Continuous Glucose Monitoring in Preterm Infants: The Role of Nutritional Management in Minimizing Glycemic Variability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Continuous Glucose Measurement (CGM)
2.3. Outcome Measurements
2.4. Statistical Analysis
3. Results
3.1. Neonatal Characteristics
3.2. Mean Amplitude Glycemic Excursions
3.3. Association between Nutrition Strategy and Blood Glucose Alterations
3.4. Association between Weight for GA and Blood Glucose Alterations Regardless of the Nutrition Strategy
3.5. Association between Weight for GAs and Blood Glucose Alterations in Patient in Intermittent Feeding
3.6. Association between Weight for GAs and Blood Glucose Alterations in Patient in Continuous Feeding
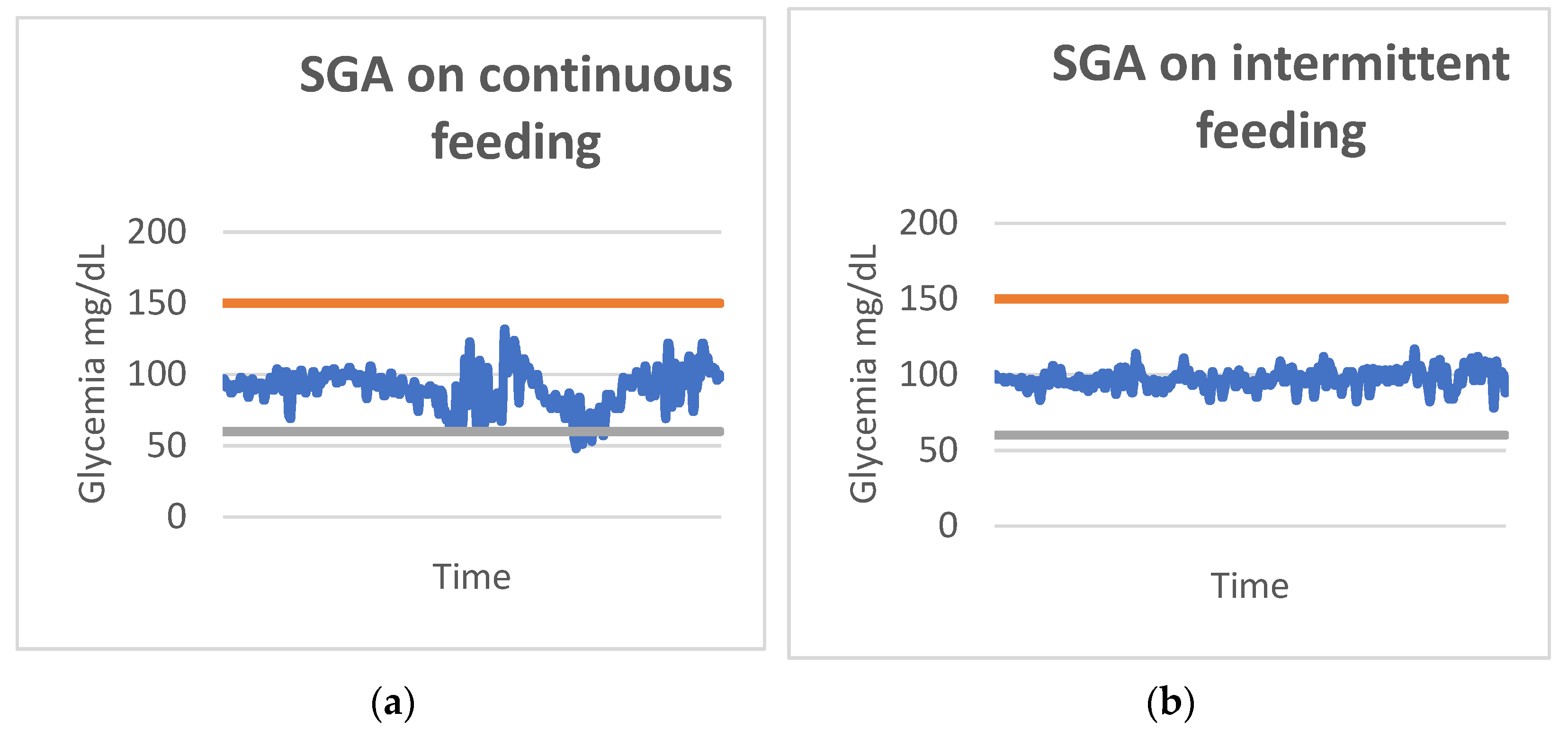
3.7. Glycemic Variability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalhan, S.; Peter-Wohl, S. Hypoglycemia: What is it for the neonate? Am. J. Perinatol. 2000, 17, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Puchalski, M.L.; Russell, T.L.; Karlsen, K.A. Neonatal hypoglycemia: Is there a sweet spot? Crit. Care Nurs. Clin. N. Am. 2018, 30, 467–480. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, C.J.D.; Alsweiler, J.M.; Anstice, N.S.; Burakevych, N.; Chakraborty, A.; Chase, J.G.; Gamble, G.D.; Harris, D.; Jacobs, R.J.; Jiang, Y.; et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 2017, 171, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Devaskar, S.U. Glucose metabolism in the late preterm infant. Clin. Perinatol. 2006, 33, 853–870. [Google Scholar] [CrossRef]
- De Angelis, L.C.; Brigati, G.; Polleri, G.; Malova, M.; Parodi, A.; Minghetti, D.; Rossi, A.; Massirio, P.; Traggiai, C.; Maghnie, M.; et al. Neonatal hypoglycemia and brain vulnerability. Front. Endocrinol. 2021, 12, 634305. [Google Scholar] [CrossRef]
- Rozance, P.J.; Hay, W.W. Hypoglycemia in newborn infants: Features associated with adverse outcomes. Biol. Neonate. 2006, 90, 74–86. [Google Scholar] [CrossRef]
- Thornton, P.S.; Stanley, C.A.; De Leon, D.D.; Harris, D.; Haymond, M.W.; Hussain, K.; Levitsky, L.L.; Murad, M.H.; Rozance, P.J.; Simmons, R.A.; et al. Recommendations from the pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J. Pediatr. 2015, 167, 238–245. [Google Scholar] [CrossRef]
- Hemachandra, A.H.; Cowett, R.M. Neonatal hyperglycemia. Pediatr. Rev. 1999, 20, e16–e24. [Google Scholar] [CrossRef]
- Hays, S.P.; Smith, E.O.; Sunehag, A.L. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics 2006, 118, 1811–1818. [Google Scholar] [CrossRef]
