Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assays
2.3. Statistical Analysis
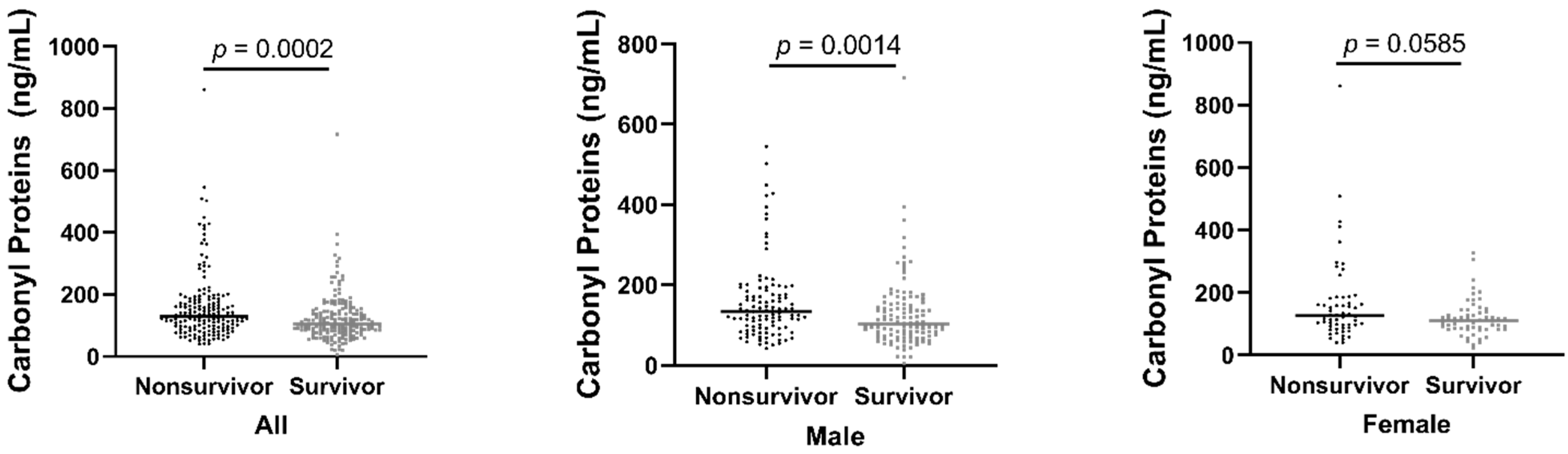
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Arici, M.; Walls, J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: Is C-reactive protein the missing link? Kidney Int. 2001, 59, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 2001, 19, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. JASN 1998, 9, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Descamps-Latscha, B.; Drüeke, T.; Witko-Sarsat, V. Dialysis-induced oxidative stress: Biological aspects, clinical consequences, and therapy. Semin. Dial. 2001, 14, 193–199. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Davies, M.J.; Fu, S.; Wang, H.; Dean, R.T. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic. Biol. Med. 1999, 27, 1151–1163. [Google Scholar] [CrossRef]
- Pryor, W.A. Oxy-radicals and related species: Their formation, lifetimes, and reactions. Annu. Rev. Physiol. 1986, 48, 657–667. [Google Scholar] [CrossRef]
- Hocher, B.; Zeng, S. Clear the Fog around Parathyroid Hormone Assays: What Do iPTH Assays Really Measure? Clin. J. Am. Soc. Nephrol. CJASN 2018, 13, 524–526. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta 2005, 1703, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Reggiani, F.; Cucchiari, D.; Astori, E.; Garavaglia, M.L.; Portinaro, N.M.; Saino, N.; Finazzi, S.; Milzani, A.; Badalamenti, S.; et al. Plasma Protein Carbonylation in Haemodialysed Patients: Focus on Diabetes and Gender. Oxidative Med. Cell. Longev. 2018, 2018, 4149681. [Google Scholar] [CrossRef] [PubMed]
- Bachi, A.; Dalle-Donne, I.; Scaloni, A. Redox proteomics: Chemical principles, methodological approaches and biological/biomedical promises. Chem. Rev. 2013, 113, 596–698. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324 Pt 1, 1–18. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Fucci, L.; Oliver, C.N.; Coon, M.J.; Stadtman, E.R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: Possible implication in protein turnover and ageing. Proc. Natl. Acad. Sci. USA 1983, 80, 1521–1525. [Google Scholar] [CrossRef]
- Starke, P.E.; Oliver, C.N.; Stadtman, E.R. Modification of hepatic proteins in rats exposed to high oxygen concentration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1987, 1, 36–39. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Gagliano, N.; Lusini, L.; Milzani, A.; Di Simplicio, P.; Colombo, R. Actin carbonylation: From a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic. Biol. Med. 2001, 31, 1075–1083. [Google Scholar] [CrossRef]
- England, K.; O’Driscoll, C.; Cotter, T.G. Carbonylation of glycolytic proteins is a key response to drug-induced oxidative stress and apoptosis. Cell Death Differ. 2004, 11, 252–260. [Google Scholar] [CrossRef]
- Magi, B.; Ettorre, A.; Liberatori, S.; Bini, L.; Andreassi, M.; Frosali, S.; Neri, P.; Pallini, V.; Di Stefano, A. Selectivity of protein carbonylation in the apoptotic response to oxidative stress associated with photodynamic therapy: A cell biochemical and proteomic investigation. Cell Death Differ. 2004, 11, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Nyström, T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Ouseph, R.; McLeish, K.R. Effects of high-flux hemodialysis on oxidant stress. Kidney Int. 2003, 63, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Brzeszczynska, J.; Kruszynska, I.; Gwozdzinski, K. Investigation of albumin properties in patients with chronic renal failure. Free Radic. Res. 2009, 43, 1008–1018. [Google Scholar] [CrossRef]
