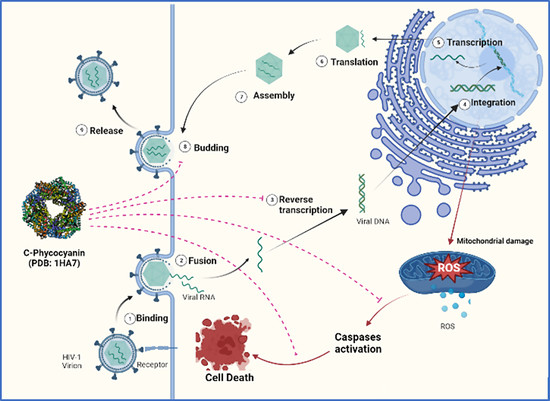
Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through In Silico and In Vitro Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Phycobiliprotein C-Phycocyanin
2.2. Protein Structure Retrieval and Preparation for Docking Simulations
2.3. Cell Lines and HIV-1 Stock
2.4. Cytotoxicity Assay by MTT
2.5. Cell Associated Anti-HIV-1 Assay
2.6. HIV-1 p24 Antigen Capture Assay
2.7. HIV-1 Reverse Transcriptase Activity Assay
2.8. HIV-1 Protease Assay
2.9. Detection of Intracellular Reactive Oxygen Species (ROS)
2.10. Confocal Microscopy
2.11. Screening of Caspase Activity
2.12. Assessment of Cell Death through FACS
3. Results
3.1. Cytotoxicity of C-Phycocyanin
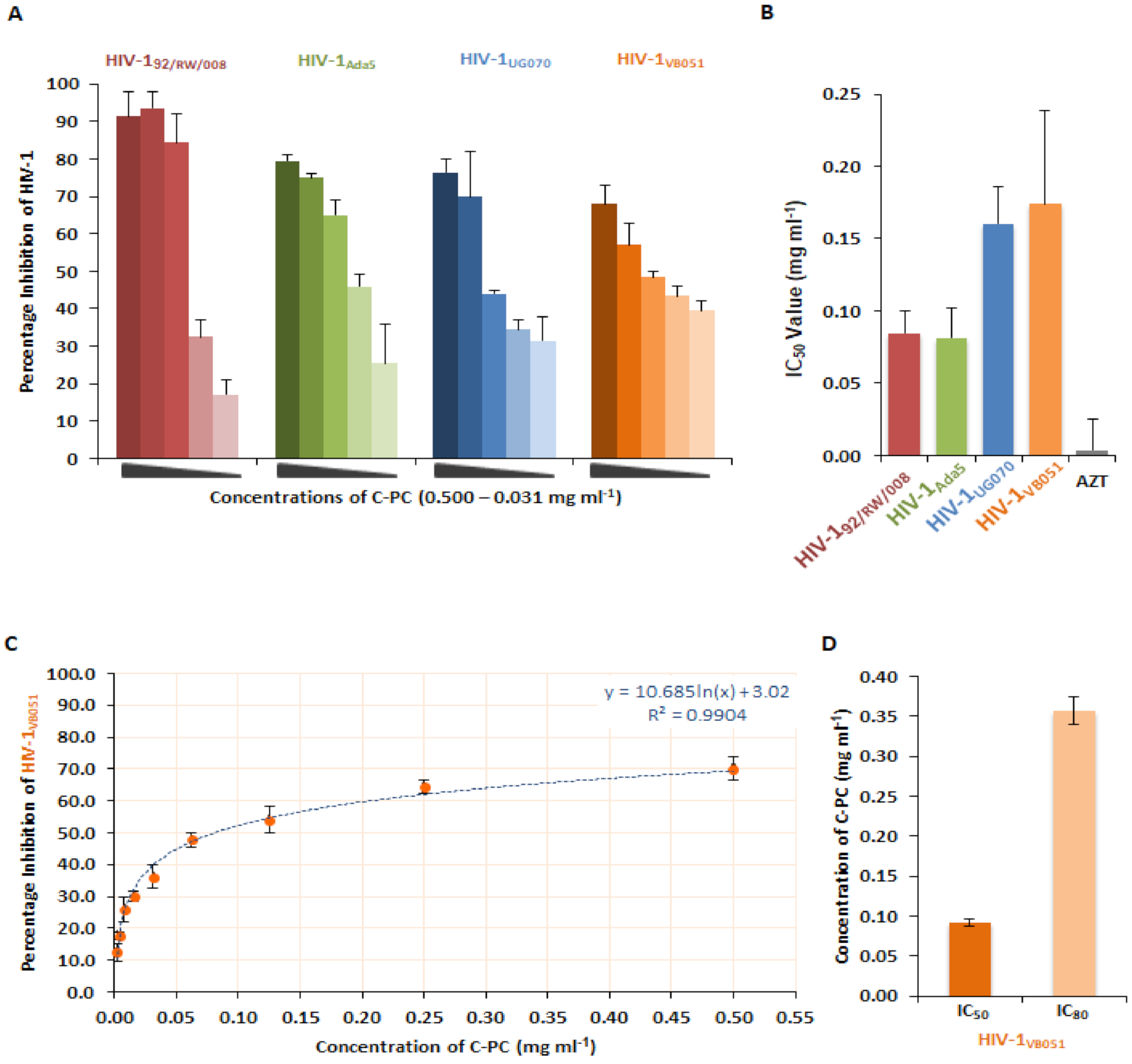
3.2. Anti-Viral Activity of C-Phycocyanin against HIV-1
3.3. Interactions of C-PC and HIV-1 Reverse Transcriptase
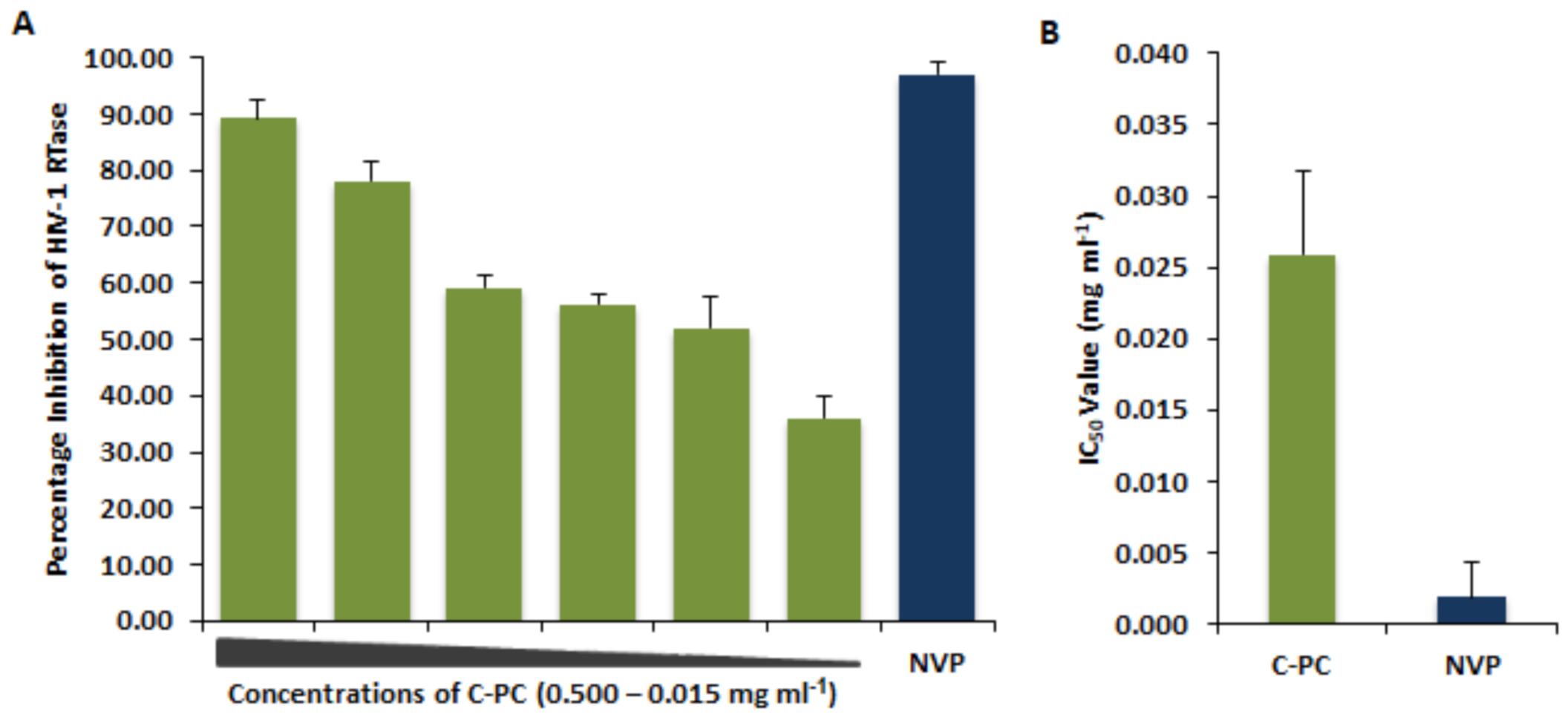
3.4. Inhibitory Effect of C-Phycocyanin on HIV-1 Reverse Transcriptase
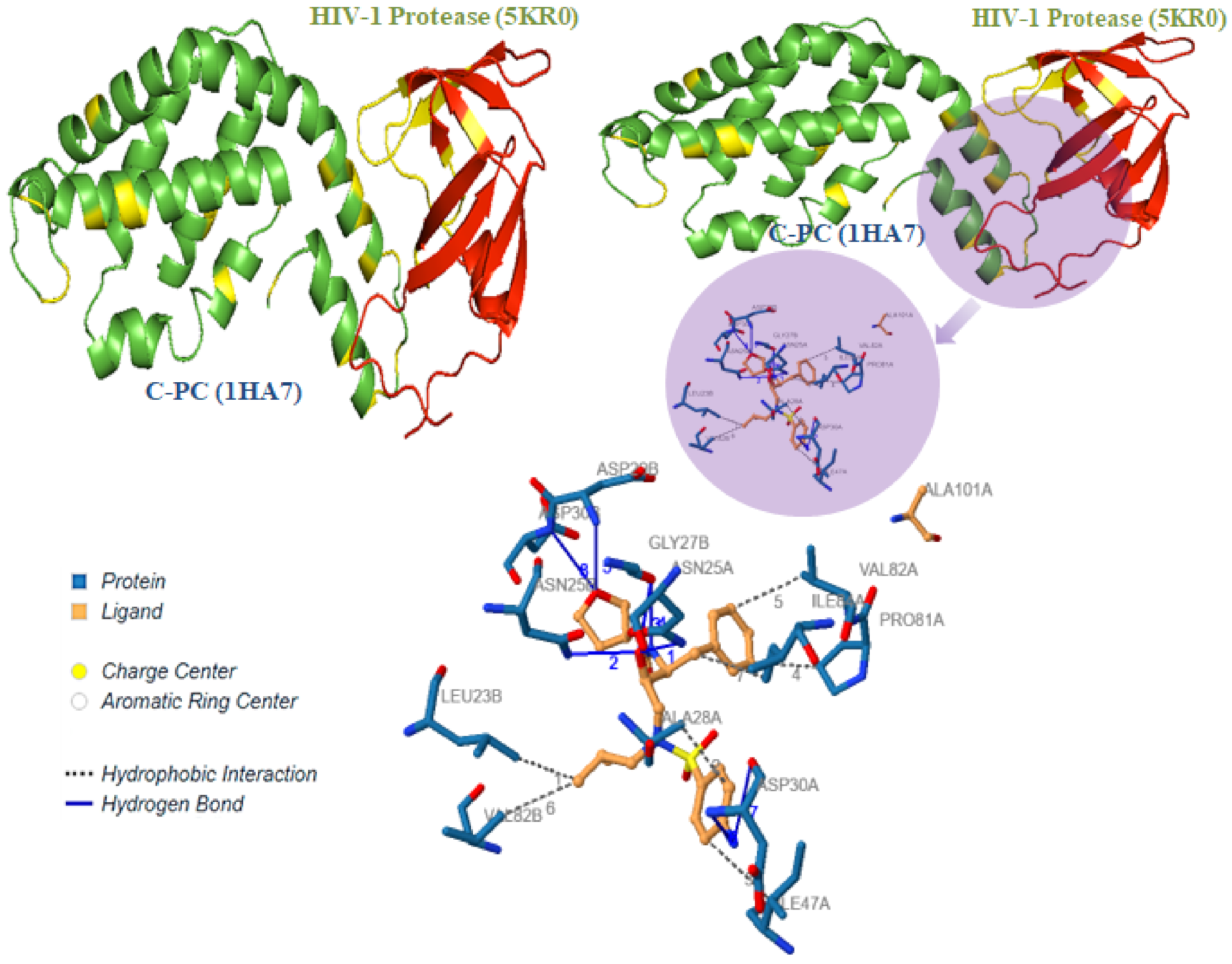
3.5. C-Phycocyanin Suppress the HIV-1 Protease Activity
3.6. In Silico Interactions of C-PC with Other Crucial HIV-1 Proteins and Viral Co-Receptor
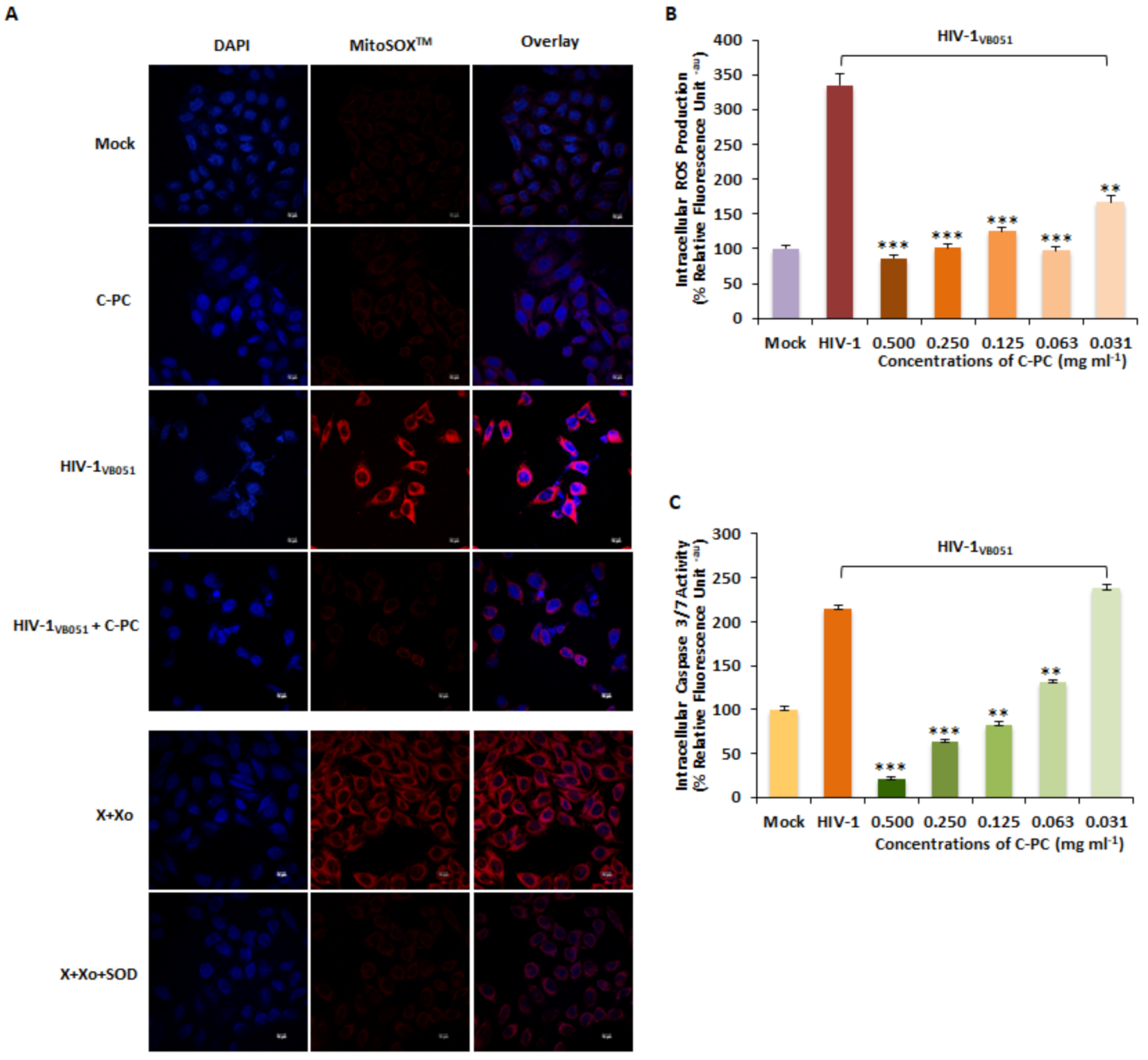
3.7. ROS Scavenging Activity of C-Phycocyanin in HIV-1 Infected Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, R.; Barré-Sinoussi, F. Controversies in HIV Cure Research. J. Int. AIDS Soc. 2012, 15, 16. [Google Scholar] [CrossRef]
- Stadeli, K.M.; Richman, D.D. Rates of Emergence of HIV Drug Resistance in Resource-Limited Settings: A Systematic Review. Antivir. Ther. 2013, 18, 115–123. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Arteni, A.A.; Ajlani, G.; Boekema, E.J. Structural Organisation of Phycobilisomes from Synechocystis Sp. Strain PCC6803 and Their Interaction with the Membrane. Biochim. Biophys. Acta BBA—Bioenerg. 2009, 1787, 272–279. [Google Scholar] [CrossRef]
