Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Supercritical Carbon Dioxide Extraction of Costus Oil
2.3. GC-MS Analysis and Conditions
2.4. MTT Assay
2.5. Microscopic Studies
2.6. Antimicrobial Activity
2.7. DPPH Radical Scavenging Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Pressures of Supercritical CO2 Extraction on the Yield of Active Compounds from S. costus
3.2. Anticancer Activity
3.3. Morphological Studies
3.4. Antimicrobial Activity
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Tsiaka, T.; Sinanoglou, V.J.; Zoumpoulakis, P. Extracting Bioactive Compounds From Natural Sources Using Green High-Energy Approaches: Trends and Opportunities in Lab- and Large-Scale Applications. In Ingredients Extraction by Physicochemical Methods in Food; Academic Press: Cambridge, MA, USA, 2017; pp. 307–365. [Google Scholar] [CrossRef]
- Elshaer, S.E.; Hamad, G.M.; Hafez, E.E.; Baghdadi, H.H.; El-Demerdash, F.M.; Simal-Gandara, J. Root Extracts of Saussurea costus as Prospective Detoxifying Food Additive against Sodium Nitrite Toxicity in Male Rats. Food Chem. Toxicol. 2022, 166, 113225. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Wani, B.; Mohammad Wani, F.; Khan, A.; Bodha, R.; Mohiddin, F.; Hamid, A. Some Herbs Mentioned in the Holy Quran and Ahadith and Their Medicinal Importance in Contemporary Times. J. Pharm. Res. 2011, 4, 3888–3891. [Google Scholar]
- Robinson, A.; Kumar, T.V.; Sreedhar, E.; Naidu, V.G.M.; Krishna, S.R.; Babu, K.S.; Srinivas, P.V.; Rao, J.M. A New Sesquiterpene Lactone from the Roots of Saussurea Lappa: Structure—Anticancer Activity Study. Bioorg. Med. Chem. Lett. 2008, 18, 4015–4017. [Google Scholar] [CrossRef] [PubMed]
- Hasson, S.S.A.; Al-Balushi, M.S.; KhazinaAlharthy; Al-Busaidi, J.Z.; MunaSulimanAldaihani; Othman, M.S.; Said, E.A.; Habal, O.; Sallam, T.A.; Aljabri, A.A.; et al. Evaluation of Anti-Resistant Activity of Auklandia (Saussurea Lappa) Root against Some Human Pathogens. Asian Pac. J. Trop. Biomed. 2013, 3, 557–562. [Google Scholar] [CrossRef]
- Zahara, K.; Tabassum, S.; Sabir, S.; Arshad, M.; Qureshi, R.; Amjad, M.S.; Chaudhari, S.K. A Review of Therapeutic Potential of Saussurea Lappa-An Endangered Plant from Himalaya. Asian Pac. J. Trop. Med. 2014, 7, S60–S69. [Google Scholar] [CrossRef]