- Song, Y.R.; Kim, J.K.; Lee, H.S.; Kim, S.G.; Choi, E.K. Serum levels of protein carbonyl, a marker of oxidative stress, are associated with overhydration, sarcopenia and mortality in hemodialysis patients. BMC Nephrol. 2020, 21, 281. [Google Scholar] [CrossRef]
- Morena, M.; Delbosc, S.; Dupuy, A.M.; Canaud, B.; Cristol, J.P. Overproduction of reactive oxygen species in end-stage renal disease patients: A potential component of hemodialysis-associated inflammation. Hemodial. Int. Int. Symp. Home Hemodial. 2005, 9, 37–46. [Google Scholar] [CrossRef]
- Rusu, C.C.; Racasan, S.; Kacso, I.M.; Moldovan, D.; Potra, A.; Patiu, I.M.; Vladutiu, D.; Caprioara, M.G. Malondialdehyde can predict survival in hemodialysis patients. Clujul Med. 2016, 89, 250–256. [Google Scholar] [CrossRef]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef]
- Savenkova, M.L.; Mueller, D.M.; Heinecke, J.W. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J. Biol. Chem. 1994, 269, 20394–20400. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef]
- Podrez, E.A.; Schmitt, D.; Hoff, H.F.; Hazen, S.L. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J. Clin. Investig. 1999, 103, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Shabani, F.; McNeil, J.; Tippett, L. The oxidative inactivation of tissue inhibitor of metalloproteinase-1 (TIMP-1) by hypochlorous acid (HOCI) is suppressed by anti-rheumatic drugs. Free Radic. Res. 1998, 28, 115–123. [Google Scholar] [CrossRef]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Febbraio, M.; Hajjar, D.P.; et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002, 277, 38517–38523. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.; Shen, Z.; Zhang, R.; Colles, S.M.; Wu, W.; Salomon, R.G.; Chen, Y.; Chisolm, G.M.; Hazen, S.L. Leukocytes utilize myeloperoxidase-generated nitrating intermediates as physiological catalysts for the generation of biologically active oxidized lipids and sterols in serum. Biochemistry 1999, 38, 16904–16915. [Google Scholar] [CrossRef]
- Himmelfarb, J.; McMenamin, M.E.; Loseto, G.; Heinecke, J.W. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic. Biol. Med. 2001, 31, 1163–1169. [Google Scholar] [CrossRef]
- Buffon, A.; Biasucci, L.M.; Liuzzo, G.; D’Onofrio, G.; Crea, F.; Maseri, A. Widespread coronary inflammation in unstable angina. N. Engl. J. Med. 2002, 347, 5–12. [Google Scholar] [CrossRef]
- Rutgers, A.; Heeringa, P.; Kooman, J.P.; van der Sande, F.M.; Cohen Travaert, J.W. Peripheral blood myeloperoxidase activity increases during hemodialysis. Kidney Int. 2003, 64, 760. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, J.S.; Wu, W.M.; Liao, T.N.; Chu, P.; Lin, S.H.; Chuang, C.H.; Lin, Y.F. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2005, 20, 1134–1139. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Brennan, M.L.; Hazen, S.L. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2006, 48, 59–68. [Google Scholar] [CrossRef]
