Transposon-Directed Insertion-Site Sequencing Reveals Glycolysis Gene gpmA as Part of the H2O2 Defense Mechanisms in Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media and Growth Conditions
2.2. TraDIS
2.3. P1 Transduction
2.4. H2O2 Susceptibiliy Assessed by Disk Diffusion Assay
2.5. H2O2 Survival Assay
2.6. Expression Levels Assessed by qRT-PCR
2.7. H2O2 Degradation Mesurements by Amplex Red
2.8. Complementation of gpmA
2.9. Software
3. Results
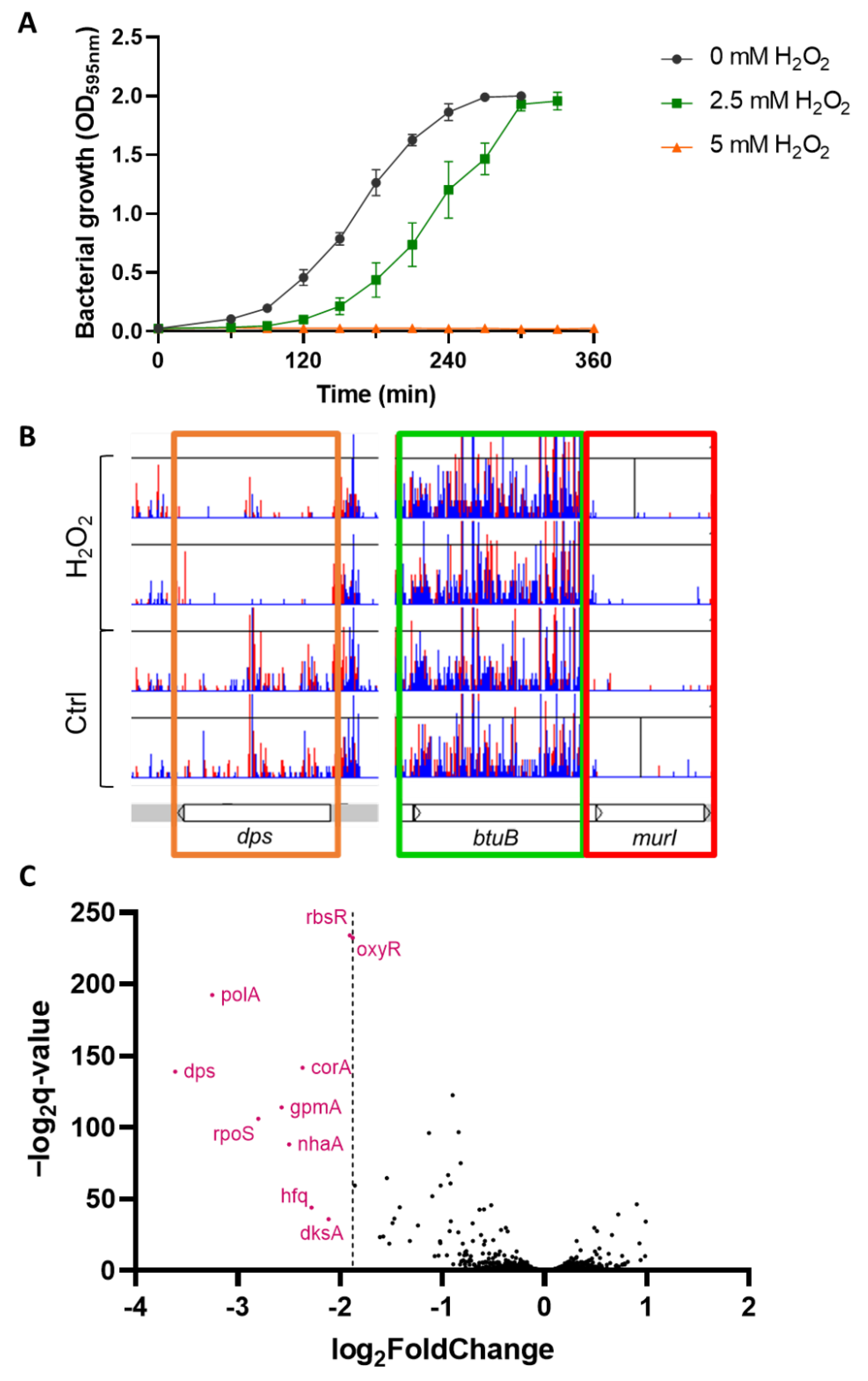
3.1. TraDIS Was Performed under Sublethal H2O2 Exposure
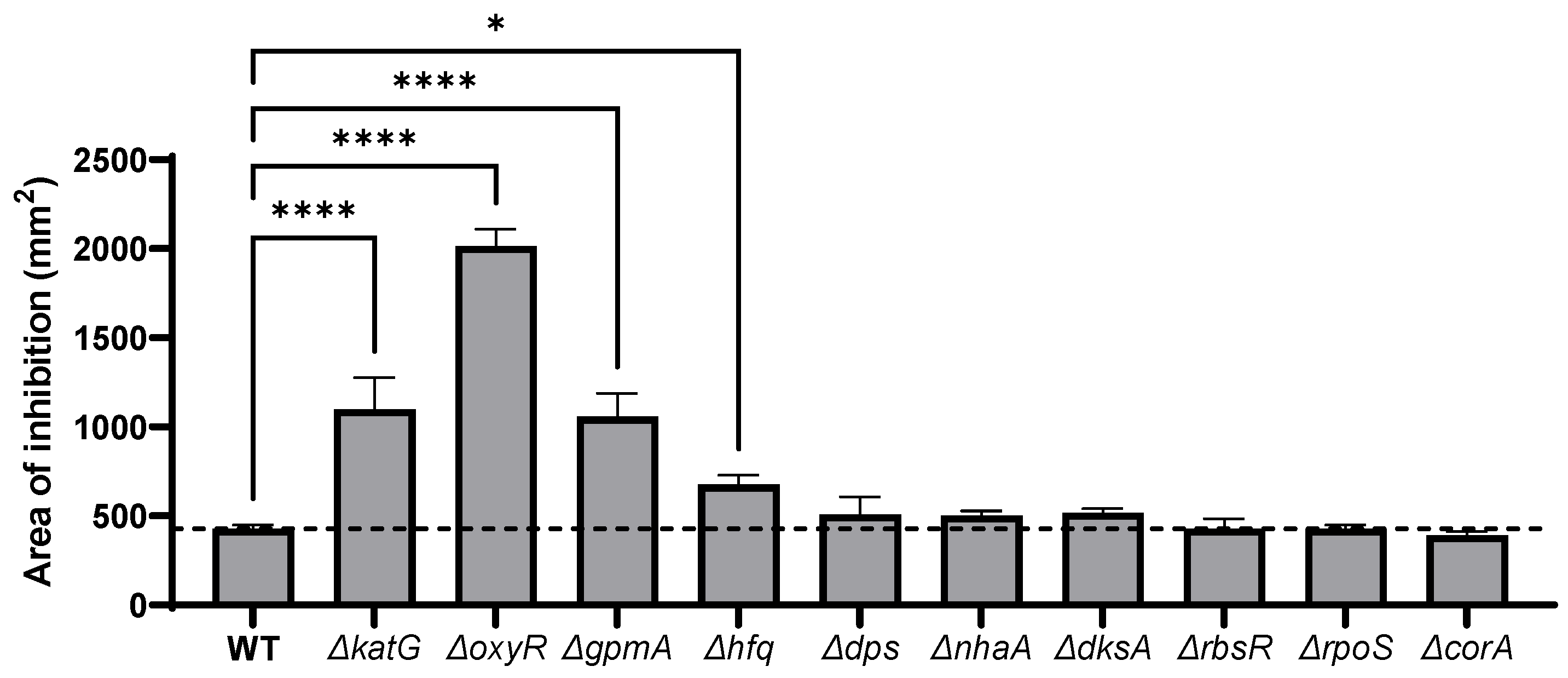
3.2. H2O2 Susceptibility of Single-Gene Deletion Identified by TraDIS
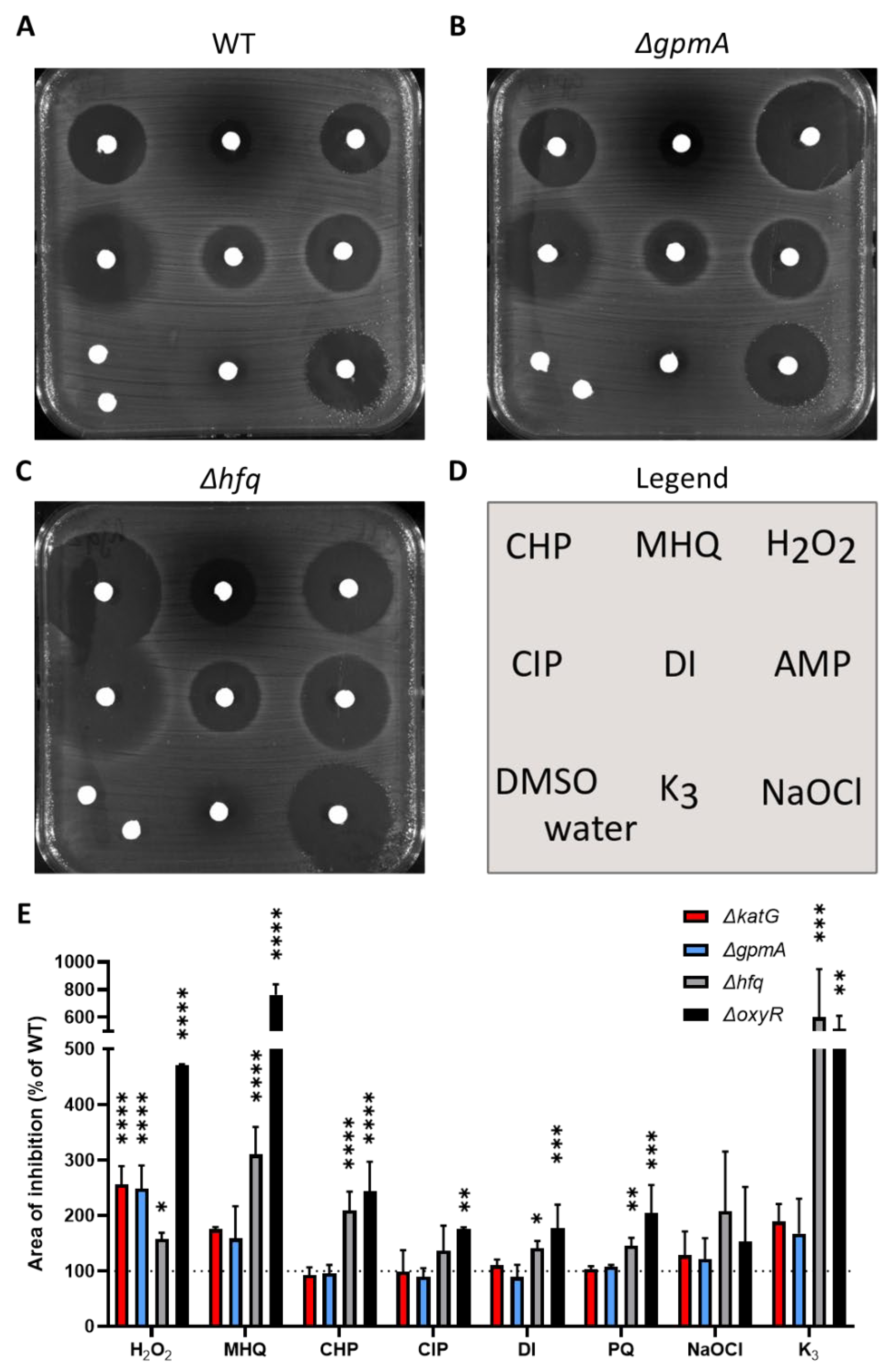
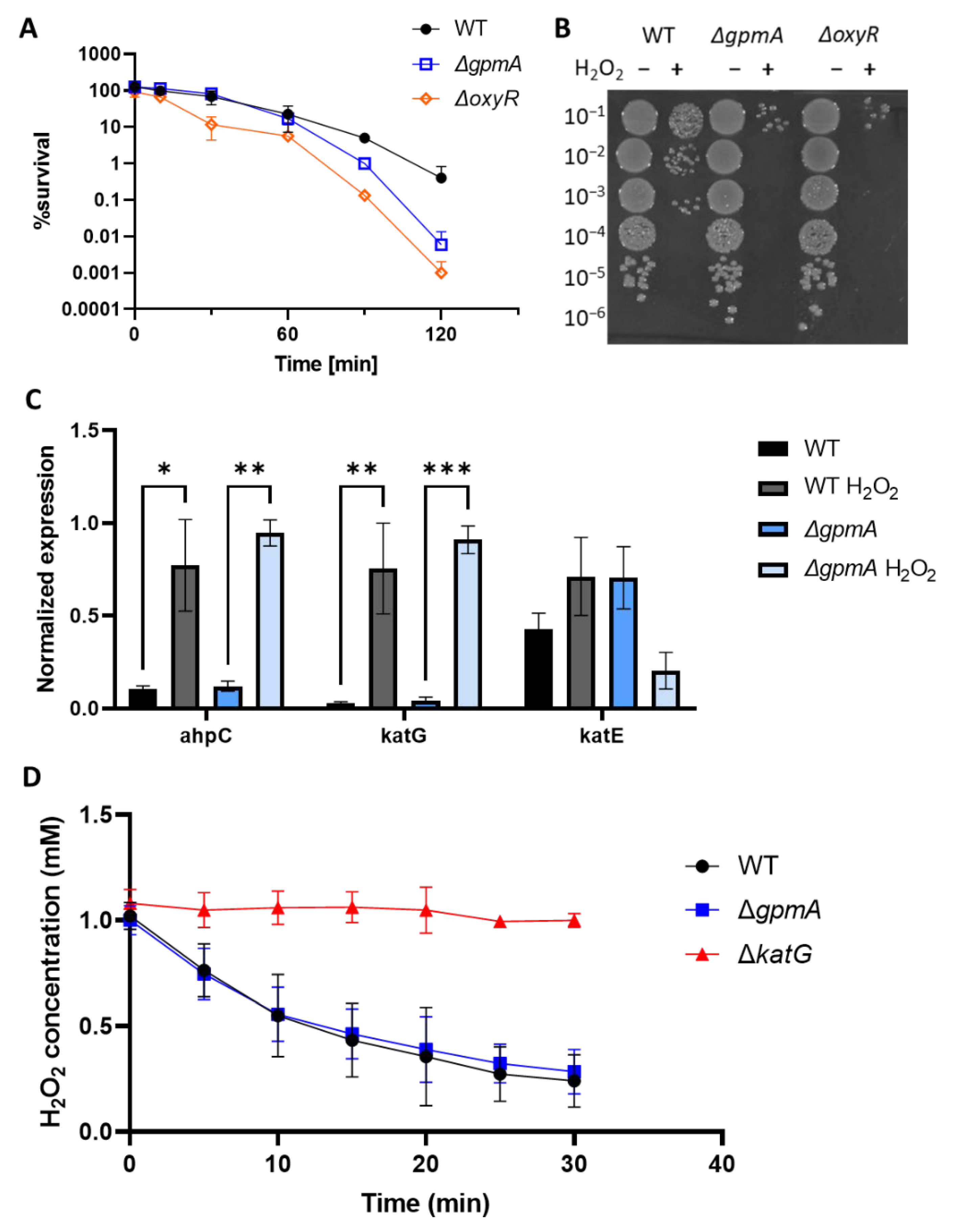
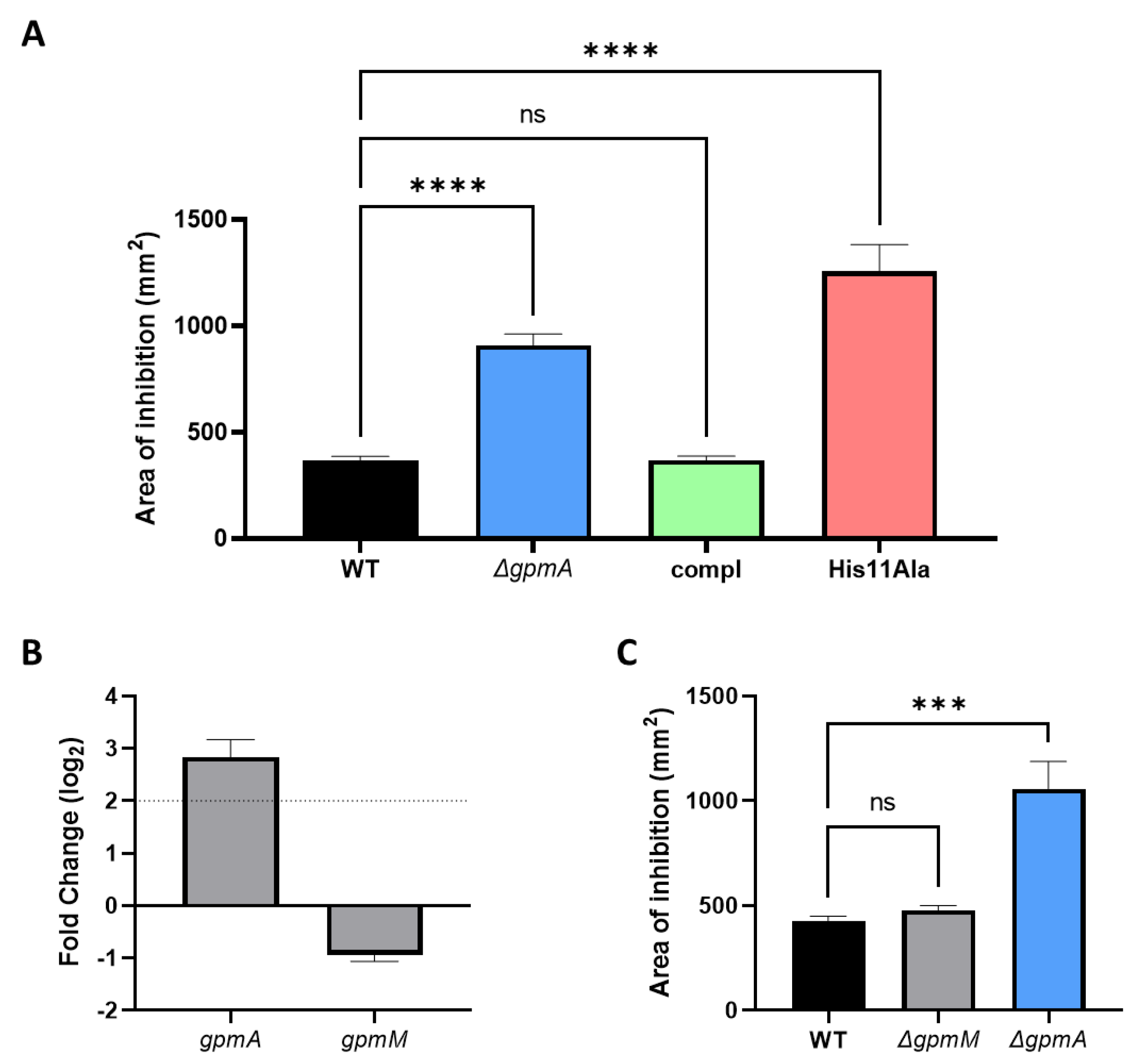
3.3. ΔgpmA Mutant Was More Sensitive to H2O2 but Not to Other Oxidants
3.4. gpmA Is Upregulated by H2O2 Exposure
3.5. Catalase Activity Is Not Involved in the Increased Sensitivity of ΔgpmA to H2O2
3.6. Other Carbon Sources Cannot Compensate the H2O2 Hypersensitivity of ΔgpmA Mutant
3.7. The Function of gpmA Is Necessary for H2O2 Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunne, K.A.; Chaudhuri, R.R.; Rossiter, A.E.; Beriotto, I.; Browning, D.F.; Squire, D.; Cunningham, A.F.; Cole, J.A.; Loman, N.; Henderson, I.R. Sequencing a Piece of History: Complete Genome Sequence of the Original Escherichia Coli Strain. Microb. Genom. 2017, 3, mgen000106. [Google Scholar] [CrossRef]
- Anderson, J.D.; Bagamian, K.H.; Muhib, F.; Amaya, M.P.; Laytner, L.A.; Wierzba, T.; Rheingans, R. Burden of Enterotoxigenic Escherichia Coli and Shigella Non-Fatal Diarrhoeal Infections in 79 Low-Income and Lower Middle-Income Countries: A Modelling Analysis. Lancet Glob. Health 2019, 7, e321–e330. [Google Scholar] [CrossRef]
- Bonten, M.; Johnson, J.R.; van den Biggelaar, A.H.J.; Georgalis, L.; Geurtsen, J.; de Palacios, P.I.; Gravenstein, S.; Verstraeten, T.; Hermans, P.; Poolman, J.T. Epidemiology of Escherichia Coli Bacteremia: A Systematic Literature Review. Clin. Infect. Dis. 2021, 72, 1211–1219. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Pridmore, R.D.; Pittet, A.-C.; Praplan, F.; Cavadini, C. Hydrogen Peroxide Production by Lactobacillus Johnsonii NCC 533 and Its Role in Anti-Salmonella Activity. FEMS Microbiol. Lett. 2008, 283, 210–215. [Google Scholar] [CrossRef]