- Beardsall, K. Hyperglycaemia in the newborn infant. Physiology verses pathology. Front. Pediatr. 2021, 9, 641306. [Google Scholar] [CrossRef]
- Blanco, C.L.; McGill-Vargas, L.L.; McCurnin, D.; Quinn, A.R. Hyperglycemia increases the risk of death in extremely preterm baboons. Pediatr. Res. 2013, 73, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez-Mokhtari, D.; Lahlou, N.; Kieffer, F.; Magny, J.F.; Roger, M.; Voyer, M. Both relative insulin resistance and defective islet beta-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics 2004, 113, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W. Strategies for feeding the preterm infant. Neonatology 2008, 94, 245–254. [Google Scholar] [CrossRef]
- Mesotten, D.; Joosten, K.; van Kempen, A.; Verbruggen, S. ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Carbohydrates. Clin. Nutr. 2018, 37, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Owens, D.R. Glycemic variability: The third component of the dysglycemia in diabetes. Is it important? How to measure it? J. Diabetes Sci. Technol. 2008, 2, 1094–1100. [Google Scholar] [CrossRef]
- Hermanides, J.; Vriesendorp, T.M.; Bosman, R.J.; Zandstra, D.F.; Hoekstra, J.B.; Devries, J.H. Glucose variability is associated with intensive care unit mortality. Crit. Care Med. 2010, 38, 838–842. [Google Scholar] [CrossRef]
- Fendler, W.; Walenciak, J.; Mlynarski, W.; Piotrowski, A. Higher glycemic variability in very low birth weight newborns is associated with greater early neonatal mortality. J. Matern. Fetal Neonatal. Med. 2012, 25, 1122–1126. [Google Scholar] [CrossRef]
- Wu, N.; Shen, H.; Liu, H.; Wang, Y.; Bai, Y.; Han, P. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc. Diabetol. 2016, 15, 109. [Google Scholar] [CrossRef]
- Galderisi, A.; Facchinetti, A.; Steil, G.M.; Ortiz-Rubio, P.; Cavallin, F.; Tamborlane, W.V.; Baraldi, E.; Cobelli, C.; Trevisanuto, D. Continuous glucose monitoring in very preterm infants: A randomized controlled trial. Pediatrics 2017, 140, e20171162. [Google Scholar] [CrossRef]
- Thomson, L.; Elleri, D.; Bond, S.; Howlett, J.; Dunger, D.B.; Beardsall, K. Targeting glucose control in preterm infants: Pilot studies of continuous glucose monitoring. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F353–F359. [Google Scholar] [CrossRef]
- Galderisi, A.; Facchinetti, A.; Steil, G.M.; Ortiz-Rubio, P.; Cavallin, F.; Tamborlane, W.V.; Baraldi, E.; Cobelli, C.; Trevisanuto, D. Procedural pain during insertion of a continuous glucose monitoring device in preterm infants. J. Pediatr. 2018, 200, 261–264.e1. [Google Scholar] [CrossRef]