- Safaei, M.; Maleki, H.; Soleimanpour, H.; Norouzy, A.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Development of a Novel Method for the Purification of C-Phycocyanin Pigment from a Local Cyanobacterial Strain Limnothrix Sp. NS01 and Evaluation of Its Anticancer Properties. Sci. Rep. 2019, 9, 9474. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina Platensis Derived C-Phycocyanin. Evid. Based Complement. Alternat. Med. 2016, 2016, 7803846. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina: Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Marles, R.J.; Barrett, M.L.; Barnes, J.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Dog, T.L.; Sarma, N.D.; Giancaspro, G.I.; et al. United States Pharmacopeia Safety Evaluation of Spirulina. Crit. Rev. Food Sci. Nutr. 2011, 51, 593–604. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Anish Zacharia, J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.; Bisen, P. Spirulina in Health Care Management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef]
- Bannu, S.M.; Lomada, D.; Gulla, S.; Chandrasekhar, T.; Reddanna, P.; Reddy, M.C. Potential Therapeutic Applications of C-Phycocyanin. Curr. Drug Metab. 2020, 20, 967–976. [Google Scholar] [CrossRef]
- Vadiraja, B.B.; Gaikwad, N.W.; Madyastha, K.M. Hepatoprotective Effect of C-Phycocyanin: Protection for Carbon Tetrachloride AndR-(+)-Pulegone-Mediated Hepatotoxicty in Rats. Biochem. Biophys. Res. Commun. 1998, 249, 428–431. [Google Scholar] [CrossRef]
- AlQranei, M.S.; Aljohani, H.; Majumdar, S.; Senbanjo, L.T.; Chellaiah, M.A. C-Phycocyanin Attenuates RANKL-Induced Osteoclastogenesis and Bone Resorption in Vitro through Inhibiting ROS Levels, NFATc1 and NF-ΚB Activation. Sci. Rep. 2020, 10, 2513. [Google Scholar] [CrossRef]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in Clinical Practice: Evidence-Based Human Applications. Evid. Based Complement. Alternat. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Reddy, M.C.; Subhashini, J.; Mahipal, S.V.K.; Bhat, V.B.; Srinivas Reddy, P.; Kiranmai, G.; Madyastha, K.M.; Reddanna, P. C-Phycocyanin, a Selective Cyclooxygenase-2 Inhibitor, Induces Apoptosis in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 385–392. [Google Scholar] [CrossRef]
