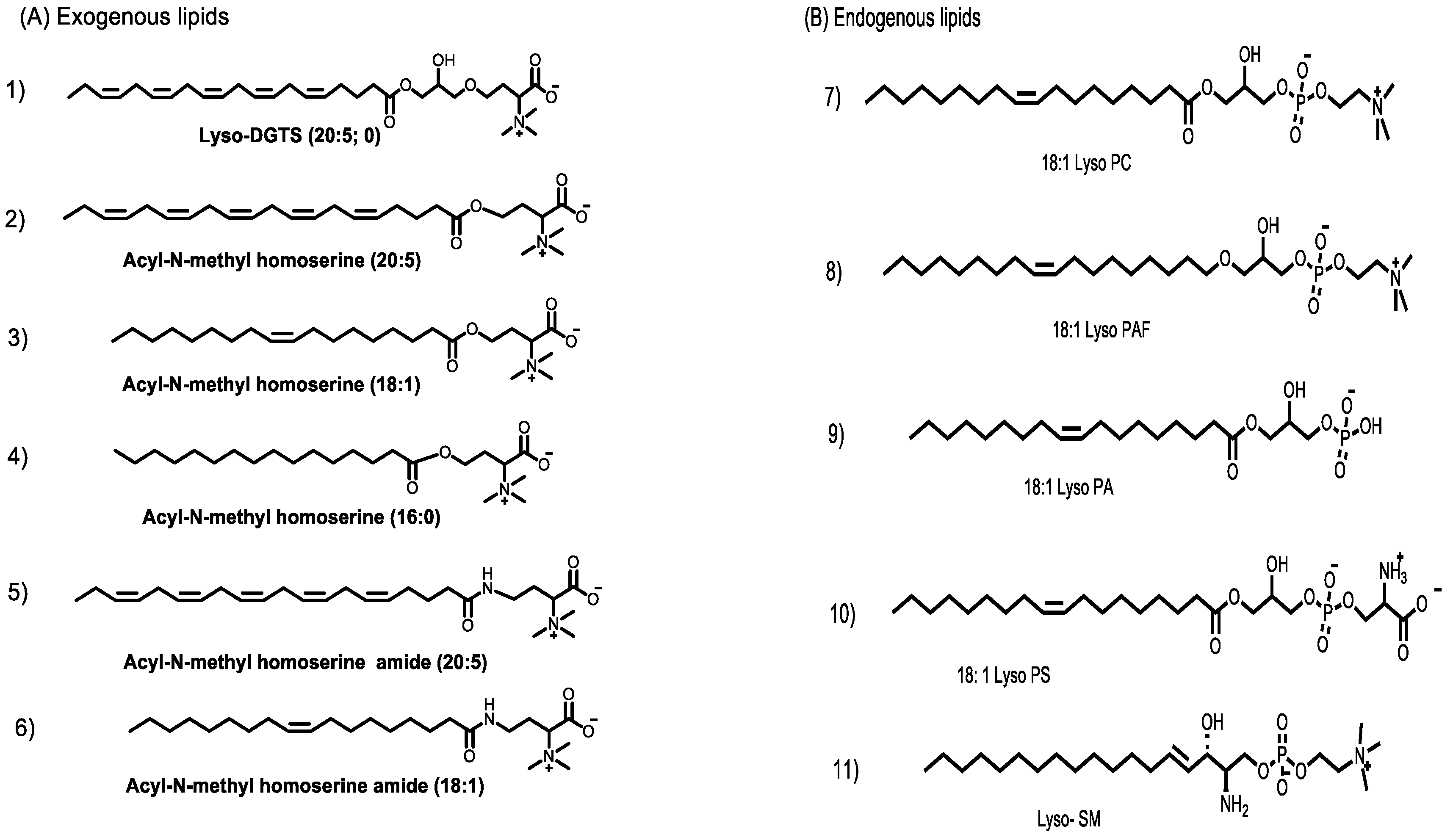
2.3. Synthesis of Lyso-DGTS Derivatives
2.3.1. General Procedure for the Synthesis of (S)-1-Carboxy-3-hydroxy-N,N,N-trimethylpropan-1-aminium (l-Homoserine Betaine) (Scheme 1)
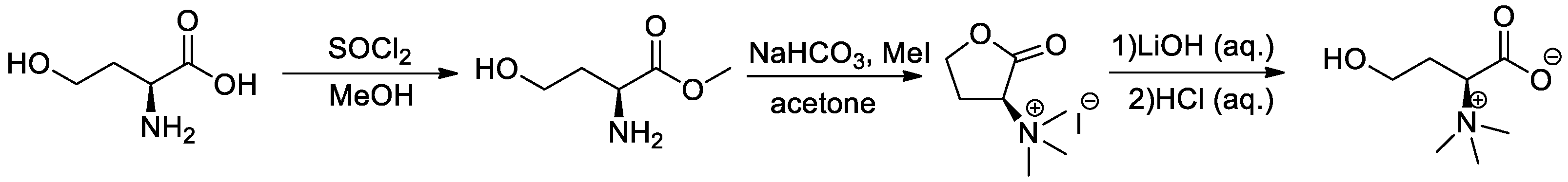
2.3.2. General Procedure for the Synthesis of l-Homoserine Betaine Fatty Acid Ester Derivatives (Scheme 2)
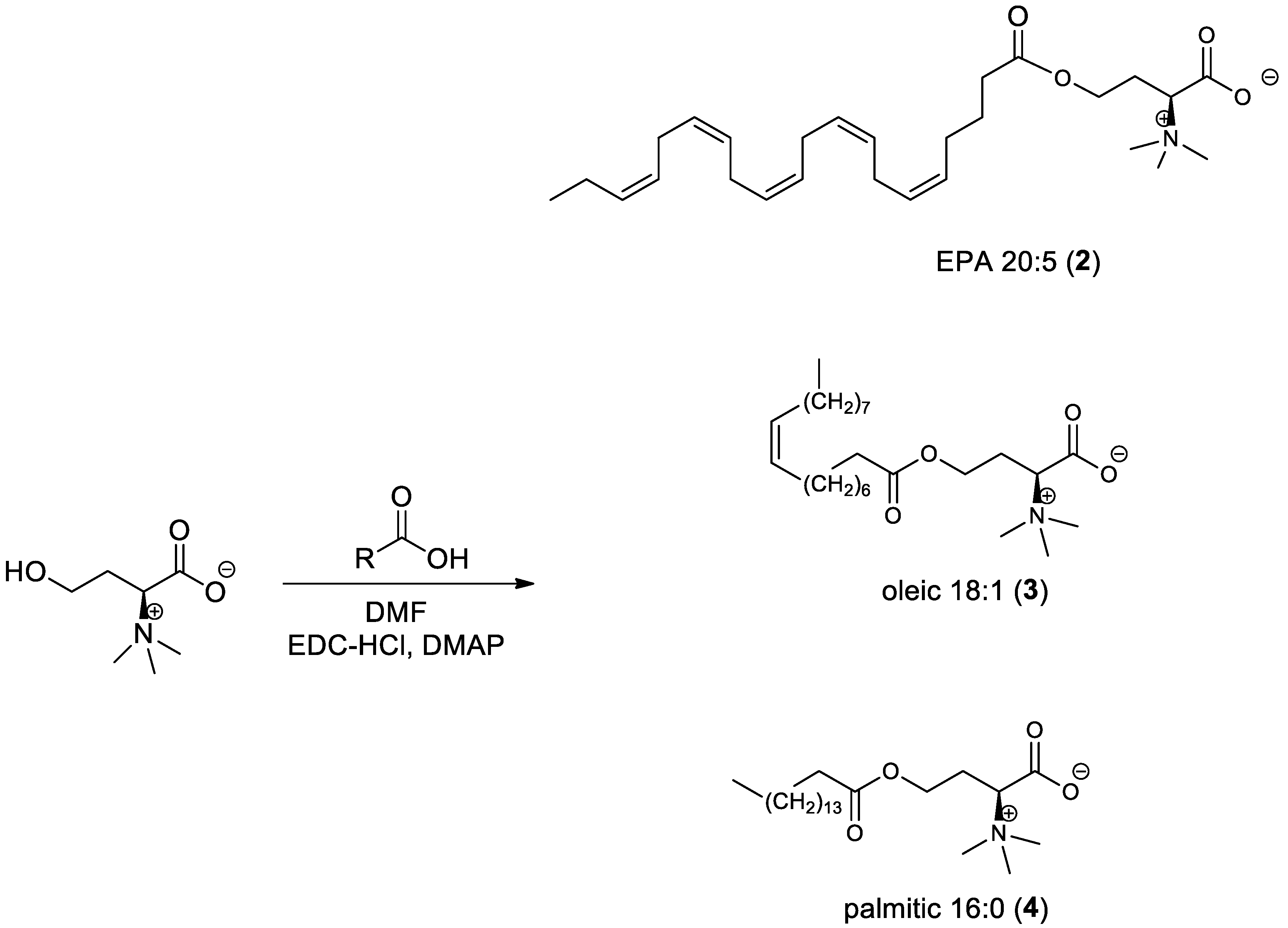
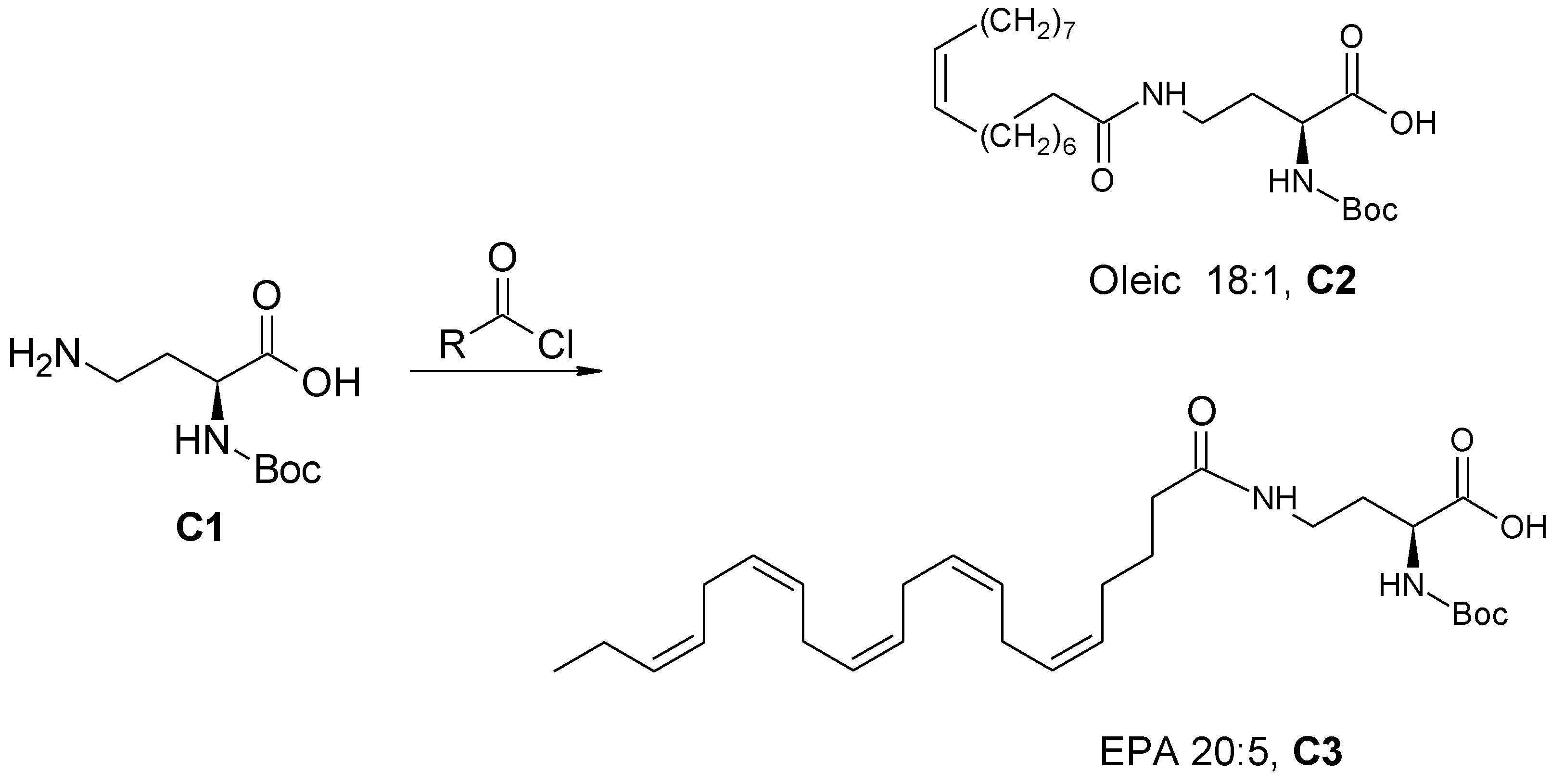
2.3.3. General Procedure for the Synthesis of Amide-Fatty Acid Derivatives
(S)-2-((Tert-butoxycarbonyl) amino)-4-oleylamido butanoic acid (C2) and (S)-2-((Tert-butoxycarbonyl)amino)-4-((5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenamido)butanoic acid (C3) (Scheme 3)
(S)-2-Amino-4-oleylamido Butanoic Acid (C4) and (S)-2-Amino-4-((5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenamido)butanoic Acid (C5) (Scheme 4)
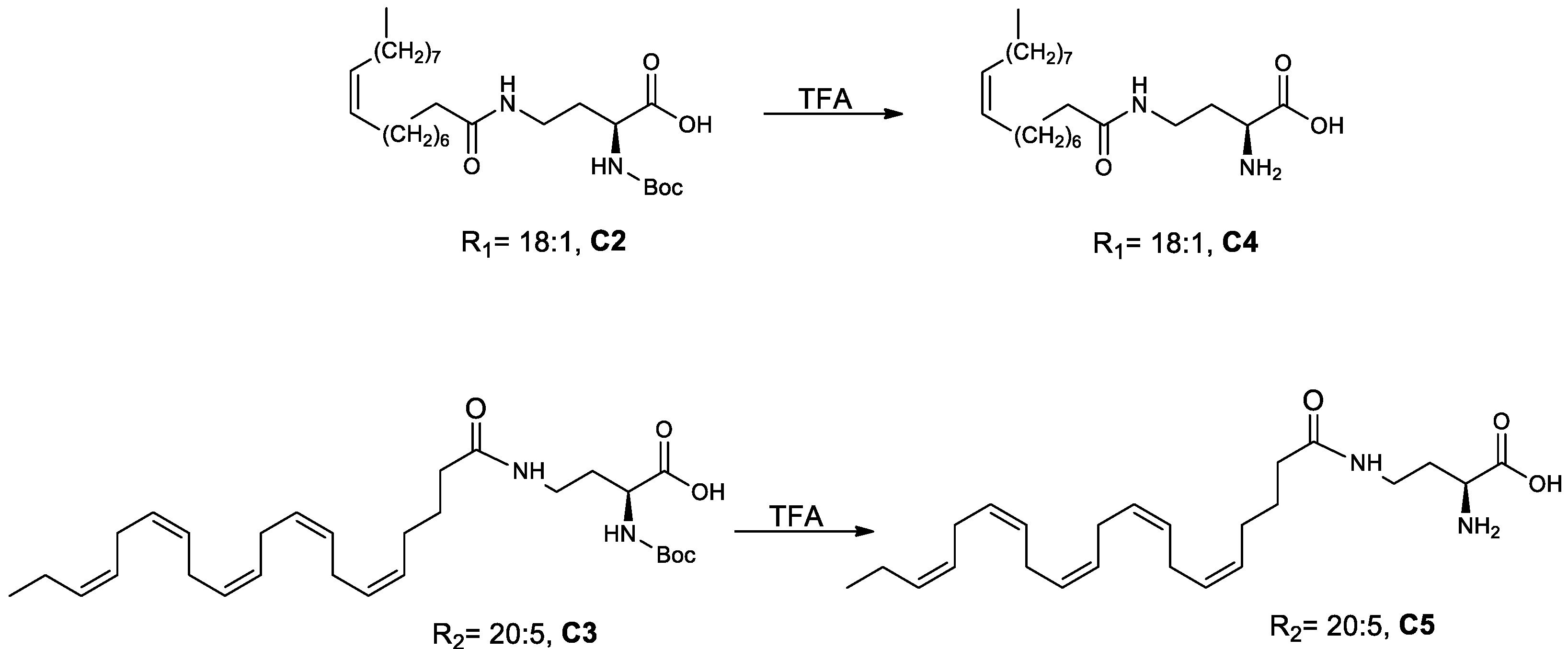
3-(S)-4-((5Z,8Z,11Z,14Z,17Z)-Eicosa-5,8,11,14,17-pentaenamido)-1-methoxy-N,N,N-trimethyl-1-oxobutan-2-aminium (5)) (Scheme 5)
2.4. Chromatography Analysis
2.4.1. HPLC Analysis
2.4.2. LC–MS Analysis
2.5. NMR Analysis
2.5.1. l-Homoserine Betaine
2.5.2. Homoserine Betaine Eicosapentaenoate (2)
2.5.3. Homoserine Betaine Oleate (3)
2.5.4. Homoserine Betaine Palmitate (4)
2.5.5. EPA Amide-Lyso (5)
2.5.6. Oleic Amide-Lyso (6)
2.6. Biological Assays
2.6.1. Effect of the Lipids on rePON1 Lactonase and Arylesterase Activities