- Boscarino, G.; Di Chiara, M.; Cellitti, R.; De Nardo, M.C.; Conti, M.G.; Parisi, P.; Spalice, A.; Di Mario, C.; Ronchi, B.; Russo, A.; et al. Effects of early energy intake on neonatal cerebral growth of preterm newborn: An observational study. Sci. Rep. 2021, 11, 18457. [Google Scholar] [CrossRef] [PubMed]
- Mangili, G.; Garzoli, E. Feeding of preterm infants and fortification of breast milk. Pediatr. Med. Chir. 2017, 39, 158. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Rosenthal, M.D.; Heyland, D.K. Intermittent versus continuous feeding in critically ill adults. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 116–120. [Google Scholar] [CrossRef]
- Premji, S.S.; Chessell, L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst Rev. 2011, 2011, CD001819. [Google Scholar] [CrossRef] [PubMed]
- Rövekamp-Abels, L.W.W.; Hogewind-Schoonenboom, J.E.; de Wijs-Meijler, D.P.M.; Maduro, M.D.; Jansen-van der Weide, M.C.; van Goudoever, J.B.; Hulst, J.M. Intermittent bolus or semicontinuous feeding for preterm infants? J. Pediatr. Gastroenterol. Nutr. 2015, 61, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Dsilna, A.; Christensson, K.; Alfredsson, L.; Lagercrantz, H.; Blennow, M. Continuous feeding promotes gastrointestinal tolerance and growth in very low birth weight infants. J. Pediatr. 2005, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Bloom, S.R.; Aynsley-Green, A. Gut hormones and ‘minimal enteral feeding’. Acta Paediatr. Scand. 1986, 75, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Toce, S.S.; Keenan, W.J.; Homan, S.M. Enteral feeding in very-low-birth-weight infants. A comparison of two nasogastric methods. Am. J. Dis Child. 1987, 141, 439–444. [Google Scholar] [CrossRef]
- Beardsall, K.; Thomson, L.; Guy, C.; Iglesias-Platas, I.; van Weissenbruch, M.M.; Bond, S.; Allison, A.; Kim, S.; Petrou, S.; Pantaleo, B.; et al. Real-time continuous glucose monitoring in preterm infants (REACT): An international, open-label, randomised controlled trial. Lancet Child. Adolesc. Health 2021, 5, 265–273. [Google Scholar] [CrossRef]