- Gupta, K.; Stapleton, A.E.; Hooton, T.M.; Roberts, P.L.; Fennell, C.L.; Stamm, W.E. Inverse Association of H2O2-Producing Lactobacilli and Vaginal Escherichia Coli Colonization in Women with Recurrent Urinary Tract Infections. J. Infect. Dis. 1998, 178, 446–450. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J. Redox Reactions and Microbial Killing in the Neutrophil Phagosome. Antioxid. Redox Signal. 2013, 18, 642–660. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Templeton, L.J.; Smulski, D.R.; LaRossa, R.A.; Storz, G. DNA Microarray-Mediated Transcriptional Profiling of the Escherichia Coli Response to Hydrogen Peroxide. J. Bacteriol. 2001, 183, 4562–4570. [Google Scholar] [CrossRef]
- Seo, S.W.; Kim, D.; Szubin, R.; Palsson, B.O. Genome-Wide Reconstruction of OxyR and SoxRS Transcriptional Regulatory Networks under Oxidative Stress in Escherichia Coli K-12 MG1655. Cell Rep. 2015, 12, 1289–1299. [Google Scholar] [CrossRef]
- Roth, M.; Jaquet, V.; Lemeille, S.; Bonetti, E.-J.; Cambet, Y.; François, P.; Krause, K.-H. Transcriptomic Analysis of E. Coli after Exposure to a Sublethal Concentration of Hydrogen Peroxide Revealed a Coordinated Up-Regulation of the Cysteine Biosynthesis Pathway. Antioxidants 2022, 11, 655. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fujikawa, M.; Kozawa, T. Oxidative Stress Sensing by the Iron–Sulfur Cluster in the Transcription Factor, SoxR. J. Inorg. Biochem. 2014, 133, 87–91. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kim, S.-J.; Mukhopadhyay, P.; Cho, S.; Woo, J.-R.; Storz, G.; Ryu, S.-E. Structural Basis of the Redox Switch in the OxyR Transcription Factor. Cell 2001, 105, 103–113. [Google Scholar] [CrossRef]
- Imlay, J.A. Transcription Factors That Defend Bacteria against Reactive Oxygen Species. Annu. Rev. Microbiol. 2015, 69, 93–108. [Google Scholar] [CrossRef]
- Farr, S.B.; D’Ari, R.; Touati, D. Oxygen-Dependent Mutagenesis in Escherichia Coli Lacking Superoxide Dismutase. Proc. Natl. Acad. Sci. USA 1986, 83, 8268–8272. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Schellhorn, H.E. Identification and Characterization of Hydrogen Peroxide-Sensitive Mutants of Escherichia Coli: Genes That Require OxyR for Expression. J. Bacteriol. 1997, 179, 330–338. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Rojas, A.; Kim, J.J.; Johnston, P.R.; Makarova, O.; Eravci, M.; Weise, C.; Hengge, R.; Rolff, J. Non-Lethal Exposure to H2O2 Boosts Bacterial Survival and Evolvability against Oxidative Stress. PLoS Genet. 2020, 16, e1008649. [Google Scholar] [CrossRef]