2.6.2. Tryptophan (Trp) Fluorescence-Quenching Measurements
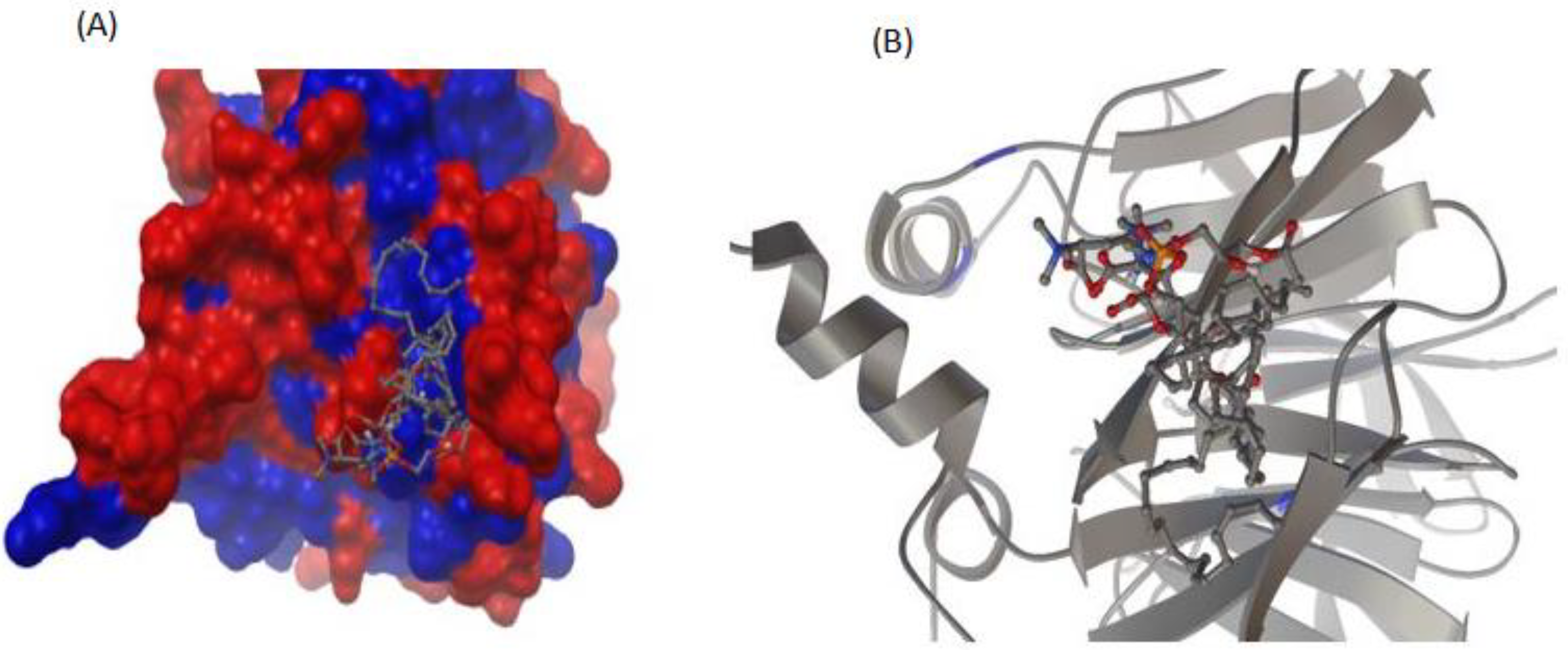
2.7. Molecular Docking
2.8. Cu2+-Induced LDL Oxidation
3. Results
3.1. Synthesis of Lyso-DGTS Derivatives
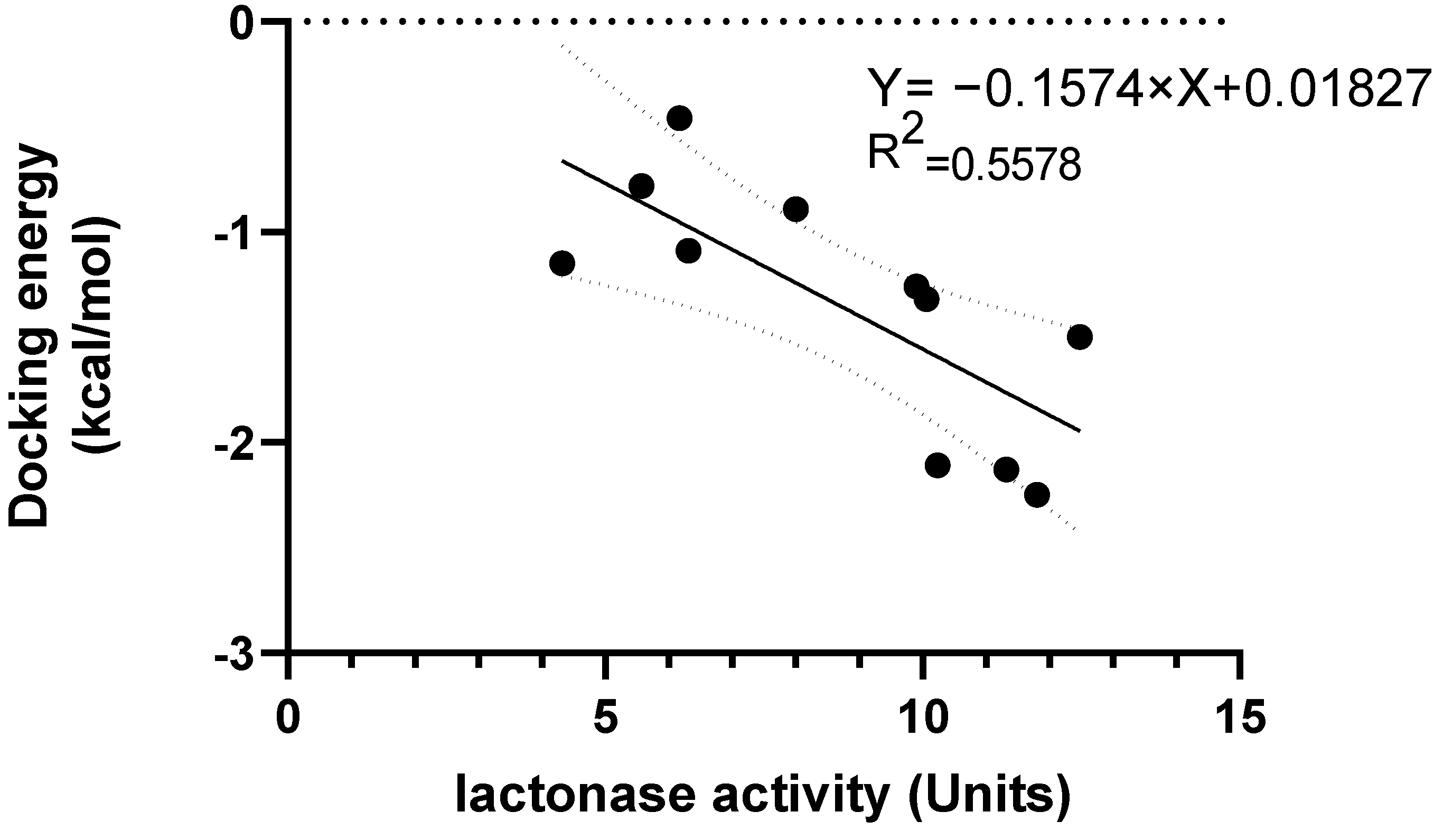
3.2. Effect of Lyso-DGTS Derivatives and Endogenous Analogs on Lactonase Activity of rePON1
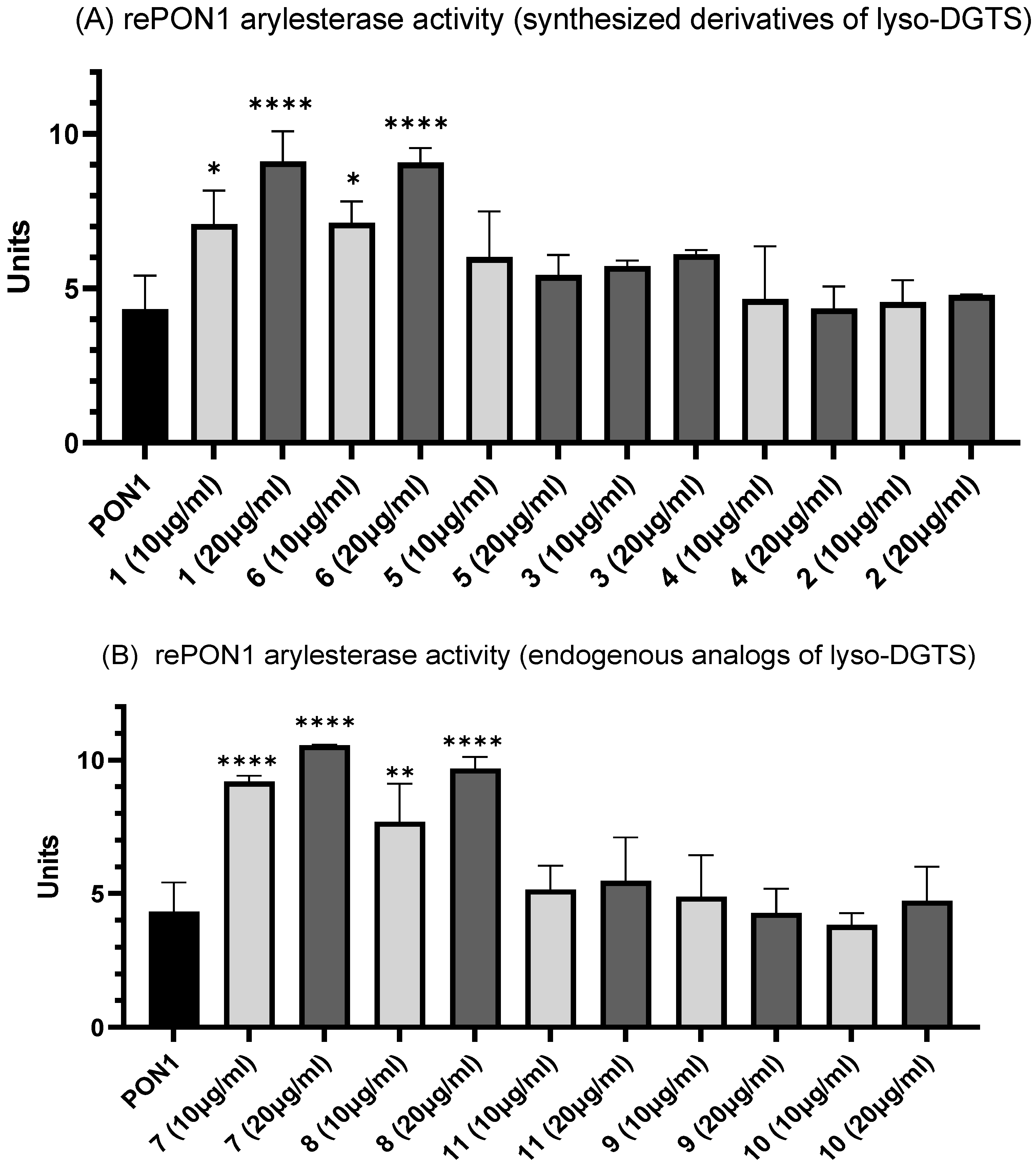
3.3. Effect of Lyso-DGTS Derivatives and Endogenous Analogs on Arylesterase Activity of rePON1
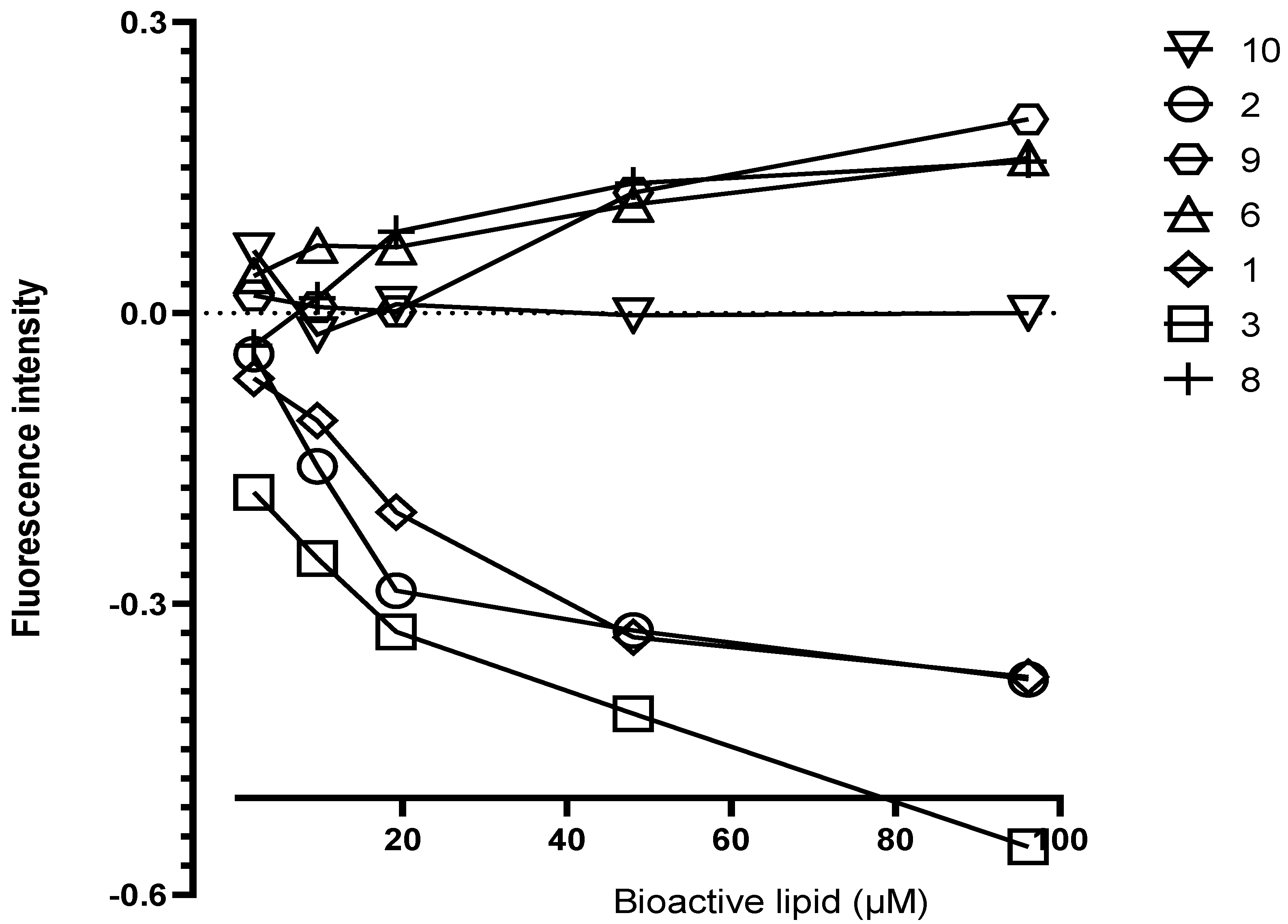
3.4. Interaction of Lyso-DGTS Derivatives and Endogenous Analogs with rePON1, Determined via the Trp Fluorescence-Quenching Method
3.5. Interaction between rePON1 and Lyso-DGTS Derivatives and Endogenous Analogs Using Molecular Modeling Calculation
3.6. Effect of rePON1–Lyso-DGTS Derivative and Analog Complexes on Cu2+-Induced LDL Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Danilo Norata, G.; Luigi Catapano, A. Treating High Density Lipoprotein Cholesterol (HDL-C): Quantity Versus Quality. Curr. Pharm. Des. 2013, 19, 3841–3857. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Modifications of low-density lipoprotein. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [PubMed]