- Thoene, M.; Anderson-Berry, A. Early enteral feeding in preterm infants: A narrative review of the nutritional, metabolic, and developmental benefits. Nutrients 2021, 13, 2289. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, H.; Kawai, M.; Yamashita, S.; Hata, D. Intraday glucose fluctuation is common in preterm infants receiving intermittent tube feeding. Pediatr. Int. 2016, 58, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Beardsall, K.; Vanhaesebrouck, S.; Ogilvy-Stuart, A.L.; Vanhole, C.; Vanweissenbruch, M.; Midgley, P.; Thio, M.; Cornette, L.; Ossuetta, I.; Palmer, C.R.; et al. Validation of the continuous glucose monitoring sensor in preterm infants. Arch. Dis. Child. Fetal Neonatal. Ed. 2013, 98, F136–F140. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, A.; Trevisanuto, D.; Russo, C.; Hall, R.; Bruschettini, M. Continuous glucose monitoring for the prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2021, 12, CD013309. [Google Scholar] [CrossRef] [PubMed]
- Uettwiller, F.; Chemin, A.; Bonnemaison, E.; Favrais, G.; Saliba, E.; Labarthe, F. Real-time continuous glucose monitoring reduces the duration of hypoglycemia episodes: A randomized trial in very low birth weight neonates. PLoS ONE 2015, 10, e0116255. [Google Scholar] [CrossRef]
- Alasaad, H.; Beyyumi, E.; Zoubeidi, T.; Khan, N.; Abu-Sa’da, O.; Khassawneh, M.; Souid, A.K. Impacts of Hypoglycemia in At-Risk Infants on Admissions to Level-3 Neonatal Units in a Tertiary-Care Hospital. Res. Rep. Neonatol. 2021, 11, 67–75. [Google Scholar] [CrossRef]
- Harris, D.L.; Battin, M.R.; Weston, P.J.; Harding, J.E. Continuous glucose monitoring in newborn babies at risk of hypoglycemia. J. Pediatr. 2010, 157, 198–202.e1. [Google Scholar] [CrossRef]
- Tortora, D.; Severino, M.; Di Biase, C.; Malova, M.; Parodi, A.; Minghetti, D.; Traggiai, C.; Uccella, S.; Boeri, L.; Morana, G.; et al. Early pain exposure influences functional brain connectivity in very preterm neonates. Front. Neurosci. 2019, 13, 899. [Google Scholar] [CrossRef]
- Ranger, M.; Chau, C.M.Y.; Garg, A.; Woodward, T.; Beg, M.F.; Bjornson, B.; Poskitt, K.; Fitzpatrick, K.; Synnes, A.R.; Miller, S.; et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS ONE 2013, 8, e76702. [Google Scholar] [CrossRef]
- Reddy, V.S.; Agarwal, B.; Ye, Z.; Zhang, C.; Roy, K.; Chinnappan, A.l.; Narayan, R.J.; Ramakrishna, S.; Ghosh, R. Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials 2022, 12, 1082. [Google Scholar] [CrossRef]
- Ahmed, I.; Jiang, N.; Xinge Shao, X.; Elsherif, M.; Alam, F.; Salih, A.E.; Butt, H.; Yetisen, A.K. Recent advances in optical sensors for continuous glucose monitoring. Sens. Diagn. 2022. [Google Scholar] [CrossRef]
- Lin, P.H.; Sheu, S.C.; Chen, C.W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef] [PubMed]