- Chao, M.C.; Abel, S.; Davis, B.M.; Waldor, M.K. The Design and Analysis of Transposon-Insertion Sequencing Experiments. Nat. Rev. Microbiol. 2016, 14, 119–128. [Google Scholar] [CrossRef]
- Goodall, E.C.A.; Robinson, A.; Johnston, I.G.; Jabbari, S.; Turner, K.A.; Cunningham, A.F.; Lund, P.A.; Cole, J.A.; Henderson, I.R. The Essential Genome of Escherichia Coli K-12. mBio 2018, 9, e02096-17. [Google Scholar] [CrossRef]
- Karash, S.; Liyanage, R.; Qassab, A.; Lay, J.O.; Kwon, Y.M. A Comprehensive Assessment of the Genetic Determinants in Salmonella Typhimurium for Resistance to Hydrogen Peroxide Using Proteogenomics. Sci. Rep. 2017, 7, 17073. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liu, L.; Fitzsimmons, L.; Porwollik, S.; Kim, J.-S.; Desai, P.; McClelland, M.; Vazquez-Torres, A. Glycolytic Reprograming in Salmonella Counters NOX2-Mediated Dissipation of ΔpH. Nat. Commun. 2020, 11, 1783. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia Coli K-12 in-Frame, Single-Gene Knockout Mutants: The Keio Collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Ezraty, B.; Vergnes, A.; Banzhaf, M.; Duverger, Y.; Huguenot, A.; Brochado, A.R.; Su, S.-Y.; Espinosa, L.; Loiseau, L.; Py, B.; et al. Fe-S Cluster Biosynthesis Controls Uptake of Aminoglycosides in a ROS-Less Death Pathway. Science 2013, 340, 1583–1587. [Google Scholar] [CrossRef]
- Wang, R.F.; Kushner, S.R. Construction of Versatile Low-Copy-Number Vectors for Cloning, Sequencing and Gene Expression in Escherichia Coli. Gene 1991, 100, 195–199. [Google Scholar] [CrossRef]
- Barquist, L.; Mayho, M.; Cummins, C.; Cain, A.K.; Boinett, C.J.; Page, A.J.; Langridge, G.C.; Quail, M.A.; Keane, J.A.; Parkhill, J. The TraDIS Toolkit: Sequencing and Analysis for Dense Transposon Mutant Libraries. Bioinformatics 2016, 32, 1109–1111. [Google Scholar] [CrossRef]