- Wang, A.Y.; Lam, C.W.; Chan, I.H.; Wang, M.; Lui, S.F.; Sanderson, J.E. Prognostic value of plasma myeloperoxidase in ESRD patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2010, 56, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.C.; Tsai, J.P.; Yang, S.F.; Lee, W.C.; Huang, J.Y.; Chang, S.C.; Hso, C.S.; Chang, H.R. MMP-2 serum concentrations predict mortality in hemodialysis patients: A 5-year cohort study. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 452, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Guo, Z.J.; Niu, H.X.; Hou, F.F.; Zhang, L.; Fu, N.; Nagai, R.; Lu, X.; Chen, B.H.; Shan, Y.X.; Tian, J.W.; et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid. Redox Signal. 2008, 10, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Descamps-Latscha, B. Are advanced oxidation protein products potential uremic toxins? Kidney Int. Suppl. 2003, 63, S11–S14. [Google Scholar] [CrossRef]
- Gonzalez, E.; Bajo, M.A.; Carrero, J.J.; Lindholm, B.; Grande, C.; Sánchez-Villanueva, R.; Del Peso, G.; Díaz-Almirón, M.; Iglesias, P.; Díez, J.J.; et al. An Increase of Plasma Advanced Oxidation Protein Products Levels Is Associated with Cardiovascular Risk in Incident Peritoneal Dialysis Patients: A Pilot Study. Oxidative Med. Cell. Longev. 2015, 2015, 219569. [Google Scholar] [CrossRef] [PubMed]
- Suvakov, S.; Jerotic, D.; Damjanovic, T.; Milic, N.; Pekmezovic, T.; Djukic, T.; Jelic-Ivanovic, Z.; Savic Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; et al. Markers of Oxidative Stress and Endothelial Dysfunction Predict Haemodialysis Patients Survival. Am. J. Nephrol. 2019, 50, 115–125. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Chen, J.; Mei, C.; Xiong, F.; Shi, W.; Zhou, W.; Liu, X.; Sun, S.; Tian, J.; et al. Association between serum advanced oxidation protein products and mortality risk in maintenance hemodialysis patients. J. Transl. Med. 2021, 19, 284. [Google Scholar] [CrossRef]
- Pachaly, M.A.; do Nascimento, M.M.; Suliman, M.E.; Hayashi, S.Y.; Riella, M.C.; Manfro, R.C.; Stenvinkel, P.; Lindholm, B. Interleukin-6 is a better predictor of mortality as compared to C-reactive protein, homocysteine, pentosidine and advanced oxidation protein products in hemodialysis patients. Blood Purif. 2008, 26, 204–210. [Google Scholar] [CrossRef]
- Sevinc Ok, E.; Kircelli, F.; Asci, G.; Altunel, E.; Ertilav, M.; Sipahi, S.; Bozkurt, D.; Duman, S.; Ozkahya, M.; Toz, H.; et al. Neither oxidized nor anti-oxidized low-density lipoprotein level is associated with atherosclerosis or mortality in hemodialysis patients. Hemodial. Int.. Int. Symp. Home Hemodial. 2012, 16, 334–341. [Google Scholar] [CrossRef]
- Epstein, M.; Vaziri, N.D. Statins in the management of dyslipidemia associated with chronic kidney disease. Nat. Rev. Nephrol. 2012, 8, 214–223. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, D.; Devaraj, S.; Islam, K.N.; Collazo, R.; McDonald, L.; Grundy, S.; Jialal, I. Low-density lipoprotein (LDL)-induced monocyte-endothelial cell adhesion, soluble cell adhesion molecules, and autoantibodies to oxidized-LDL in chronic renal failure patients on dialysis therapy. Metab. Clin. Exp. 2001, 50, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Cwiklińska, A.; Ziętkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press. Res. 2011, 34, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Takahashi, K.; Kobayashi, T.; Oshima, E.; Iwasaki, S.; Suzuki, H. Oxidized low density lipoprotein (Ox-LDL) as a marker of atherosclerosis in hemodialysis (HD) patients. Clin. Nephrol. 