- Win, M.; Beckett, R.; Thomson, L.; Thankamony, A.; Beardsal, K. Continuous Glucose Monitoring in the Management of Neonates with Persistent Hypoglycemia and Congenital Hyperinsulinism. J. Clin. Endocrinol. Metab. 2022, 107, e246–e253. [Google Scholar] [CrossRef] [PubMed]
- Perea, V.; Picón, M.J.; Megia, A.; Goya, M.; Wägner, A.M.; Vega, B.; Seguí, N.; Montañez, M.D.; Vinagre, I. Addition of intermittently scanned continuous glucose monitoring to standard care in a cohort of pregnant women with type 1 diabetes: Effect on glycaemic control and pregnancy outcomes. Diabetologia 2022, 65, 1302–1314. [Google Scholar] [CrossRef]
- Shah, R.; McKinlay, C.J.D.; Harding, J.E. Neonatal hypoglycemia: Continuous glucose monitoring. Curr. Opin. Pediatr. 2018, 30, 204–208. [Google Scholar] [CrossRef]
- Jagła, M.; Szymońska, I.; Starzec, K.; Gach, O.; Włodarczyk, A.; Kwinta, P. Defining glycemic variability in very low-birthweight infants: Data from a continuous glucose monitoring system. Diabetes Technol. Ther. 2018, 20, 725–730. [Google Scholar] [CrossRef]
- Hay, W.W.; Rozance, P.J. Continuous glucose monitoring for diagnosis and treatment of neonatal hypoglycemia. J. Pediatr. 2010, 157, 180–182. [Google Scholar] [CrossRef]
- Cornblath, M.; Ichord, R. Hypoglycemia in the neonate. Semin. Perinatol. 2000, 24, 136–149. [Google Scholar] [CrossRef]
- Bouyssi-Kobar, M.; du Plessis, A.J.; McCarter, R.; Brossard-Racine, M.; Murnick, J.; Tinkleman, L.; Robertson, R.L.; Limperopoulos, C. Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics. 2016, 138, e20161640. [Google Scholar] [CrossRef]
- Sannia, A.; Natalizia, A.R.; Parodi, A.; Malova, M.; Fumagalli, M.; Rossi, A.; Ramenghi, L.A. Different gestational ages and changing vulnerability of the premature brain. J. Matern. Fetal Neonatal. Med. 2015, 28, 2268–2272. [Google Scholar] [CrossRef]
- Alexandrou, G.; Skiöld, B.; Karlén, J.; Tessma, M.K.; Norman, M.; Adén, U.; Vanpée, M. Early hyperglycemia is a risk factor for death and white matter reduction in preterm infants. Pediatrics 2010, 125, e584–e591. [Google Scholar] [CrossRef] [PubMed]
- Tottman, A.C.; Alsweiler, J.M.; Bloomfield, F.H.; Pan, M.; Harding, J.E. Relationship between measures of neonatal glycemia, neonatal illness, and 2-year outcomes in very preterm infants. J. Pediatr. 2017, 188, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Beardsall, K. Real time continuous glucose monitoring in neonatal intensive care. Early Hum. Dev. 2019, 138, 104844. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef]