- Thomason, L.C.; Costantino, N.; Court, D.L.E. Coli Genome Manipulation by P1 Transduction. Curr. Protoc. Mol. Biol. 2007, 1, 1.17.1–1.17.8. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Rocha, D.J.P.G.; Castro, T.L.P.; Aguiar, E.R.G.R.; Pacheco, L.G.C. Gene Expression Analysis in Bacteria by RT-QPCR. In Quantitative Real-Time PCR: Methods and Protocols; Biassoni, R., Raso, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; pp. 119–137. ISBN 978-1-4939-9833-3. [Google Scholar]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An Integrated Platform for Visualization and Analysis of High-Throughput Sequence-Based Experimental Data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef]
- Keseler, I.M.; Collado-Vides, J.; Santos-Zavaleta, A.; Peralta-Gil, M.; Gama-Castro, S.; Muñiz-Rascado, L.; Bonavides-Martinez, C.; Paley, S.; Krummenacker, M.; Altman, T.; et al. EcoCyc: A Comprehensive Database of Escherichia Coli Biology. Nucleic Acids Res. 2011, 39, D583–D590. [Google Scholar] [CrossRef]
- Keseler, I.M.; Gama-Castro, S.; Mackie, A.; Billington, R.; Bonavides-Martínez, C.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Muñiz-Rascado, L.; et al. The EcoCyc Database in 2021. Front. Microbiol. 2021, 12, 711077. [Google Scholar] [CrossRef]
- Christman, M.F.; Morgan, R.W.; Jacobson, F.S.; Ames, B.N. Positive Control of a Regulon for Defenses against Oxidative Stress and Some Heat-Shock Proteins in Salmonella Typhimurium. Cell 1985, 41, 753–762. [Google Scholar] [CrossRef]
- Seaver, L.C.; Imlay, J.A. Hydrogen Peroxide Fluxes and Compartmentalization inside Growing Escherichia Coli. J. Bacteriol. 2001, 183, 7182–7189. [Google Scholar] [CrossRef]
- Schellhorn, H.E.; Hassan, H.M. Transcriptional Regulation of KatE in Escherichia Coli K-12. J. Bacteriol. 1988, 170, 4286–4292. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia Coli Physiology in Luria-Bertani Broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef]
- Guarino, V.A.; Oldham, W.M.; Loscalzo, J.; Zhang, Y.-Y. Reaction Rate of Pyruvate and Hydrogen Peroxide: Assessing Antioxidant Capacity of Pyruvate under Biological Conditions. Sci. Rep. 2019, 9, 19568. [Google Scholar] [CrossRef]
- Bond, C.S.; White, M.F.; Hunter, W.N. High Resolution Structure of the Phosphohistidine-Activated Form of Escherichia Coli Cofactor-Dependent Phosphoglycerate Mutase*. J. Biol. Chem. 2001, 276, 3247–3253. [Google Scholar] [CrossRef]