2002, 58, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Van Tits, L.; De Graaf, J.; Hak-Lemmers, H.; Bredie, S.; Demacker, P.; Holvoet, P.; Stalenhoef, A. Increased levels of low-density lipoprotein oxidation in patients with familial hypercholesterolemia and in end-stage renal disease patients on hemodialysis. Lab. Investig. A J. Tech. Methods Pathol. 2003, 83, 13–21. [Google Scholar] [CrossRef][Green Version]
- Nissel, R.; Faraj, S.; Sommer, K.; Henning, L.; van der Giet, M.; Querfeld, U. Oxidative stress markers in young hemodialysis patients—A pilot study. Clin. Nephrol. 2008, 70, 135–143. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Oxidized low-density lipoprotein (oxLDL) plasma levels and oxLDL to LDL ratio—Are they real oxidative stress markers in dialyzed patients? Life Sci. 2013, 92, 253–258. [Google Scholar] [CrossRef]
- Johnson-Davis, K.L.; Fernelius, C.; Eliason, N.B.; Wilson, A.; Beddhu, S.; Roberts, W.L. Blood enzymes and oxidative stress in chronic kidney disease: A cross sectional study. Ann. Clin. Lab. Sci. 2011, 41, 331–339. [Google Scholar]
- Diepeveen, S.H.; Verhoeven, G.H.; van der Palen, J.; Dikkeschei, B.L.; van Tits, L.J.; Kolsters, G.; Offerman, J.J.; Bilo, H.J.; Stalenhoef, A.F. Oxidative stress in patients with end-stage renal disease prior to the start of renal replacement therapy. Nephron. Clin. Pract. 2004, 98, c3–c7. [Google Scholar] [CrossRef]
- Tavridou, A.; Georgoulidou, A.; Roumeliotis, A.; Roumeliotis, S.; Giannakopoulou, E.; Papanas, N.; Passadakis, P.; Manolopoulos, V.G.; Vargemezis, V. Association of Plasma Adiponectin and Oxidized Low-Density Lipoprotein with Carotid Intima-Media Thickness in Diabetic Nephropathy. J. Diabetes Res. 2015, 2015, 507265. [Google Scholar] [CrossRef]
- Tsimikas, S.; Brilakis, E.S.; Miller, E.R.; McConnell, J.P.; Lennon, R.J.; Kornman, K.S.; Witztum, J.L.; Berger, P.B. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 2005, 353, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, D.H.; Kim, J.K.; Park, M.J.; Yan, J.J.; Song, D.K.; Vaziri, N.D.; Noh, J.W. Lysophosphatidylcholine, oxidized low-density lipoprotein and cardiovascular disease in Korean hemodialysis patients: Analysis at 5 years of follow-up. J. Korean Med. Sci. 2013, 28, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kraśniak, A.; Drozdz, M.; Pasowicz, M.; Chmiel, G.; Michałek, M.; Szumilak, D.; Podolec, P.; Klimeczek, P.; Konieczyńska, M.; Wicher-Muniak, E.; et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2007, 22, 515–521. [Google Scholar] [CrossRef]
- Wagner, S.; Apetrii, M.; Massy, Z.A.; Kleber, M.E.; Delgado, G.E.; Scharnagel, H.; März, W.; Metzger, M.; Rossignol, P.; Jardine, A.; et al. Oxidized LDL, statin use, morbidity, and mortality in patients receiving maintenance hemodialysis. Free Radic. Res. 2017, 51, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002, 8, 1211–1217. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef]