- Panfoli, I.; Candiano, G.; Malova, M.; De Angelis, L.; Cardiello, V.; Buonocore, G.; Ramenghi, L.A. Oxidative stress as a primary risk factor for brain damage in preterm newborns. Front. Pediatr. 2018, 6, 369. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative stress in preterm newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Satya Krishna, S.V.; Kota, S.K.; Modi, K.D. Glycemic variability: Clinical implications. Indian J. Endocrinol. Metab. 2013, 17, 611–619. [Google Scholar] [CrossRef]
- Colella, M.; Panfoli, I.; Doglio, M.; Cassanello, M.; Bruschi, M.; De Angelis, L.C.; Candiano, G.; Parodi, A.; Malova, M.; Petretto, A.; et al. Adenosine blood level: A biomarker of white matter damage in very low birth weight infants. Curr. Pediatr. Rev. 2022, 18, 153–163. [Google Scholar] [CrossRef]







| Infant Characteristics | Continuous Nutrition (n = 10) | Intermittent Nutrition (n = 10) | p-Value |
|---|---|---|---|
| AGA | 7 | 7 | NS |
| SGA | 1 | 1 | NS |
| LGA | 2 | 2 | NS |
| GA (weeks) at FEF | 33.5 ± 1.49 (32–36.4) | 34.67 ± 0.95 (33.56–35.7) | 0.98 |
| Weight at FEF | 1.59 ± 0.3 (1.23–2.17) | 1.79 ± 0.34 (1.25–2.54) | 0.17 |
| Two-Sample t Test with Equal Variances | |||||
|---|---|---|---|---|---|
| Type of Nutrition | N° | Mean (mg/dL) | Std. Err. (mg/dL) | Std. Dev. (mg/dL) | [95% Conf. Interval] |
| Intermittent | 4 | 11.44 | 2.20 | 4.41 | 4.42–18.45 |
| Continuous | 6 | 25.25 | 7.39 | 18.09 | 6.26–44.23 |
| Glycemia | Intermittent | Continuous | Total | |
|---|---|---|---|---|
| Normal | Obs | 12,741 | 14,002 | 26,743 |
| % | 98.4 | 96.01 | 97.13 | |
| Hypoglycemia | Obs | 207 | 566 | 773 |
| % | 1.6 | 3.88 | 2.81 | |
| Hyperglycemia | Obs | 0 | 16 | 16 |
| % | 0 | 0.11 | 0.06 | |
| Total | Obs | 12,948 | 14,584 | 27,532 |
| % | 100 | 100 | 100 | |
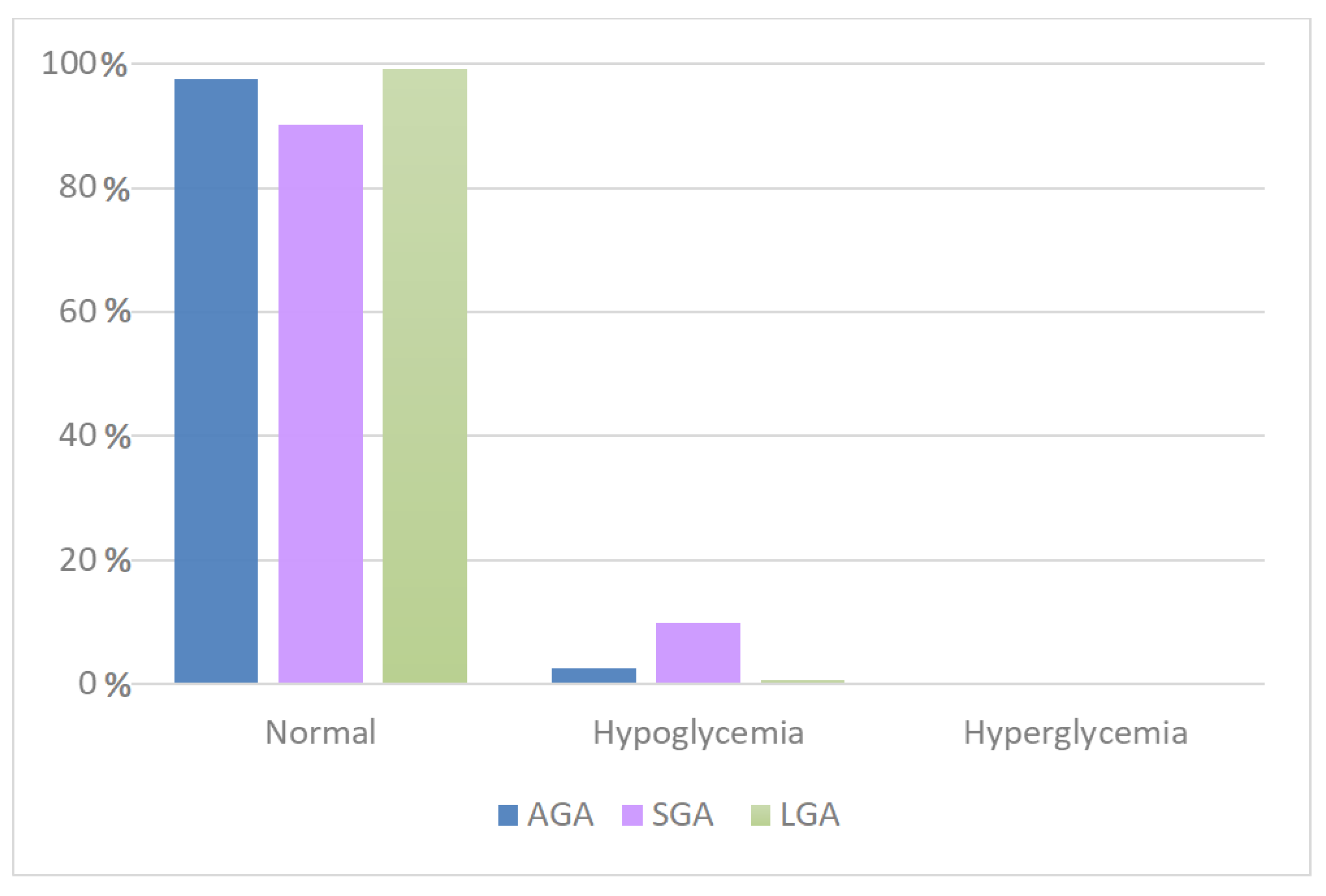
| Glycemia | AGA | SGA | LGA | Total | |
|---|---|---|---|---|---|
| Normal | Obs | 18,265 | 2322 | 6156 | 26,743 |
| % | 97.42 | 90.17 | 99.16 | 97.13 | |
| Hypoglycemia | Obs | 476 | 253 | 44 | 773 |
| % | 2.54 | 9.83 | 0.71 | 2.81 | |
| Hyperglycemia | Obs | 8 | 0 | 8 | 16 |
| % | 0.04 | 0 | 0.13 | 0.06 | |
| Total | Obs | 18,749 | 2575 | 6208 | 27,532 |
| % | 100 | 100 | 100 | 100 | |
| Glycemia | AGA | SGA | LGA | Total | |
|---|---|---|---|---|---|
| Normal | Obs | 7805 | 1524 | 3412 | 12,741 |
| % | 97.88 | 98.32 | 99.65 | 98.40 | |
| Hypoglycemia | Obs | 169 | 26 | 12 | 207 |
| % | 2.12 | 1.68 | 0.35 | 1.60 | |
| Total | Obs | 7974 | 1550 | 3424 | 12,948 |
| % | 100 | 100 | 100 | 100 | |
| Glycemia | AGA | LGA | SGA | Total | |
|---|---|---|---|---|---|