- Fraser, H.I.; Kvaratskhelia, M.; White, M.F. The Two Analogous Phosphoglycerate Mutases of Escherichia Coli. FEBS Lett. 1999, 455, 344–348. [Google Scholar] [CrossRef]
- Martinez, A.; Kolter, R. Protection of DNA during Oxidative Stress by the Nonspecific DNA-Binding Protein Dps. J. Bacteriol. 1997, 179, 5188–5194. [Google Scholar] [CrossRef]
- Sen, A.; Imlay, J.A. How Microbes Defend Themselves From Incoming Hydrogen Peroxide. Front. Immunol. 2021, 12, 1104. [Google Scholar] [CrossRef]
- Lange, R.; Hengge-Aronis, R. Identification of a Central Regulator of Stationary-Phase Gene Expression in Escherichia Coli. Mol. Microbiol. 1991, 5, 49–59. [Google Scholar] [CrossRef]
- Loewen, P.C.; Triggs, B.L. Genetic Mapping of KatF, a Locus That with KatE Affects the Synthesis of a Second Catalase Species in Escherichia Coli. J. Bacteriol. 1984, 160, 668–675. [Google Scholar] [CrossRef]
- Henard, C.A.; Tapscott, T.; Crawford, M.A.; Husain, M.; Doulias, P.-T.; Porwollik, S.; Liu, L.; McClelland, M.; Ischiropoulos, H.; Vázquez-Torres, A. The 4-Cysteine Zinc-Finger Motif of the RNA Polymerase Regulator DksA Serves as a Thiol Switch for Sensing Oxidative and Nitrosative Stress. Mol. Microbiol. 2014, 91, 790–804. [Google Scholar] [CrossRef]
- Zhang, A.; Wassarman, K.M.; Ortega, J.; Steven, A.C.; Storz, G. The Sm-like Hfq Protein Increases OxyS RNA Interaction with Target MRNAs. Mol. Cell 2002, 9, 11–22. [Google Scholar] [CrossRef]
- Muffler, A.; Fischer, D.; Hengge-Aronis, R. The RNA-Binding Protein HF-I, Known as a Host Factor for Phage Qbeta RNA Replication, Is Essential for RpoS Translation in Escherichia Coli. Genes Dev. 1996, 10, 1143–1151. [Google Scholar] [CrossRef]
- Liu, Y.; Imlay, J.A. Cell Death from Antibiotics without the Involvement of Reactive Oxygen Species. Science 2013, 339, 1210–1213. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. Mutagenesis and Stress Responses Induced in Escherichia Coli by Hydrogen Peroxide. J. Bacteriol. 1987, 169, 2967–2976. [Google Scholar] [CrossRef]
- Demple, B.; Halbrook, J.; Linn, S. Escherichia Coli Xth Mutants Are Hypersensitive to Hydrogen Peroxide. J. Bacteriol. 1983, 153, 1079–1082. [Google Scholar] [CrossRef]
- Sermon, J.; Wevers, E.M.-R.P.; Jansen, L.; De Spiegeleer, P.; Vanoirbeek, K.; Aertsen, A.; Michiels, C.W. CorA Affects Tolerance of Escherichia Coli and Salmonella Enterica Serovar Typhimurium to the Lactoperoxidase Enzyme System but Not to Other Forms of Oxidative Stress. Appl. Environ. Microbiol. 2005, 71, 6515–6523. [Google Scholar] [CrossRef]