| Characteristics | All (n = 347) | Survivors (n = 170) | Non-Survivors (n = 168) | p-Value |
|---|---|---|---|---|
| Age (years) | 66.0 (56.0–75.0) | 60.50 (49.00–69.00) | 71.00 (66.00–78.00) | <0.001 |
| Sex (M/F/Unknown) | 229/117/1 | 114/56/0 | 110/58/0 | 0.759 |
| Body mass index, kg/m2 | 24.40 (22.01–27.60) | 24.20 (22.12–28.30) | 24.57 (21.71–26.99) | 0.541 |
| Drinker, n (%) | 62 (17.90%) | 30 (17.60%) | 32 (9.10%) | 0.740 |
| Smoker, n (%) | 108 (31.10%) | 54 (31.80%) | 52 (14.80%) | 0.872 |
| Diabetes mellitus, n (%) | 130 (37.50%) | 55 (32.40%) | 74 (21.10%) | 0.027 |
| Hypertension, n (%) | 269 (77.50%) | 134 (78.80%) | 135 (38.50%) | 0.727 |
| Cardiovascular disease, n (%) | 161 (46.40%) | 82 (48.20%) | 101 (28.80%) | <0.001 |
| Dialysis vintage (days) | 263.00 (31.00–1219.25) | 221.00 (31.00–939.25) | 351.00 (31.00–1461.00) | 0.004 |
| Dialysis dose (Kt/V) | 1.04 (0.91–1.16) | 1.03 (0.91–1.16) | 1.04 (0.91–1.17) | 0.749 |
| Medication, n (%) | ||||
| RAAS inhibitors | 88 (25.40%) | 46 (27.1%) | 41 (11.70%) | 0.577 |
| Beta-blockers | 204 (58.8%) | 116 (68.2%) | 86 (24.50%) | 0.001 |
| Calcium channel blockers | 104 (30.00%) | 60 (35.3%) | 43 (12.30%) | 0.053 |
| Erythropoietin | 171 (49.30%) | 82 (48.2%) | 89 (25.40%) | 0.414 |
| Diuretics | 194 (55.90%) | 98 (57.6%) | 95 (27.10%) | 0.838 |
| Hemoglobin (g/dL) | 10.20 (9.10–11.63) | 10.25 (9.00–11.67) | 10.20 (9.20–11.70) | 0.865 |
| Ferritin (ng/mL) | 532.00 (253.25–1125.88) | 527.50 (225.00–1065.75) | 532.00 (281.00–1235.00) | 0.540 |
| Transferrin (µg/mL) | 138.00(106.00–173.00) | 145.00 (121.00–173.50) | 128.50 (99.00–172.25) | 0.003 |
| Fasting blood glucose (mg/dL) | 108.00 (90.00–134.00) | 114.50 (94.50–143.60) | 104.00 (87.00–123.60) | 0.006 |
| Creatinine (mg/dL) | 6.62 (4.23–8.34) | 6.67 (4.15–8.53) | 6.60 (4.23–7.96) | 0.007 |
| Potassium (mmol/L) | 4.70 (4.10–5.28) | 4.60 (4.00–5.30) | 4.77 (4.21–5.26) | 0.734 |
| Calcium (mmol/L) | 2.24 (2.10–2.40) | 2.20 (2.09–2.40) | 2.27 (2.10–2.47) | 0.414 |
| Phosphorus (mmol/L) | 1.61 (1.19–2.10) | 1.70 (1.22–2.12) | 1.54 (1.11–2.06) | 0.051 |
| iPTH (ng/L) | 49.90 (18.68–124.60) | 68.19 (21.75–171.05) | 39.76 (14.47–101.90) | 0.003 |
| n-ox PTH (ng/L) | 5.86 (2.38–14.01) | 7.18 (3.05–16.26) | 4.99 (1.98–11.11) | 0.003 |
| Albumin (g/dL) | 3.30 (2.90–3.70) | 3.40 (3.05–3.80) | 3.10 (2.80–3.60) | 0.001 |
| BUN (mg/dL) | 195.12 (146.70–267.67) | 201.05 (152.64–267.67) | 189.63 (131.73–279.50) | 0.822 |
| LDL (mg/dL) | 92.70 (72.20–121.20) | 100.80 (75.05–127.40) | 89.00 (70.70–112.00) | 0.013 |
| HDL (mg/dL) | 39.90 (32.20–50.80) | 38.60 (31.00–50.20) | 42.30 (34.30–54.00) | 0.435 |
| hsCRP (mg/L) | 2.60 (1.00–5.20) | 2.30 (0.70–4.50) | 2.80 (1.20–6.63) | 0.006 |
| MPO (ng/mL) | 106.84 (67.71–188.38) | 102.27 (67.37–176.37) | 118.90 (69.46–199.24) | 0.176 |
| AOPPs (µmol/L) | 107.79 (78.79–149.94) | 109.99 (80.59–156.72) | 107.57 (79.53–146.80) | 0.588 |
| ox-LDL (mg/dL) | 84.90 (44.80–180.55) | 87.55 (45.85–197.63) | 83.10 (44.53–176.35) | 0.779 |
| Carbonyl proteins (ng/mL) | 117.85 (84.73–163.18) | 105.40 (81.30–147.85) | 129.65 (93.20–180.33) | <0.001 |
| Analyses | HR (95% CI) | p-Value |
|---|---|---|
| Age(years) | 1.062 (1.047–1.077) | <0.001 |
| Male/Female | 0.981 (0.714–1.349) | 0.908 |