- Al Ghasham, A.; Al Muzaini, M.; Ahmad Qureshi, K.; Osman Elhassan, G.; Ahmed Khan, R.; Ayesha Farhana, S.; Hashmi, S.; El-Agamy, E.; Abdallah, W.E. Phytochemical Screening, Antioxidant and Antimicrobial Activities of Methanolic Extract of Ziziphus Mauritiana Lam. Leaves Collected from Unaizah, Saudi Arabia. Int. J. Pharm. Res. Allied Sci. 2017, 6, 33–46. Available online: www.ijpras.com (accessed on 21 January 2017).
- Naseer, S.; Iqbal, J.; Naseer, A.; Kanwal, S.; Hussain, I.; Tan, Y.; Aguilar-Marcelino, L.; Cossio-Bayugar, R.; Zajac, Z.; bin Jardan, Y.A.; et al. Deciphering Chemical Profiling, Pharmacological Responses and Potential Bioactive Constituents of Saussurea Lappa Decne. Extracts through in Vitro Approaches. Saudi J. Biol. Sci. 2022, 29, 1355–1366. [Google Scholar] [CrossRef]
- Lee, B.K.; Park, S.J.; Nam, S.Y.; Kang, S.; Hwang, J.; Lee, S.J.; Im, D.S. Anti-Allergic Effects of Sesquiterpene Lactones from Saussurea costus (Falc.) Lipsch. Determined Using in Vivo and in Vitro Experiments. J. Ethnopharmacol. 2018, 213, 256–261. [Google Scholar] [CrossRef]
- Deabes, M.M.; El-Fatah, S.I.A.; Salem, S.H.; Naguib, K.M. Antimicrobial Activity of Bioactive Compounds Extract from Saussurea costus against Food Spoilage Microorganisms. Egypt J. Chem. 2021, 64, 2833–2843. [Google Scholar] [CrossRef]
- Mohamed, R.; Alaagib, O.; Mohamed, S.; Ayoub, H. The Pharma Innovation Journal 2015; 4(2): 73-76 On the Chemical Composition and Antibacterial Activity of Saussurea Lappa (Asteraceae). TPI 2015, 4, 73–76. [Google Scholar]
- Alshubaily, F.A. Enhanced Antimycotic Activity of Nanoconjugates from Fungal Chitosan and Saussurea costus Extract against Resistant Pathogenic Candida Strains. Int. J. Biol. Macromol. 2019, 141, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Tag, H.M.; Khaled, H.E.; Ismail, H.A.A.; El-Shenawy, N.S. Evaluation of Anti-Inflammatory Potential of the Ethanolic Extract of the Saussurea Lappa Root (Costus) on Adjuvant-Induced Monoarthritis in Rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.; El-Darra, N.; Saleh, F.A.; Rajha, H.N.; Louka, N. Optimization of Infrared-Assisted Extraction of Bioactive Lactones from Saussurea lappa L. and Their Effects against Gestational Diabetes. Pharmacogn. Mag. 2019, 15, 208. [Google Scholar] [CrossRef]
- Chai, Y.H.; Yusup, S.; Kadir, W.N.A.; Wong, C.Y.; Rosli, S.S.; Ruslan, M.S.H.; Chin, B.L.F.; Yiin, C.L. Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology. Sustainability 2020, 13, 233. [Google Scholar] [CrossRef]