- Hafiane, A.; Genest, J. HDL, Atherosclerosis, and Emerging Therapies. Hindawi 2013, 2013, 891403. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Translation of high-density lipoprotein function into clinical practice: Current prospects and future challenges. Circulation 2013, 128, 1256–1267. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Gür, M.; Çayli, M.; Uçar, H.; Elbasan, Z.; Şahin, D.Y.; Gözükara, M.Y.; Selek, Ş.; Koyunsever, N.Y.; Şeker, T.; Türkoǧlu, C.; et al. Paraoxonase (PON1) activity in patients with subclinical thoracic aortic atherosclerosis. Int. J. Cardiovasc. Imaging 2014, 30, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Caccavello, R.; Nassar, H.; Ahmad, W.A.; Sinnreich, R.; Kark, J.D. Low protective PON1 lactonase activity in an Arab population with high rates of coronary heart disease and diabetes. Clin. Chim. Acta 2015, 445, 41–47. [Google Scholar] [CrossRef]
- Guns, P.J.; Van Assche, T.; Verreth, W.; Fransen, P.; Mackness, B.; Mackness, M.; Holvoet, P.; Bult, H. Paraoxonase 1 gene transfer lowers vascular oxidative stress and improves vasomotor function in apolipoprotein E-deficient mice with pre-existing atherosclerosis. Br. J. Pharmacol. 2008, 153, 508–516. [Google Scholar] [CrossRef]
- Tavori, H.; Khatib, S.; Aviram, M.; Vaya, J. Bioorganic & Medicinal Chemistry Characterization of the PON1 active site using modeling simulation, in relation to PON1 lactonase activity. Bioorganic Med. Chem. 2008, 16, 7504–7509. [Google Scholar] [CrossRef]