| Normal | Obs | 10,460 | 2744 | 798 | 14,002 |
| % | 97.08 | 98.56 | 77.85 | 96.01 | |
| Hypoglycemia | Obs | 307 | 32 | 227 | 566 |
| % | 2.85 | 1.15 | 22.15 | 3.88 | |
| Hyperglycemia | Obs | 8 | 8 | 0 | 16 |
| % | 0.07 | 0.29 | 0 | 0.11 | |
| Total | Obs | 18,749 | 2575 | 1025 | 14,584 |
| % | 100 | 100 | 100 | 100 | |
| Variance Ratio Test | |||||
|---|---|---|---|---|---|
| Weight for GA | Obs | Mean (mg/dL) | Std. Err (mg/dL) | St. Dev. (mg/dL) | [95% Confidence Interval] |
| AGA | 18,749 | 84.46 | 0.095 | 13.02 | 84.27–84.65 |
| LGA | 6208 | 89.33 | 0.17 | 13.06 | 89.00–89.65 p-value 0.7262 |
| SGA | 2575 | 78.61 | 0.31 | 15.78 | 77.45–78.67 p-value 0.001 |
| Variance Ratio Test | |||||
|---|---|---|---|---|---|
| Type of Nutrition | Obs | Mean (mg/dL) | Std. Err (mg/dL) | St. Dev. (mg/dL) | [95% Confidence Interval] |
| Intermittent | 12,948 | 85.76 | 0.11 | 12.65 | 85.54–85.98 |
| Continuous | 14,584 | 84.25 | 0.12 | 14.43 | 84.01–84.48 p-value 0.001 |
| Variance Ratio Test | |||||
|---|---|---|---|---|---|
| Type of Nutrition | Obs | Mean (mg/dL) | Std. Err (mg/dL) | St. Dev. (mg/dL) | [95% Confidence Interval] |
| Intermittent | 1025 | 68.52 | 0.45 | 14.31 | 67.97–69.73 |
| Continuous | 1550 | 84.15 | 0.35 | 13.62 | 83.47–84.83 p-value 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musso, V.; Panfoli, I.; Battaglini, M.; Brigati, G.; Minghetti, D.; Andreato, C.; Ramenghi, L.A. Continuous Glucose Monitoring in Preterm Infants: The Role of Nutritional Management in Minimizing Glycemic Variability. Antioxidants 2022, 11, 1945. https://doi.org/10.3390/antiox11101945
Musso V, Panfoli I, Battaglini M, Brigati G, Minghetti D, Andreato C, Ramenghi LA. Continuous Glucose Monitoring in Preterm Infants: The Role of Nutritional Management in Minimizing Glycemic Variability. Antioxidants. 2022; 11(10):1945. https://doi.org/10.3390/antiox11101945
Chicago/Turabian StyleMusso, Valeria, Isabella Panfoli, Marcella Battaglini, Giorgia Brigati, Diego Minghetti, Chiara Andreato, and Luca A. Ramenghi. 2022. "Continuous Glucose Monitoring in Preterm Infants: The Role of Nutritional Management in Minimizing Glycemic Variability" Antioxidants 11, no. 10: 1945. https://doi.org/10.3390/antiox11101945
APA StyleMusso, V., Panfoli, I., Battaglini, M., Brigati, G., Minghetti, D., Andreato, C., & Ramenghi, L. A. (2022). Continuous Glucose Monitoring in Preterm Infants: The Role of Nutritional Management in Minimizing Glycemic Variability. Antioxidants, 11(10), 1945. https://doi.org/10.3390/antiox11101945