- Khanyile, A.T.; Andrew, J.E.; Paul, V.; Sithole, B.B. A Comparative Study of Supercritical Fluid Extraction and Accelerated Solvent Extraction of Lipophilic Compounds from Lignocellulosic Biomass. Sustain. Chem. Pharm. 2022, 26, 100608. [Google Scholar] [CrossRef]
- Ghasemi, E.; Raofie, F.; Najafi, N.M. Application of Response Surface Methodology and Central Composite Design for the Optimisation of Supercritical Fluid Extraction of Essential Oils from Myrtus communis L. Leaves. Food Chem. 2011, 126, 1449–1453. [Google Scholar] [CrossRef]
- Kate, A.E.; Singh, A.; Shahi, N.C.; Pandey, J.P.; Prakash, O.; Singh, T.P. Singh Novel Eco-Friendly Techniques for Extraction of Food Based Lipophilic Compounds from Biological Materials. Nat. Prod. Chem. Res. 2016, 4, 231–237. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- López-Hortas, L.; Rodríguez, P.; Díaz-Reinoso, B.; Gaspar, M.C.; de Sousa, H.C.; Braga, M.E.M.; Domínguez, H. Supercritical Fluid Extraction as a Suitable Technology to Recover Bioactive Compounds from Flowers. J. Supercrit. Fluids 2022, 188, 105652. [Google Scholar] [CrossRef]
- Ruan, X.; Cui, W.X.; Yang, L.; Li, Z.H.; Liu, B.; Wang, Q. Extraction of Total Alkaloids, Peimine and Peiminine from the Flower of Fritillaria thunbergii Miq Using Supercritical Carbon Dioxide. J. CO2 Util. 2017, 18, 283–293. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Supercritical CO2 Extraction of Bioactive Compounds from Hibiscus sabdariffa. J. Supercrit. Fluids 2019, 147, 213–221. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chang, W.L.; Lee, S.C.; Liu, Y.G.; Lin, H.C.; Chen, C.J.; Yen, C.Y.; Yu, D.S.; Lin, S.Z.; Harn, H.J. Acetone Extract of Bupleurum Scorzonerifolium Inhibits Proliferation of A549 Human Lung Cancer Cells via Inducing Apoptosis and Suppressing Telomerase Activity. Life Sci. 2003, 73, 2383–2394. [Google Scholar] [CrossRef]
- Smania, A., Jr.; Monache, F.D.; Smania, E.d.F.A.; Cuneo, R.S. Antibacterial Activity of Steroidal Compounds Isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) Fruit Body. Int. J. Med. Mushrooms 1999, 1, 325–330. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P. der Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 2002, 42, 629–632. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Supercritical CO2 Extraction of Rosehip Seed Oil: Fatty Acids Composition and Process Optimization. J. Supercrit. Fluids 2007, 41, 421–428. [Google Scholar] [CrossRef]