- Engelman, A.; Cherepanov, P. The Structural Biology of HIV-1: Mechanistic and Therapeutic Insights. Nat. Rev. Microbiol. 2012, 10, 279–290. [Google Scholar] [CrossRef]
- Montessori, V.; Press, N.; Harris, M.; Akagi, L.; Montaner, J.S.G. Adverse Effects of Antiretroviral Therapy for HIV Infection. CMAJ Can. Med. Assoc. J. J. Assoc. Medicale Can. 2004, 170, 229–238. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An Environment for Comparative Protein Modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 25 May 2022).
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Recio, J.; Totrov, M.; Abagyan, R. Identification of Protein-Protein Interaction Sites from Docking Energy Landscapes. J. Mol. Biol. 2004, 335, 843–865. [Google Scholar] [CrossRef] [PubMed]
- Chemical Computing Group ULC Molecular Operating Environment (MOE) 2022. Available online: https://www.chemcomp.com/Products.htm (accessed on 7 June 2022).
- Mascola, J.R. Neutralization of HIV-1 Infection of Human Peripheral Blood Mononuclear Cells (PBMC): Antibody Dilution Method. In HIV Protocols; Humana Press: Totowa, NJ, USA, 1999; Volume 17, pp. 309–316. ISBN 978-0-89603-369-6. [Google Scholar]
- Kumar, S.; Gupta, S.; Gaikwad, S.; Abadi, L.F.; Bhutani, L.K.K.; Kulkarni, S.; Singh, I.P. Design, Synthesis and In Vitro Evaluation of Novel Anti-HIV 3-Pyrazol-3- Yl-Pyridin-2-One Analogs. Med. Chem. 2019, 15, 561–570. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Production and Detection of Reactive Oxygen Species (ROS) in Cancers. J. Vis. Exp. 2011, 57, 3357. [Google Scholar] [CrossRef]
- Zhang, B.; Davidson, M.M.; Zhou, H.; Wang, C.; Walker, W.F.; Hei, T.K. Cytoplasmic Irradiation Results in Mitochondrial Dysfunction and DRP1-Dependent Mitochondrial Fission. Cancer Res. 2013, 73, 6700–6710. [Google Scholar] [CrossRef]
- Jadaun, P.; Yadav, D.; Bisen, P.S. Spirulina Platensis Prevents High Glucose-Induced Oxidative Stress Mitochondrial Damage Mediated Apoptosis in Cardiomyoblasts. Cytotechnology 2018, 70, 523–536. [Google Scholar] [CrossRef]