- Bargota, R.S.; Akhtar, M.; Biggadike, K.; Gani, D.; Allemann, R.K. Structure-activity relationship on human serum paraoxonase (PON1) using substrate analogues and inhibitors. Bioorganic Med. Chem. Lett. 2003, 13, 1623–1626. [Google Scholar] [CrossRef]
- Rosenblat, M.; Gaidukov, L.; Khersonsky, O.; Vaya, J.; Oren, R.; Tawfik, D.S.; Aviram, M. The Catalytic Histidine Dyad of High Density Lipoprotein- associated Serum Paraoxonase-1 ( PON1 ) Is Essential for PON1-mediated Inhibition of Low Density Lipoprotein Oxidation and Stimulation of Macrophage Cholesterol Efflux. J. Biol. Chem. 2006, 281, 7657–7665. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, M.; Elias, M.; Filippi, J.; Duñach, E.; Silman, I.; Sussman, J.L.; Tawfik, D.S. Catalytic Versatility and Backups in Enzyme Active Sites: The Case of Serum Paraoxonase 1. J. Mol. Biol. 2012, 418, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.S.; Chu, T.; Esposito, B.; Hui, P.; Connelly, P.W.; Gross, P.L. Paraoxonase-1 deficiency in mice predisposes to vascular inflammation, oxidative stress, and thrombogenicity in the absence of hyperlipidemia. Cardiovasc. Pathol. 2008, 17, 226–232. [Google Scholar] [CrossRef]
- Shekhanawar, M.; Shekhanawar, S.M.; Krisnaswamy, D.; Indumati, V.; Satishkumar, D.; Vijay, V.; Rajeshwari, T.; Amareshwar, M. The role of “paraoxonase-1 activity” as an antioxidant in coronary artery diseases. J. Clin. Diagnostic Res. 2013, 7, 1284–1287. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T. Effect of dietary lipids on paraoxonase-1 activity and gene expression. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 88–94. [Google Scholar] [CrossRef]