- Cassel, E.; Vargas, R.M.F.; Brun, G.W.; Almeida, D.E.; Cogoi, L.; Ferraro, G.; Filip, R. Supercritical Fluid Extraction of Alkaloids from Ilex Paraguariensis St. Hil. J. Food Eng. 2010, 100, 656–661. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, J.; Gao, H.; Wang, Y. Optimization and Composition of Volatile Oil from Polygonatum odoratum (Mill Druce) Using Supercritical Fluid Extraction. Trop. J. Pharm. Res. 2014, 13, 779–786. [Google Scholar] [CrossRef][Green Version]
- Adib, K.; Rahimi-Nasrabadi, M.; Rezvani, Z.; Pourmortazavi, S.M.; Ahmadi, F.; Naderi, H.R.; Ganjali, M.R. Facile Chemical Synthesis of Cobalt Tungstates Nanoparticles as High Performance Supercapacitor. J. Mater. Sci. Mater. Electron. 2016, 27, 4541–4550. [Google Scholar] [CrossRef]
- Hamburger, M.; Baumann, D.; Adler, S. Supercritical Carbon Dioxide Extraction of Selected Medicinal Plants—Effects of High Pressure and Added Ethanol on Yield of Extracted Substances. Phytochem. Anal. 2004, 15, 46–54. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved Extraction of Vegetable Oils under High-Intensity Ultrasound and/or Microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, C.; D’Auria, M.; Mecca, M.; Prasad, P.; Singh, P.; Singh, S.; Sinisgalli, C.; Milella, L. Chemical and Biological Evaluation of Essential Oil from Saussurea costus (Falc.) Lipsch. from Garhwal Himalaya Collected at Different Harvesting Periods. Nat. Prod. Res. 2019, 33, 2355–2358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef]
- Ansari, M.; Emami, S. β-Ionone and Its Analogs as Promising Anticancer Agents. Eur. J. Med. Chem. 2016, 123, 141–154. [Google Scholar] [CrossRef]
- A, A.R.; Mary, A.; Sabapathy, I.; Rajalakshmi, M. In Vitro and In Silico Studies on Evaluating Eremanthin as Potential Therapeutic Drug against Breast Cancer. Asian J. Pharm. Clin. Res. 2020, 131, 132–137. [Google Scholar] [CrossRef]
- Brito, M.T.; Ferreira, R.C.; Beltrão, D.M.; Moura, A.P.G.; Xavier, A.L.; Pita, J.C.L.R.; Batista, T.M.; Longato, G.B.; Ruiz, A.L.T.G.; de Carvalho, J.E.; et al. Antitumor Activity and Toxicity of Volatile oil from the Leaves of Annona leptopetala. Rev. Bras. De Farmacogn. 2018, 28, 602–609. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, Chemical and Pharmacological Review of an Ayurvedic Medicinal Plant. J. Ethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef]