- Das, K.; Ding, J.; Hsiou, Y.; Clark, A.D.; Moereels, H.; Koymans, L.; Andries, K.; Pauwels, R.; Janssen, P.A.; Boyer, P.L.; et al. Crystal Structures of 8-Cl and 9-Cl TIBO Complexed with Wild-Type HIV-1 RT and 8-Cl TIBO Complexed with the Tyr181Cys HIV-1 RT Drug-Resistant Mutant. J. Mol. Biol. 1996, 264, 1085–1100. [Google Scholar] [CrossRef]
- Ding, J.; Das, K.; Moereels, H.; Koymans, L.; Andries, K.; Janssen, P.A.; Hughes, S.H.; Arnold, E. Structure of HIV-1 RT/TIBO R 86183 Complex Reveals Similarity in the Binding of Diverse Nonnucleoside Inhibitors. Nat. Struct. Biol. 1995, 2, 407–415. [Google Scholar] [CrossRef]
- Seniya, C.; Yadav, A.; Khan, G.J.; Sah, N.K. In-Silico Studies Show Potent Inhibition of HIV-1 Reverse Transcriptase Activity by a Herbal Drug. IEEE/ACM Trans. Comput. Biol. Bioinform. 2015, 12, 1355–1364. [Google Scholar] [CrossRef]
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-Oxidant Activity of Holo- and Apo-c-Phycocyanin and Their Protective Effects on Human Erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; Falcón-Cama, V. C-Phycocyanin and Phycocyanobilin as Remyelination Therapies for Enhancing Recovery in Multiple Sclerosis and Ischemic Stroke: A Preclinical Perspective. Behav. Sci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Huesemann, M.; Edmundson, S.; Sims, A.; Hurst, B.; Cady, S.; Beirne, N.; Freeman, J.; Berger, A.; Gao, S. Viral Inhibitors Derived from Macroalgae, Microalgae, and Cyanobacteria: A Review of Antiviral Potential throughout Pathogenesis. Algal Res. 2021, 57, 102331. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chang, G.-K.; Kuo, S.-M.; Huang, S.-Y.; Hu, I.-C.; Lo, Y.-L.; Shih, S.-R. Well-Tolerated Spirulina Extract Inhibits Influenza Virus Replication and Reduces Virus-Induced Mortality. Sci. Rep. 2016, 6, 24253. [Google Scholar] [CrossRef]