- Freese, R.; Alfthan, G.; Jauhiainen, M.; Basu, S.; Erlund, I.; Salminen, I.; Aro, A.; Mutanen, M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. Am. J. Clin. Nutr. 2002, 76, 950–960. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Aviram, M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: Studies in vitro and in diabetic patients. Nutrition 2010, 26, 359–366. [Google Scholar] [CrossRef]
- Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Martínez-Pérez, M.M.; Calderón-Ramos, Z.G.; Belefant-Miller, H.; Cancino-Diaz, J.C. Pomegranate juice increases levels of paraoxonase1 (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res. Int. 2011, 44, 1381–1385. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-macromolecules interactions in human diseases with focus on alzheimer, atherosclerosis and cancer. Antioxidants 2021, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Atrahimovich, D.; Samson, A.O.; Khattib, A.; Vaya, J.; Khatib, S. Punicalagin Decreases Serum Glucose Levels and Increases PON1 Activity and HDL Anti-Inflammatory Values in Balb/c Mice Fed a High-Fat Diet. Oxid. Med. Cell. Longev. 2018, 2018, 2673076. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Artoul, F.; Paluy, I.; Boluchevsky, L.; Kvitnitsky, E.; Vaya, J. Nannochloropsis sp. ethanol extract prevents macrophage and LDL oxidation and enhances PON1 activity through the principal active compound lyso-diacylglyceryltrimethylhomoserine (lyso-DGTS). J. Appl. Phycol. 2018, 30, 1679–1689. [Google Scholar] [CrossRef]
- Dahli, L.; Atrahimovich, D.; Vaya, J.; Khatib, S. Lyso-DGTS lipid isolated from microalgae enhances PON1 activities in vitro and in vivo, increases PON1 penetration into macrophages and decreases cellular lipid accumulation. BioFactors 2018, 44, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Khattib, A.; Atrahimovich, D.; Dahli, L.; Vaya, J.; Khatib, S. Lyso-diacylglyceryltrimethylhomoserine (lyso-DGTS) isolated from Nannochloropsis microalgae improves high-density lipoprotein (HDL) functions. BioFactors 2020, 46, 146–157. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Khatib, S.; Sela, S.; Vaya, J.; Samson, A.O. Punicalagin Induces Serum Low-Density Lipoprotein Influx to Macrophages. Oxid. Med. Cell. Longev. 2016, 2016, 7124251. [Google Scholar] [CrossRef]
- Aviram, M.; Vaya, J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr. Opin. Lipidol. 2013, 24, 339–344. [Google Scholar] [CrossRef]
- Cohen, E.; Aviram, M.; Khatib, S.; Artoul, F.; Rabin, A. Free Radical Biology and Medicine Human carotid plaque phosphatidylcholine speci fi cally interacts with paraoxonase 1, increases its activity, and enhances its uptake by macrophage at the expense of its binding to HDL. Free Radic. Biol. Med. 2014, 76, 14–24. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Sparks, C.E.; James, R.W.; Bakker, S.J.L.; Dullaart, R.P.F. Low serum paraoxonase-1 activity associates with incident cardiovascular disease risk in subjects with concurrently high levels of high-density lipoprotein cholesterol and c-reactive protein. J. Clin. Med. 2019, 8, 1357. [Google Scholar] [CrossRef]
- Khersonsky, O.; Tawfik, D.S. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005, 44, 6371–6382. [Google Scholar] [CrossRef]
- Niki, E. Do free radicals play causal role in atherosclerosis? Low density lipoprotein oxidation and vitamin E revisited. J. Clin. Biochem. Nutr. 2011, 48, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, H.; Naderi, G.A.; Asgary, S.; Pakmehr, F.; Sadeghi, M.; Javadi, E. Correlation Between Lag Time of Ldl To in Vitro Oxidation and in Vivo Ox-Ldl in the Patients With Cad. Atheroscler. Suppl. 2010, 11, 130. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Juliano, R.A.; Mason, R.P. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183254. [Google Scholar] [CrossRef] [PubMed]

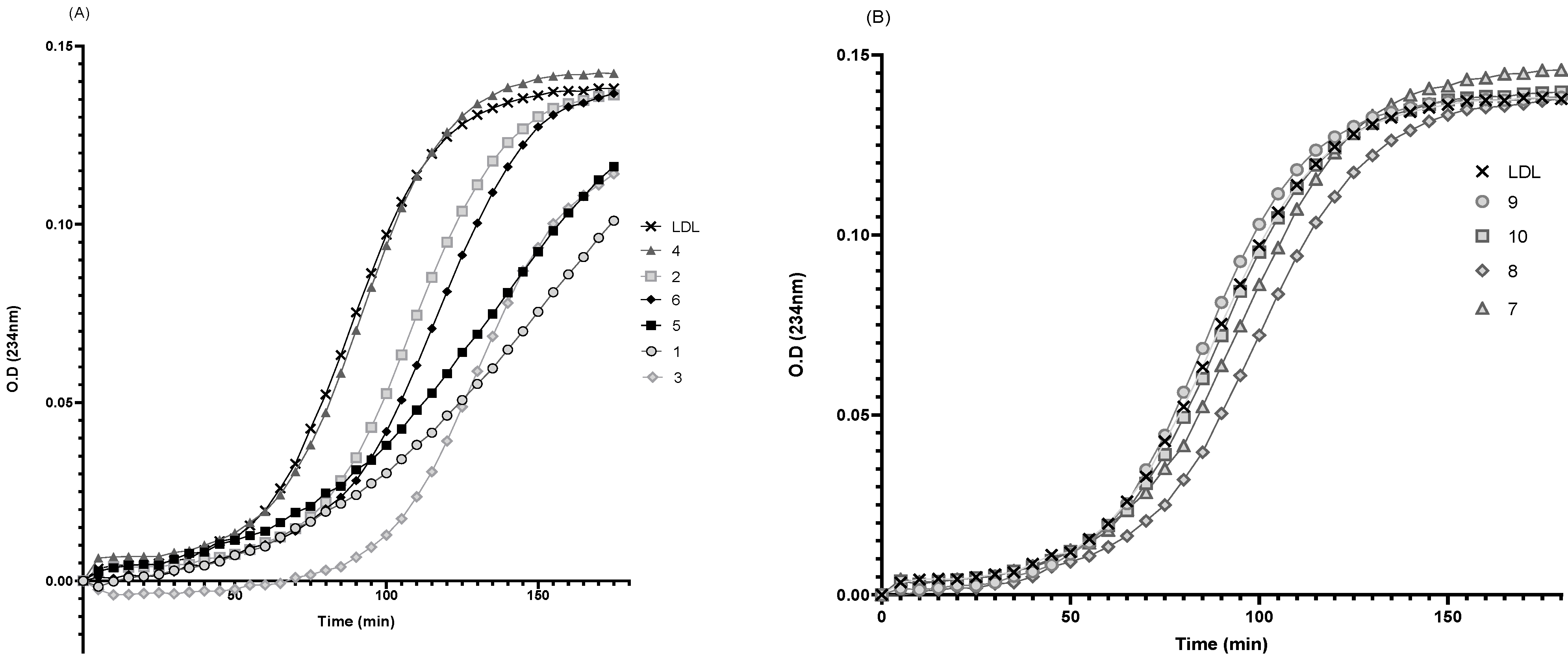
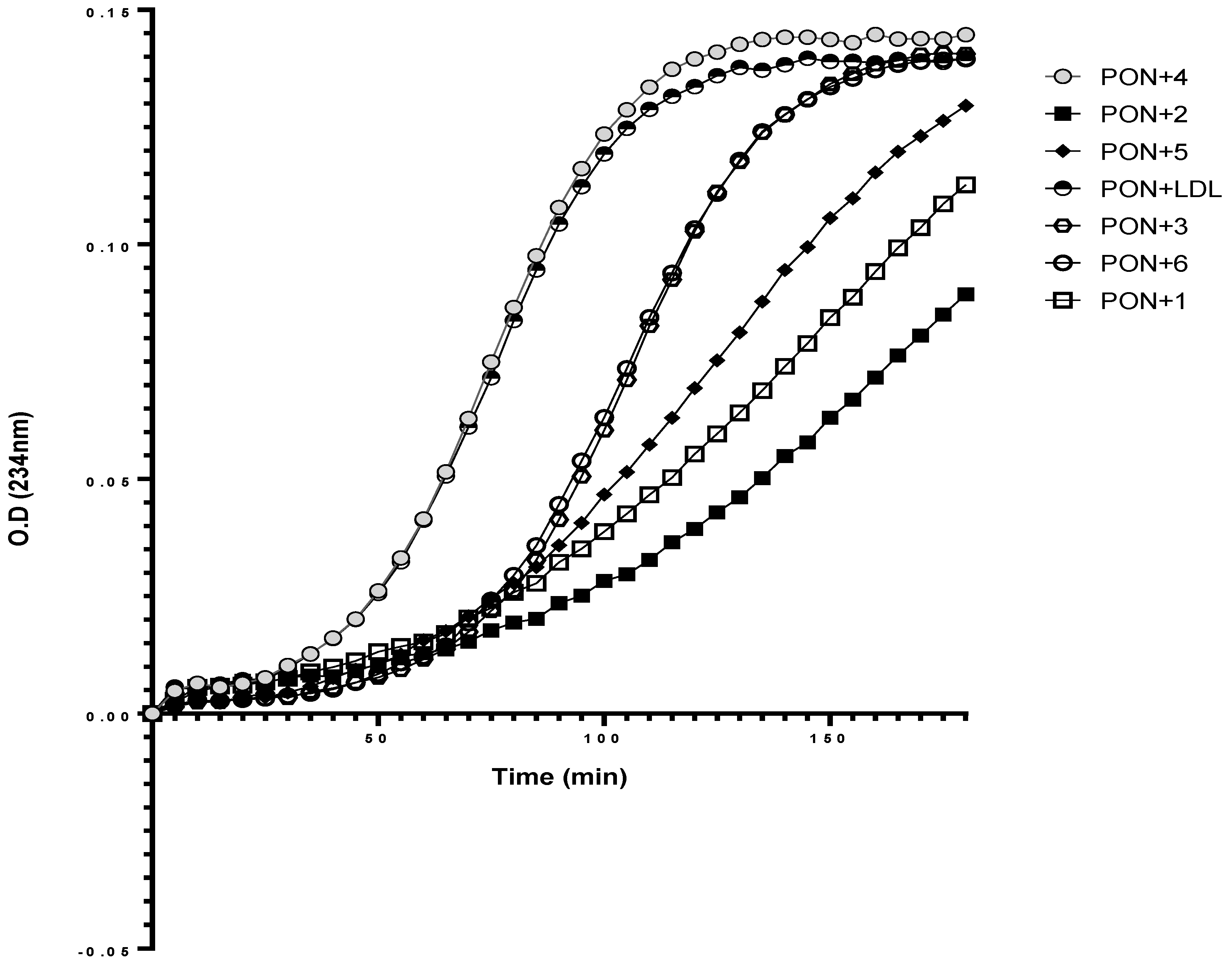
| Compound | Lag Time | K | % Inhibition |
|---|---|---|---|
| LDL | 24.22 | 0.04128 | 0 |
| 1 | 96.8 | 0.0103 | 75.04 |
| 5 | 99.4 | 0.01006 | 75.62 |
| 6 | 35.37 | 0.02827 | 31.51 |
| 2 | 29.37 | 0.03405 | 17.51 |