- Butturini, E.; Cavalieri, E.; de Prati, A.C.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H.; et al. Two Naturally Occurring Terpenes, Dehydrocostuslactone and Costunolide, Decrease Intracellular GSH Content and Inhibit STAT3 Activation. PLoS ONE 2011, 6, e20174. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chang, H.-S.; Chen, I.-S.; Chen, C.-J.; Hsu, M.-L.; Fu, S.-L.; Chen, Y.-J. Costunolide Causes Mitotic Arrest and Enhances Radiosensitivity in Human Hepatocellular Carcinoma Cells. Radiat. Oncol. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.-H. Evaluation of Anticancer Activity of Dehydrocostuslactone in Vitro. Mol. Med. Rep. 2010, 3, 185–188. [Google Scholar] [CrossRef]
- Pitchai, D.; Roy, A.; Banu, S. In Vitro and In Silico Evaluation of NF-ΚB Targeted Costunolide Action on Estrogen Receptor-Negative Breast Cancer Cells—A Comparison with Normal Breast Cells. Phytother. Res. 2014, 28, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Jasna, I.; Irena, O. Investigation of Antibacterial Activity of Supercritical Extracts of Plants, as Well as of Extracts Obtained by Other Technological Processes on Some Bacteria Isolated from Animals. Acta Vet. 2009, 59, 557–568. [Google Scholar] [CrossRef]
- Karakaya, S.; El, S.N.; Karagözlü, N.; Şahin, S. Antioxidant and Antimicrobial Activities of Essential Oils Obtained from Oregano (Origanum vulgare Ssp. Hirtum) by Using Different Extraction Methods. J. Med. Food 2011, 14, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Yang, C.-S.; Hwang, M.-L.; Chang, C.-C.; Li, R.-X.; Chuang, L.-Y. Antimicrobial Activity of Various Parts of Cinnamomum Cassia Extracted with Different Extraction Methods. J. Food Biochem. 2012, 36, 690–698. [Google Scholar] [CrossRef]
- Liang, M.-T.; Yang, C.-H.; Li, S.-T.; Yang, C.-S.; Chang, H.-W.; Liu, C.-S.; Cham, T.-M.; Chuang, L.-Y. Antibacterial and Antioxidant Properties of Ramulus Cinnamomi Using Supercritical CO2 Extraction. Eur. Food Res. Technol. 2008, 227, 1387–1396. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, M.; Luo, W.; Yang, B.; Jiang, Y. Identification of Volatile Components in Phyllanthus emblica L. and Their Antimicrobial Activity. J. Med. Food 2009, 12, 423–428. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; de Martino, L.; Coppola, R.; Feo, V. de Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Lamponi, S.; Mammate, N.; Ezzahra El Oumari, F.; Imtara, H.; Belchkar, S.; Lahrichi, A.; Alqahtani, A.S.; Noman, O.M.; Tarayrah, M.; Houssaini, T.S. Antioxidant and Anti-Urolithiatic Activity of Aqueous and Ethanolic Extracts from Saussurea costus (Falc) Lispich Using Scanning Electron Microscopy. Life 2022, 12, 1026. [Google Scholar] [CrossRef]