- Shih, S.-R.; Tsai, K.-N.; Li, Y.-S.; Chueh, C.-C.; Chan, E.-C. Inhibition of Enterovirus 71-Induced Apoptosis by Allophycocyanin Isolated from a Blue-Green Algaspirulina Platensis. J. Med. Virol. 2003, 70, 119–125. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Morita, N.; Kojima, I. An Extract FromSpirulina Platensis Is a Selective Inhibitor of Herpes Simplex Virus Type 1 Penetration into HeLa Cells. Phytother. Res. 1993, 7, 76–80. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Kojima, I. A Natural Sulfated Polysaccharide, Calcium Spirulan, Isolated from Spirulina Platensis: In Vitro and Ex Vivo Evaluation of Anti-Herpes Simplex Virus and Anti-Human Immunodeficiency Virus Activities. AIDS Res. Hum. Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina Platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef]
- Sarafianos, S.G.; Marchand, B.; Das, K.; Himmel, D.M.; Parniak, M.A.; Hughes, S.H.; Arnold, E. Structure and Function of HIV-1 Reverse Transcriptase: Molecular Mechanisms of Polymerization and Inhibition. J. Mol. Biol. 2009, 385, 693–713. [Google Scholar] [CrossRef]
- Lv, Z.; Chu, Y.; Wang, Y. HIV Protease Inhibitors: A Review of Molecular Selectivity and Toxicity. HIVAIDS Auckl. NZ 2015, 7, 95–104. [Google Scholar] [CrossRef]
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Jucá, M.B.; Aoyama, H.; Leite, E.L. Inhibition of Reverse Transcriptase Activity of HIV by Polysaccharides of Brown Algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Lin, S.; Zhang, B.; Jin, H.; Ding, L. Antiviral Potential of Natural Products from Marine Microbes. Eur. J. Med. Chem. 2020, 207, 112790. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Salomon, P.S.; Hamerski, L.; Walter, J.; Menezes, R.B.; Siqueira, J.E.; Santos, A.; Santos, J.A.M.; Ferme, N.; Guimarães, T.; et al. Inhibitory Effect of Microalgae and Cyanobacteria Extracts on Influenza Virus Replication and Neuraminidase Activity. PeerJ 2018, 6, e5716. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Dash, S.R.; Ki, J.-S.; Adhikary, S.P.; Ragusa, A.; Jena, M. Cyanobacteria and Algae-Derived Bioactive Metabolites as Antiviral Agents: Evidence, Mode of Action, and Scope for Further Expansion; A Comprehensive Review in Light of the SARS-CoV-2 Outbreak. Antioxid. Basel Switz. 2022, 11, 354. [Google Scholar] [CrossRef]