- Shimada, T.; Kori, A.; Ishihama, A. Involvement of the Ribose Operon Repressor RbsR in Regulation of Purine Nucleotide Synthesis in Escherichia Coli. FEMS Microbiol. Lett. 2013, 344, 159–165. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, M.; Chan, E.W.C.; Chen, S. Membrane Transporters of the Major Facilitator Superfamily Are Essential for Long-Term Maintenance of Phenotypic Tolerance to Multiple Antibiotics in E. Coli. Microbiol. Spectr. 2021, 9, e0184621. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Liu, X.; Pomposiello, P.J. Activation of Glucose Transport under Oxidative Stress in Escherichia Coli. Arch. Microbiol. 2008, 190, 41–49. [Google Scholar] [CrossRef]
- Varghese, S.; Tang, Y.; Imlay, J.A. Contrasting Sensitivities of Escherichia Coli Aconitases A and B to Oxidation and Iron Depletion. J. Bacteriol. 2003, 185, 221–230. [Google Scholar] [CrossRef]
- Park, S.J.; Gunsalus, R.P. Oxygen, Iron, Carbon, and Superoxide Control of the Fumarase FumA and FumC Genes of Escherichia Coli: Role of the ArcA, Fnr, and SoxR Gene Products. J. Bacteriol. 1995, 177, 6255–6262. [Google Scholar] [CrossRef]
- Jozefczuk, S.; Klie, S.; Catchpole, G.; Szymanski, J.; Cuadros-Inostroza, A.; Steinhauser, D.; Selbig, J.; Willmitzer, L. Metabolomic and Transcriptomic Stress Response of Escherichia Coli. Mol. Syst. Biol. 2010, 6, 364. [Google Scholar] [CrossRef]
- Radin, J.N.; Kelliher, J.L.; Solórzano, P.K.P.; Grim, K.P.; Ramezanifard, R.; Slauch, J.M.; Kehl-Fie, T.E. Metal-Independent Variants of Phosphoglycerate Mutase Promote Resistance to Nutritional Immunity and Retention of Glycolysis during Infection. PLoS Pathog. 2019, 15, e1007971. [Google Scholar] [CrossRef]
- Foster, J.M.; Davis, P.J.; Raverdy, S.; Sibley, M.H.; Raleigh, E.A.; Kumar, S.; Carlow, C.K.S. Evolution of Bacterial Phosphoglycerate Mutases: Non-Homologous Isofunctional Enzymes Undergoing Gene Losses, Gains and Lateral Transfers. PLoS ONE 2010, 5, e13576. [Google Scholar] [CrossRef]
- Imlay, J.A. The Molecular Mechanisms and Physiological Consequences of Oxidative Stress: Lessons from a Model Bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]