- Singh, R.; Chahal, K.K.; Khushminder, C.; Chahal, K. Phytochemical Analysis and in Vitro Antioxidant Capacity of Different Solvent Extracts of Saussurea lappa L. Roots. J. Pharmacogn. Phytochem. 2018, 7, 427–432. [Google Scholar]
- Ijaz, S.; Perveen, A.; Ghaffar, N. Phytochemical Screening an Antioxidant Activity of Seeds of Saussurea costus (Lalc.) Lipsch: An Indigenous Medicinal Plant of Neelum Valley Azad Kashmir. Asian J. Pharmacogn. 2021, 4, 24–30. [Google Scholar]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of Extraction Methods on Yield, Phytochemical Constituents and Antioxidant Activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Petrelli, R.; Orsomando, G.; Sorci, L.; Maggi, F.; Ranjbarian, F.; Biapa Nya, P.C.; Petrelli, D.; Vitali, L.A.; Lupidi, G.; Quassinti, L.; et al. Biological Activities of the Essential oil from Erigeron floribundus. Molecules 2016, 21, 1065. [Google Scholar] [CrossRef] [PubMed]


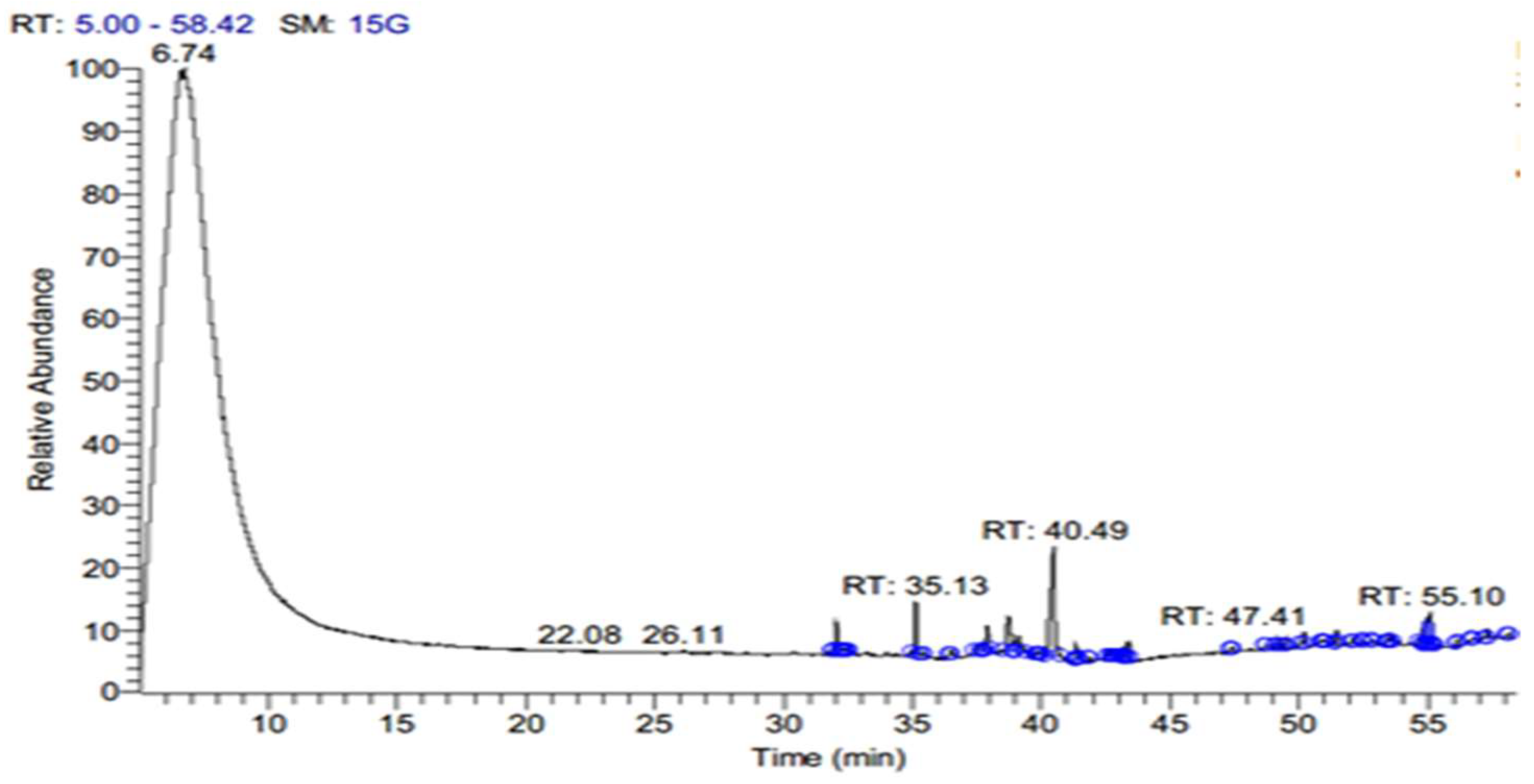
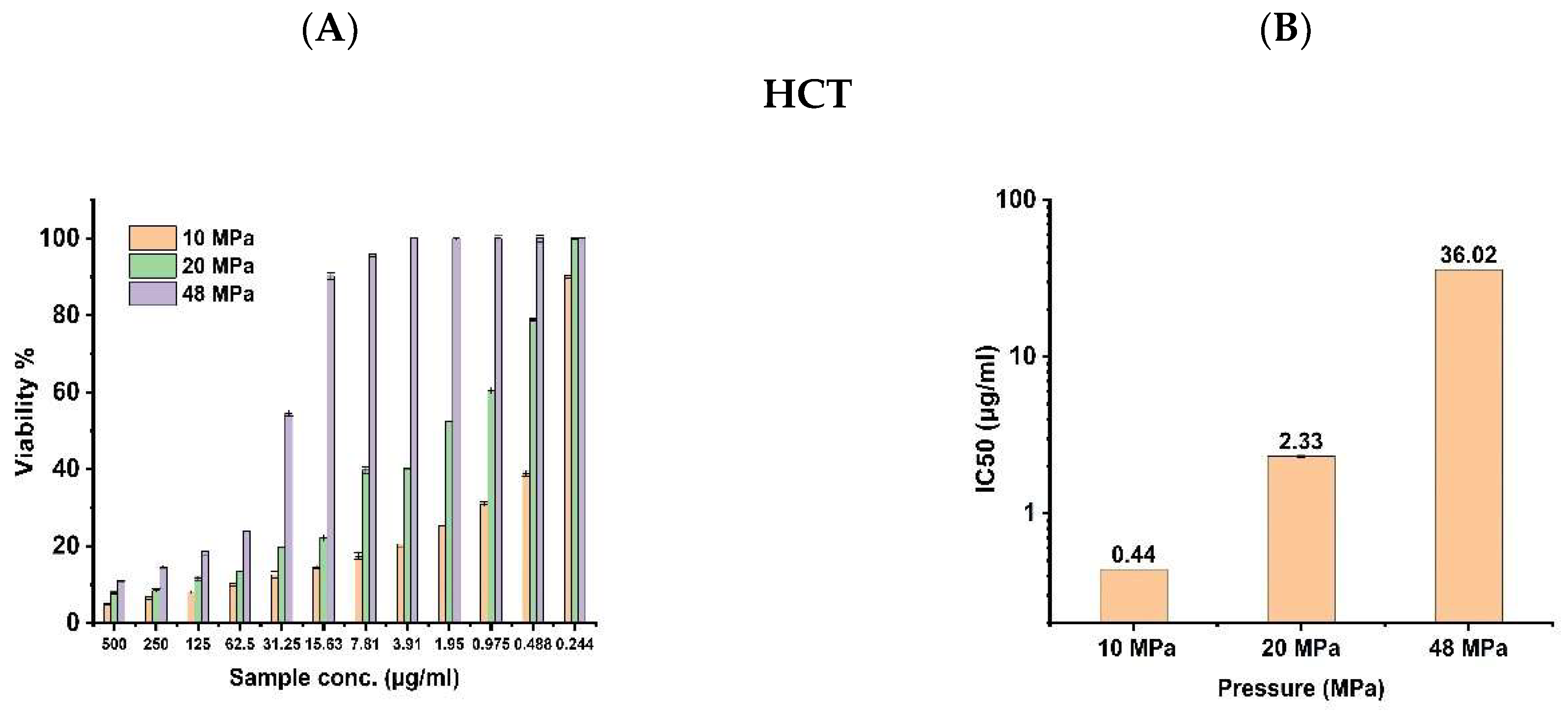

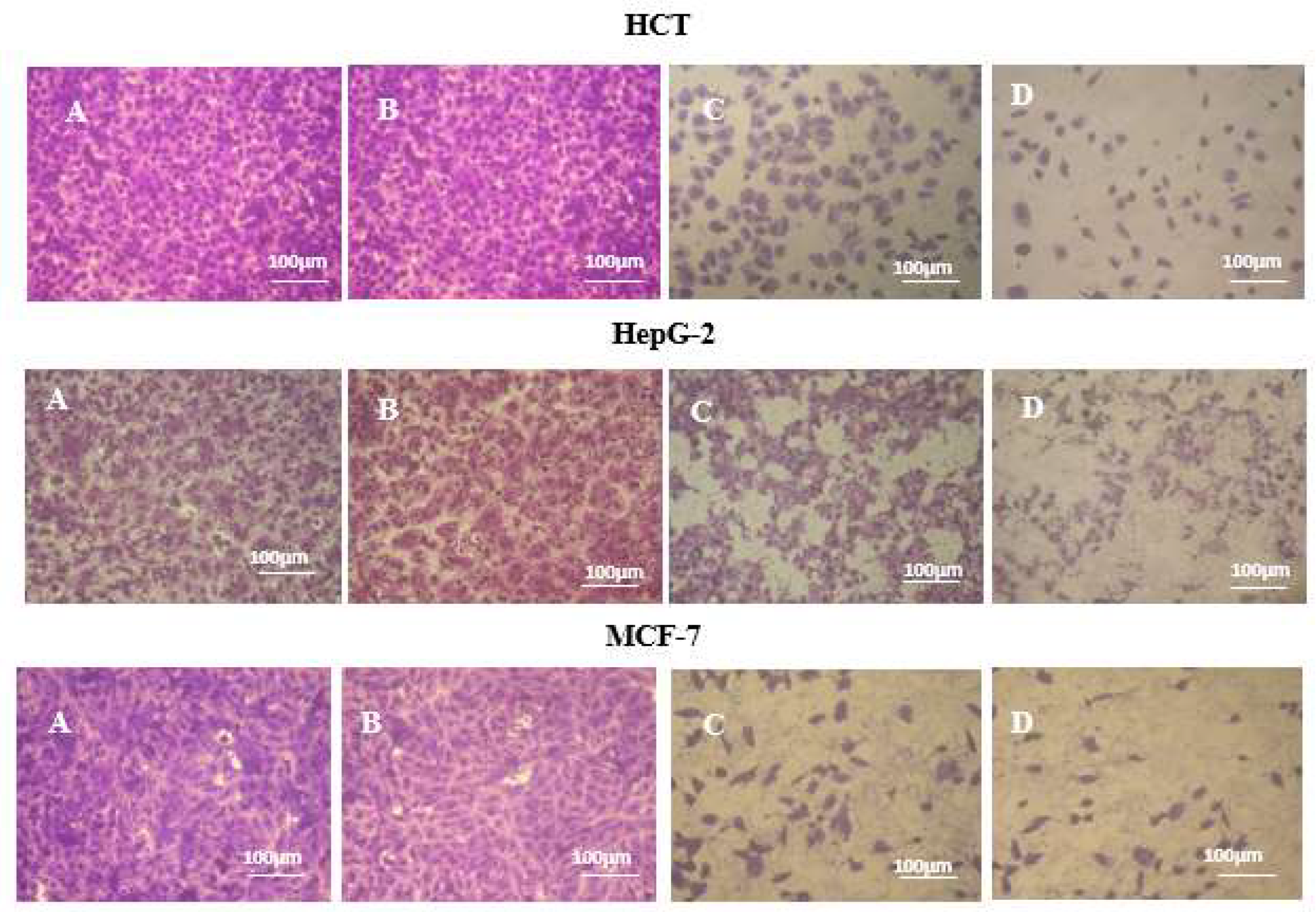
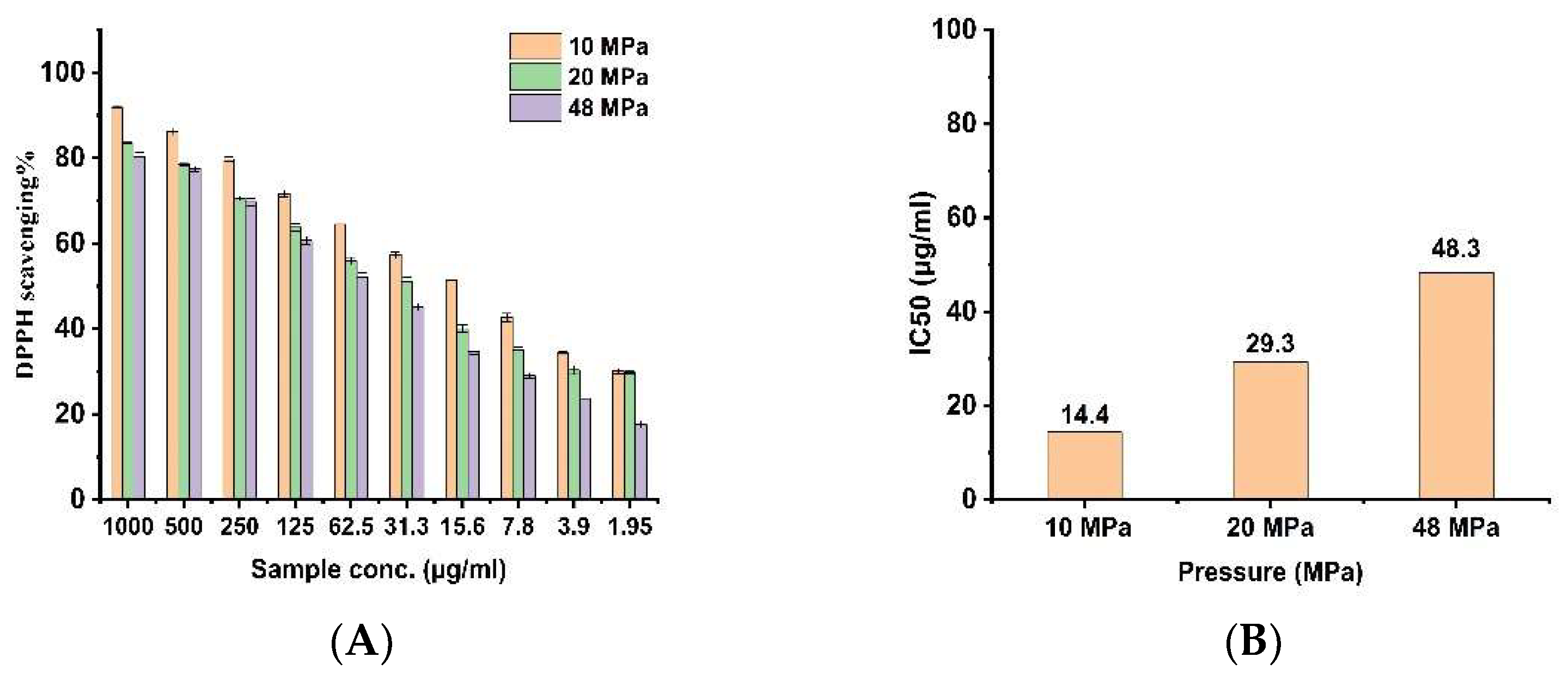
| Chemical Name | 10 MPa | 20 MPa | 48 MPa | |||
|---|---|---|---|---|---|---|
| Peak Area (%) | RT | Peak Area (%) | RT | Peak Area (%) | RT | |
| á-elemene | 0.40 | 24.79 | - | - | - | - |
| Dihydro-à-ionone | 0.64 | 25.84 | - | - | - | - |
| à-Ionone | 1.56 | 26.17 | - | - | - | - |
| Patchoulene | 0.41 | 26.92 | - | - | - | - |
| á-Maaliene | 0.69 | 27.36 | - | - | - | - |
| á-Guaiene | 0.40 | 27.70 | - | - | - | - |
| (-)-à-Selinene | 0.15 | 28.02 | - | - | - | - |
| (-)-Spathulenol | 0.09 | 28.13 | - | - | - | - |
| Cedran-diol, 8S,13 | 0.33 | 28.60 | - | - | - | - |
| á-Maaliene | 0.22 | 28.92 | - | - | - | - |
| á-Guaiene | 0.4 | 29.07 | - | - | - | - |
| Elemol | 0.23 | 29.56 | - | - | - | - |
| Methyl2-hydroxy-octadeca-9,12,15-trienoate | 0.11 | 29.66 | - | - | - | - |
| á-ylangene | 0.71 | 30.50 | - | - | - | - |
| gama-eudesmol | 0.08 | 31.69 | - | - | - | - |
| 9,12,15-Octadecatrien-1-ol,(Z, Z, Z) | 18.61 | 32.43 | - | - | - | - |
| ç-Gurjunenepoxide-(2) | 2.06 | 32.79 | - | - | - | - |
| ç-Gurjunenepoxide-(2) | 0.71 | 32.98 | - | - | - | |