- Nunez Aguilar, E. HIV-1 Protease Inhibitors From Marine Brown Alga: A Literature Review. McNair Res. J. SJSU 2017, 13, 4. [Google Scholar] [CrossRef]
- Guzmán, E. Regulated Cell Death Signaling Pathways and Marine Natural Products That Target Them. Mar. Drugs 2019, 17, 76. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-Mediated Oxidative Stress during Viral Infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Couret, J.; Chang, T.L. Reactive Oxygen Species in HIV Infection. EC Microbiol. 2016, 3, 597–604. [Google Scholar]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Ramirez, R.; Estrada-Perez, A.; Alcalde-Vazquez, R.; Muñoz, J.L. Antioxidant Effect of Phycobiliproteins on Proteins and DNA Exposed to a Reactive Oxygen Species Generating System. FASEB J. 2013, 27, 890.15. [Google Scholar] [CrossRef]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential Microalgae Derived Pharmaceutical and Biological Reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Min, S.K.; Park, J.S.; Luo, L.; Kwon, Y.S.; Lee, H.C.; Jung Shim, H.; Kim, I.-D.; Lee, J.-K.; Shin, H.S. Assessment of C-Phycocyanin Effect on Astrocytes-Mediated Neuroprotection against Oxidative Brain Injury Using 2D and 3D Astrocyte Tissue Model. Sci. Rep. 2015, 5, 14418. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.M.; Boppana, N.B.; Asokan, D.; Sekaran, S.D.; Shankar, E.M.; Li, C.; Gopal, K.; Bakar, S.A.; Karthik, H.S.; Ebrahim, A.S. C-Phycocyanin Confers Protection against Oxalate-Mediated Oxidative Stress and Mitochondrial Dysfunctions in MDCK Cells. PLoS ONE 2014, 9, e93056. [Google Scholar] [CrossRef] [PubMed]
- Young, I.-C.; Chuang, S.-T.; Hsu, C.-H.; Sun, Y.-J.; Lin, F.-H. C-Phycocyanin Alleviates Osteoarthritic Injury in Chondrocytes Stimulated with H2O2 and Compressive Stress. Int. J. Biol. Macromol. 2016, 93, 852–859. [Google Scholar] [CrossRef]