| Body mass index, kg/m2 | 0.992 (0.961–1.024) | 0.615 |
| Drinker, n (%) | 1.108 (0.754–1.629) | 0.601 |
| Smoker, n (%) | 0.879 (0.634–1.220) | 0.441 |
| Diabetes mellitus, n (%) | 1.203 (0.887–1.632) | 0.235 |
| Hypertension, n (%) | 0.723 (0.493–1.059) | 0.096 |
| Cardiovascular disease, n (%) | 1.440 (1.056–1.963) | 0.021 |
| Dialysis vintage (days) | 0.999869 (0.999716–1.000022) | 0.093 |
| Dialysis dose (Kt/V) | 0.731 (0.377–1.417) | 0.353 |
| Hemoglobin (g/dL) | 0.950 (0.868–1.038) | 0.256 |
| Ferritin (ng/mL) | 1.000 (1.000–1.000) | 0.846 |
| Transferrin (µg/mL) | 0.995 (0.992–0.998) | 0.002 |
| Fasting blood glucose (mg/dL) | 0.999 (0.995–1.002) | 0.449 |
| Creatinine (mg/dL) | 0.875 (0.819–0.935) | <0.001 |
| Potassium (mmol/L) | 0.895 (0.744–1.076) | 0.238 |
| Calcium (mmol/L) | 0.832 (0.493–1.403) | 0.490 |
| Phosphorus (mmol/L) | 0.771 (0.595–1.000) | 0.0503 |
| iPTH (ng/L) | 0.998 (0.997–1.000) | 0.012 |
| n-ox PTH | 0.986 (0.973–1.000) | 0.043 |
| Albumin (g/dL) | 0.663 (0.515–0.855) | 0.001 |
| BUN (mg/dL) | 1.000 (0.999–1.001) | 0.479 |
| LDL (mg/dL) | 0.997 (0.993–1.002) | 0.250 |
| HDL (mg/dL) | 1.005 (0.996–1.015) | 0.302 |
| hsCRP (mg/L) | 1.018 (0.992–1.044) | 0.179 |
| MPO (ng/mL) | 1.000035 (1.000020–1.000051) | <0.001 |
| AOPPs (µmol/L) | 1.001 (0.998–1.004) | 0.445 |
| ox-LDL (mg/dL) | 1.000 (0.999–1.000) | 0.451 |
| Carbonyl proteins (ng/mL) | 1.002 (1.001–1.003) | 0.001 |
| Analyses | HR (95% CI) | p-Value |
|---|---|---|
| Univariate Cox regression | ||
| Continuous Carbonyl proteins | 1.002 (1.001–1.003) | 0.001 |
| Binary Carbonyl proteins | 0.564 (0.414–0.767) | <0.001 |
| Log Carbonyl proteins | 3.162 (1.684–5.937) | <0.001 |
| Multivariable Cox regression | ||
| Model A | 1.002 (1.001–1.004) | 0.001 |
| Model B | 1.002 (1.000–1.003) | 0.027 |
| Model C | 1.002 (1.000–1.004) | 0.015 |
| Analyses | HR (95% CI) | p-Value |
|---|---|---|
| Univariate Cox regression | ||
| Continuous MPO | 1.000035 (1.000020–1.000051) | <0.001 |
| Binary MPO | 1.363 (0.998–1.862) | 0.052 |
| Log MPO | 2.123 (1.394–3.234) | <0.001 |
| Multivariable Cox regression | ||
| Model A | 1.000033 (1.000018–1.000049) | <0.001 |
| Model B | 1.000028 (1.000012–1.000044) | <0.001 |
| Model C | 1.000024 (1.000008–1.000040) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Chaykovska, L.; Chu, C.; Chen, X.; Hasan, A.A.; Krämer, B.K.; Tepel, M.; Hocher, B. Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients. Antioxidants 2022, 11, 1975. https://doi.org/10.3390/antiox11101975
Zuo J, Chaykovska L, Chu C, Chen X, Hasan AA, Krämer BK, Tepel M, Hocher B. Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients. Antioxidants. 2022; 11(10):1975. https://doi.org/10.3390/antiox11101975
Chicago/Turabian StyleZuo, Jiao, Lyubov Chaykovska, Chang Chu, Xin Chen, Ahmed A. Hasan, Bernhard K. Krämer, Martin Tepel, and Berthold Hocher. 2022. "Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients" Antioxidants 11, no. 10: 1975. https://doi.org/10.3390/antiox11101975
APA StyleZuo, J., Chaykovska, L., Chu, C., Chen, X., Hasan, A. A., Krämer, B. K., Tepel, M., & Hocher, B. (2022). Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients. Antioxidants, 11(10), 1975. https://doi.org/10.3390/antiox11101975