| 4 | 25.56 | 0.03913 | 5.20 |
| 3 | 80.50 | 0.02759 | 33.16 |
| Compound | Lag Time | K | % Inhibition |
|---|---|---|---|
| LDL | 24.08 | 0.04152 | 0 |
| 1 | 118 | 0.00844 | 79.67 |
| 5 | 67.87 | 0.01473 | 64.52 |
| 6 | 29.65 | 0.03373 | 18.76 |
| 2 | 199 | 0.005013 | 87.9 |
| 4 | 23.73 | 0.04215 | 0 |
| 3 | 90.30 | 0.03481 | 16.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattib, A.; Musa, S.; Halabi, M.; Hayek, T.; Khatib, S. Lyso-DGTS Lipid Derivatives Enhance PON1 Activities and Prevent Oxidation of LDL: A Structure–Activity Relationship Study. Antioxidants 2022, 11, 2058. https://doi.org/10.3390/antiox11102058
Khattib A, Musa S, Halabi M, Hayek T, Khatib S. Lyso-DGTS Lipid Derivatives Enhance PON1 Activities and Prevent Oxidation of LDL: A Structure–Activity Relationship Study. Antioxidants. 2022; 11(10):2058. https://doi.org/10.3390/antiox11102058
Chicago/Turabian StyleKhattib, Ali, Sanaa Musa, Majdi Halabi, Tony Hayek, and Soliman Khatib. 2022. "Lyso-DGTS Lipid Derivatives Enhance PON1 Activities and Prevent Oxidation of LDL: A Structure–Activity Relationship Study" Antioxidants 11, no. 10: 2058. https://doi.org/10.3390/antiox11102058
APA StyleKhattib, A., Musa, S., Halabi, M., Hayek, T., & Khatib, S. (2022). Lyso-DGTS Lipid Derivatives Enhance PON1 Activities and Prevent Oxidation of LDL: A Structure–Activity Relationship Study. Antioxidants, 11(10), 2058. https://doi.org/10.3390/antiox11102058