| ç-Gurjunenepoxide-(2) | 0.56 | 33.53 | - | - | - | |
| 12-Oxatetracyclo[4.3.1.1(2,5).1 (4,10)]dodecane,11-isopropylidene- | 0.49 | 34.17 | - | - | - | - |
| Bicyclo[4.4.0]dec-2-ene-4-ol,2-methyl-9-(prop-1-en-3-ol-2-yl)- | 11.85 | 35.55 | - | - | - | - |
| Methyl 8,10-octadecadiynoate | 2.42 | 35.76 | - | - | - | - |
| Begonanline | 2.89 | 36.82 | - | - | - | - |
| ISO-VELLERAL | 0.09 | 37.25 | - | - | - | - |
| Hexadecanoic acid, methylester (CAS) | 8.25 | 38.31 | - | - | - | - |
| Testosterone | 0.29 | 38.73 | - | - | - | - |
| Eremanthin | 14.71 | 39.57 | - | - | ||
| ISO-VELLERAL | 1.40 | 40.78 | - | - | 0.27 | 37.71 |
| Methyl4,7,10,13,16-docosapentaenoate | 3.10 | 40.89 | - | - | - | |
| Furoscrobiculin B | 1.33 | 41.04 | - | - | - | - |
| Eremanthin | 0.24 | 42.95 | - | - | - | - |
| Eremanthin | 0.36 | 43.02 | - | - | - | - |
| Eremanthin | 0.19 | 43.23 | - | - | - | - |
| Chiapin B | 1.09 | 43.35 | - | - | - | - |
| Azuleno[4,5-b]furan-2(3H)-one, | 1.18 | 43.49 | - | - | - | - |
| Furoscrobiculin B | 0.09 | 43.56 | - | - | - | - |
| Carda-5,20(22)-dienolide, 3,14,19-trihydroxy-, (3á)-(CAS) | 0.18 | 44.73 | - | - | - | - |
| Cyclodecasiloxane,eicosamethyl- | 0.20 | 45.39 | - | - | - | - |
| Myricanene B | 0.21 | 47.80 | - | - | - | - |
| Di-(2-ethylhexyl)phthalate | 1.10 | 49.55 | - | - | - | - |
| Eremanthin | - | - | - | 5.41 | 39.10 | |
| Eremanthin | - | - | - | - | 33.87 | 40.49 |
| Chiapin B | - | - | ||||
| Furoscrobiculin B | - | - | - | 0.27 | 39.78 | |
| Furoscrobiculin B | - | - | - | - | 0.05 | 39.85 |
| Furoscrobiculin B | - | - | - | - | 0.10 | 39.92 |
| Furoscrobiculin B | - | - | - | - | 0.04 | 39.98 |
| 1,3-Bis(4-chlorobenzyl)-5,6-dihydrobenzo[f]quinazoline | - | 6.23 | 22.09 | - | ||
| Cycloheptasiloxane | - | 10.33 | 26.64 | - | ||
| Cyclooctasiloxane | - | 11.81 | 30.73 | - | ||
| Methylstearidonate | - | 10.01 | 32.01 | 4.04 | 32.04 | |
| Cyclononasiloxane | - | 8.74 | 34.25 | - | ||
| Cyclodecasiloxane | - | 5.75 | 37.38 | - | ||
| Cholic acid | - | 15.14 | 40.33 | - | ||
| Pleiocarpamine | - | - | 0.02 | |||
| Gitoxigenin | - | - | 0.05 | 32.29 | ||
| Effusanin B | - | - | 0.08 | 23.39 | ||
| 1H-Imidazole,1-[(4-methylphenyl)sulfonyl]- | - | - | 0.38 | 32.55 | ||
| Xanthumin | - | - | 6.87 | 35.13 | ||
| QUERCETIN7,3′,4′-TRIMETHOXY | - | - | 3.36 | 36.49 | ||
| Card-20(22)-enolide | - | - | 0.87 | 37.56 | ||
| Prednisone | - | - | 0.01 | 41.90 | ||