- Daussy, C.F.; Galais, M.; Pradel, B.; Robert-Hebmann, V.; Sagnier, S.; Pattingre, S.; Biard-Piechaczyk, M.; Espert, L. HIV-1 Env Induces Pexophagy and an Oxidative Stress Leading to Uninfected CD4+ T Cell Death. Autophagy 2021, 17, 2465–2474. [Google Scholar] [CrossRef]
- Safe, I.P.; Amaral, E.P.; Araújo-Pereira, M.; Lacerda, M.V.G.; Printes, V.S.; Souza, A.B.; Beraldi-Magalhães, F.; Monteiro, W.M.; Sampaio, V.S.; Barreto-Duarte, B.; et al. Adjunct N-Acetylcysteine Treatment in Hospitalized Patients with HIV-Associated Tuberculosis Dampens the Oxidative Stress in Peripheral Blood: Results From the RIPENACTB Study Trial. Front. Immunol. 2021, 11, 602589. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial Control of Apoptosis: The Role of Cytochrome c. Biochim. Biophys. Acta BBA—Bioenerg. 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssière, J.-L. Mitochondrial Reactive Oxygen Species in Cell Death Signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Cicala, C.; Arthos, J.; Rubbert, A.; Selig, S.; Wildt, K.; Cohen, O.J.; Fauci, A.S. HIV-1 Envelope Induces Activation of Caspase-3 and Cleavage of Focal Adhesion Kinase in Primary Human CD4+ T Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1178–1183. [Google Scholar] [CrossRef] [PubMed]




| Parameters | HADDOCK Score | Cluster Size | RMSD Score | Van der Waals Energy | Electrostatic Energy | Desolvation Energy | Restraints Violation Energy | Buried Surface Area | Z-Score |
|---|---|---|---|---|---|---|---|---|---|
| Cluster 9 | −58.3 ± 5.8 | 5 | 18.9 ± 0.0 | −47.6 ± 0.6 | −242.2± 16.1 | 13.8 ± 1.1 | 239.2 ± 57.1 | 1482.6 ± 25.7 | −1.9 |
| Cluster 1 | −57.6 ± 2.7 | 45 | 20.7 ± 0.0 | −50.2 ± 2.7 | −131.8 ± 8.6 | 2.3 ± 0.4 | 166.3 ± 49.8 | 1637.4 ± 32.1 | −1.8 |
| Cluster 8 | −48.8 ± 4.2 | 5 | 19.3 ± 0.2 | −35.6 ± 3.6 | −218.3 ± 13.0 | 6.7 ± 1.5 | 238.0 ± 43.1 | 1476.2 ± 25.2 | −0.3 |
| Cluster 6 | −47.2 ± 4.7 | 6 | 17.7 ± 0.1 | 31.8 ± 1.8 | −176.8 ± 13.1 | −4.2 ± 3.3 | 241.6 ± 82.2 | 1363.2 ± 197.9 | −0.0 |
| Cluster 15 | −45.2 ± 1.6 | 4 | 16.1 ± 0.1 | −24.3 ± 1.1 | −249.8 ± 5.3 | −0.2 ± 1.7 | 293.7 ±16.3 | 1438.7 ± 29.9 | 0.3 |
| Hydrophobic Bond Interactions | |||||||||