| Name | Genotype | Source or Reference |
|---|---|---|
| BW25113 | F-, ∆(araD-araB)567, ∆lacZ4787(::rrnB-3), rph-1, ∆(rhaD-rhaB)568, hsdR514 | CGSC 1 [21] |
| MG1655 | F-, λ−, rph-1 | CGSC 1 |
| BEFB02 | MG1655, ΔoxyR::Cmr | [22] |
| JW3914 | BW25113, ΔkatG::kan | [21] |
| ΔkatG | MG1655, ΔkatG::kan | This study |
| JW0738 | BW25113, ΔgpmA::kan | [21] |
| ΔgpmA | MG1655, ΔgpmA::kan | This study |
| JW4130 | BW25113, Δhfq::kan | [21] |
| Δhfq | MG1655, Δhfq::kan | This study |
| JW0797 | BW25113, Δdps::kan | [21] |
| Δhfq | MG1655, Δdps::kan | This study |
| JW3789 | BW25113, ΔcorA::kan | [21] |
| Δhfq | MG1655, ΔcorA::kan | This study |
| JW5437 | BW25113, ΔrpoS::kan | [21] |
| ΔrpoS | MG1655, ΔrpoS::kan | This study |
| JW3732 | BW25113, ΔrbsR::kan | [21] |
| ΔrbsR | MG1655, ΔrbsR::kan | This study |
| JW0141 | BW25113, ΔdksA::kan | [21] |
| ΔdksA | MG1655, ΔdksA::kan | This study |
| JW0018 | BW25113, ΔnhaA::kan | [21] |
| ΔnhaA | MG1655, ΔnhaA::kan | This study |
| JW3587 | BW25113, ΔgpmM::kan | [21] |
| ΔgpmM | MG1655, ΔgpmM::kan | This study |
| pWSK29 | AmpR | [23] |
| Name | Sequence | Gene Accession ID Ecocyc | Efficiency (RT-qPCR Primers) | Reference |
|---|---|---|---|---|
| RT-qPCR primers | ||||
| gyrB_N_qPCR_F | GTCCTGAAAGGGCTGGATG | EG10424 | 1.89 (89.37%) | [27] |
| gyrB_N_qPCR_R | CGAATACCATGTGGTG-CAGA | |||
| gyrB_V_qPCR_F | GAAATTCTCCTCCCAGACCA | EG10424 | 1.83 (82.56%) | [27] |
| gyrB_V_qPCR_R | GCAGTTCGTTCATCTGCTGT | |||
| katG_qPCR_F | GGGCCGACCTGTTTATCCTC | EG10511 | 1.92 (92.09%) | [10] |
| katG_qPCR_R | ATCCAGATCCGGTTCCCAGA | |||
| gpmA_qPCR_F | AGCCATGCCTGATCCAGTTC | EG11699 | 2.00 (100.45%) | This study |
| gpmA_qPCR_R | TTTCACCGGTTGGTACGACG | |||
| hfq_qPCR_F | CTACTGTTGTCCCGTCTCGC | EG10438 | 2.01 (101.14%) | This study |
| hfq_qPCR_R | TCGGTTTCTTCGCTGTCCTG | |||
| ahpC_qPCR_F | TGCGACCTTCGTTGTTGACC | EG11384 | 2.00 (100.23%) | This study |
| ahpC_qPCR_R | CGGAGCCAGAGTTGCTTCAC | |||
| katE_qPCR_F | TCCGGAATACGAACTGGGCT | EG10509 | 2.08 (108.44%) | This study |
| katE_qPCR_R | ATTTTGCCGACACGCTGAAC | |||
| Cloning primers for gpmA (EG11699) | ||||
| pWSK_gpmA_KpnI_R | GGGGTACCCCGACGTTTACTTCGCTTTACCCTG | This study | ||
| pWSK_EcoRI_gpmA_F | GGAATTCCATCACCAGCAAACACCGAC | This study | ||
| gpmA_His11Ala_F | CTGGTTCTGGTTCGTGCGGGCGAAAGTCAG | This study | ||