| Lucenin 2 | 0.26 | 55.35 | 3.79 | 39.12 | 0.65 | 42.81 |
| 25-Norisopropyl-9,19-cyclolanostan-22-en-24-one, | - | - | 0.09 | 43.01 | ||
| Propanedioic acid | - | 0.11 | 46.35 | |||
| 1,4-Pentadien-3-one,1,5-diphenyl- | - | - | 0.01 | 48.71 | ||
| Tested Organisms | Inhibitory Activity against the Tested Organism (Zone of Inhibition in mm) | |||
|---|---|---|---|---|
| 10 MPa | 20 MPa | 48 MPa | St. | |
| Gram-positive bacteria | Gentamycin | |||
| S. Saureus (MRSA)—ATCC43300 | 52 ± 0.25 | 41 ± 0.42 | 40 ± 0.74 | 15 ± 0.60 |
| B. subtilis ATCC6633 | 50 ± 0.58 | 46 ± 0.79 | 45 ± 0.96 | 26 ± 0.52 |
| Gram-negative bacteria | Gentamicin | |||
| E. coli ATCC25922 | 40 ± 0.64 | 35 ± 85 | 32 ± 0.75 | 23 ± 0.95 |
| P. aeruginosa (ATCC27853) | 42 ± 0.85 | 30 ± 0.75 | 28 ± 0.56 | 12 ± 0.64 |
| K. pneumonia RCMB005 001 “2” | 50 ± 0.98 | 50 ± 68 | 49 ± 0.79 | 16 ± 0.53 |
| Fungi | Ketoconazole | |||
| C. albicans ATCC 10231 | 18 ± 0.83 | 17 ± 0.59 | 17 ± 0.88 | 20 ± 0.26 |
| C. tropicalis RCMB005 004 | 15 ± 0.34 | 12 ± 0.76 | 12 ± 0.96 | 18 ± 0.49 |
| F. oxysporium RCMB008 001 “2” | NA | NA | NA | 26 ± 0.55 |
| A. flavus RCMB 002002 | NA | NA | NA | 16 ± 0.36 |
| NA | No activity | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.Y.; Kareem, S.M.; Atef, A.; Safwat, N.A.; Shehata, R.M.; Yosri, M.; Youssef, M.; Baakdah, M.M.; Sami, R.; Baty, R.S.; et al. Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities. Antioxidants 2022, 11, 1960. https://doi.org/10.3390/antiox11101960
Ahmed HY, Kareem SM, Atef A, Safwat NA, Shehata RM, Yosri M, Youssef M, Baakdah MM, Sami R, Baty RS, et al. Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities. Antioxidants. 2022; 11(10):1960. https://doi.org/10.3390/antiox11101960
Chicago/Turabian StyleAhmed, Hanaa Y., Sayed M. Kareem, Ahmed Atef, Nesreen A. Safwat, Reda M. Shehata, Mohammed Yosri, Mahmoud Youssef, Morooj M. Baakdah, Rokayya Sami, Roua S. Baty, and et al. 2022. "Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities" Antioxidants 11, no. 10: 1960. https://doi.org/10.3390/antiox11101960
APA StyleAhmed, H. Y., Kareem, S. M., Atef, A., Safwat, N. A., Shehata, R. M., Yosri, M., Youssef, M., Baakdah, M. M., Sami, R., Baty, R. S., Alsubhi, N. H., Alrefaei, G. I., Shati, A. A., & Elsaid, F. G. (2022). Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities. Antioxidants, 11(10), 1960. https://doi.org/10.3390/antiox11101960