| gpmA_His11Ala_R | CTGACTTTCGCCCGCACGAACCAGAACCAG | This study | ||
| Gene Name | Function | Log2 FC | q Value |
|---|---|---|---|
| corA | magnesium/nickel/cobalt transporter | −2.37 | 2.11 × 10−43 |
| dksA | transcriptional regulator of rRNA transcription, DnaK suppressor protein | −2.11 | 1.56 × 10−11 |
| dps | Fe-binding and storage protein; stress-inducible DNA-binding protein | −3.61 | 1.37 × 10−42 |
| gpmA | phosphoglyceromutase 1 | −2.57 | 4.52 × 10−35 |
| hfq | global sRNA chaperone; HF-I, host factor for RNA phage Q beta replication | −2.28 | 5.73 × 10−14 |
| nhaA | sodium-proton antiporter | −2.50 | 2.71 × 10−27 |
| oxyR | oxidative and nitrosative stress transcriptional regulator | −1.88 | 1.17 × 10−70 |
| polA | fused DNA polymerase I 5′->3′ polymerase/3′->5′ exonuclease/5’->3’ exonuclease | −3.25 | 1.29 × 10−58 |
| rbsR | transcriptional repressor of ribose metabolism | −1.90 | 3.27 × 10−71 |
| rpoS | RNA polymerase, sigma S (sigma 38) factor | −2.80 | 1.11 × 10−32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, M.; Goodall, E.C.A.; Pullela, K.; Jaquet, V.; François, P.; Henderson, I.R.; Krause, K.-H. Transposon-Directed Insertion-Site Sequencing Reveals Glycolysis Gene gpmA as Part of the H2O2 Defense Mechanisms in Escherichia coli. Antioxidants 2022, 11, 2053. https://doi.org/10.3390/antiox11102053
Roth M, Goodall ECA, Pullela K, Jaquet V, François P, Henderson IR, Krause K-H. Transposon-Directed Insertion-Site Sequencing Reveals Glycolysis Gene gpmA as Part of the H2O2 Defense Mechanisms in Escherichia coli. Antioxidants. 2022; 11(10):2053. https://doi.org/10.3390/antiox11102053
Chicago/Turabian StyleRoth, Myriam, Emily C. A. Goodall, Karthik Pullela, Vincent Jaquet, Patrice François, Ian R. Henderson, and Karl-Heinz Krause. 2022. "Transposon-Directed Insertion-Site Sequencing Reveals Glycolysis Gene gpmA as Part of the H2O2 Defense Mechanisms in Escherichia coli" Antioxidants 11, no. 10: 2053. https://doi.org/10.3390/antiox11102053
APA StyleRoth, M., Goodall, E. C. A., Pullela, K., Jaquet, V., François, P., Henderson, I. R., & Krause, K.-H. (2022). Transposon-Directed Insertion-Site Sequencing Reveals Glycolysis Gene gpmA as Part of the H2O2 Defense Mechanisms in Escherichia coli. Antioxidants, 11(10), 2053. https://doi.org/10.3390/antiox11102053