| Index | Residue | AA | Distance | Ligand Atom | Protein Atom | ||||
| 1 | 95A | PRO | 3.79 | 4907 | 585 | ||||
| 2 | 100A | LEU | 3.79 | 4907 | 625 | ||||
| 3 | 100A | LEU | 3.45 | 4909 | 624 | ||||
| 4 | 100A | LEU | 3.81 | 4920 | 624 | ||||
| 5 | 106A | VAL | 3.69 | 4917 | 693 | ||||
| 6 | 106A | VAL | 3.34 | 4916 | 692 | ||||
| 7 | 181A | TYR | 3.51 | 4910 | 1302 | ||||
| 8 | 188A | TYR | 3.30 | 4912 | 1379 | ||||
| 9 | 318A | TYR | 3.20 | 4918 | 2685 | ||||
| Hydrogen Bond Interactions | |||||||||
| Index | Residue | AA | Distance H-A | Distance D-A | Donor Angle | Protein Donor | Side Chain | Donor Atom | Acceptor Atom |
| 1 | 279A | LEU | 3.03 | 3.66 | 125.22 | ✔ | ☓ | 2306 [Nam] | 2318 [N3] |
| 2 | 281A | LYS | 3.42 | 4.01 | 118.54 | ✔ | ☓ | 2327 [N3] | 2315 [O2] |
| 3 | 281A | LYS | 2.55 | 3.40 | 143.88 | ☓ | ☓ | 2318 [N3] | 2327 [N3] |
| Parameter Cluster No. | HADDOCK Score | RMSD Score | Van der Waals Energy | Electrostatic Energy | Desolvation Energy | Restraints Violation Energy | Z-Score |
|---|---|---|---|---|---|---|---|
| Cluster 6 | −83.1 ± 12.6 | 1.3 ± 0.9 | −57.8 ± 4.8 | −138.0 ± 16.7 | −23.1 ± 6.5 | 254.1 ±44.92 | −1.6 |
| Cluster 5 | −77.5 ± 9.9 | 15.0 ± 0.2 | −30.8 ±3.6 | −301.4 ± 65.8 | −3.5 ± 2.4 | 170.9 ± 41.40 | −1.1 |
| Cluster 7 | −74.7 ± 5.4 | 6.9 ± 0.7 | −36.5 ± 5.7 | −174.9 ±34.9 | −13.9 ± 6.1 | 106.6 ± 23.59 | −0.8 |
| Cluster 2 | −72.3 ± 4.2 | 10.4 ± 0.3 | −48.7 ± 5.7 | −92.3 ± 8.7 | −18.9 ± 3.1 | 137.8 ± 24.64 | −0.6 |
| Cluster 1 | −69.8 ± 8.5 | 11.0 ± 0.5 | −50.2 ± 6.7 | −104.7 ±16.7 | −7.4 ± 1.7 | 88.1 ± 17.47 | −0.4 |
| Hydrophobic Bond Interactions | |||||||||
| Index | Residue | AA | Distance | Ligand Atom | Protein Atom | ||||
| 1 | 23B | LEU | 3.97 | 1536 | 939 | ||||
| 2 | 28A | ALA | 3.60 | 1531 | 215 | ||||
| 3 | 47A | ILE | 3.95 | 1533 | 366 | ||||
| 4 | 81A | PRO | 3.56 | 1524 | 616 | ||||
| 5 | 82A | VAL | 3.71 | 1523 | 624 | ||||
| 6 | 82B | VAL | 3.72 | 1536 | 1379 | ||||
| 7 | 84A | ILE | 3.85 | 1519 | 640 | ||||
| Hydrogen Bond Interactions | |||||||||
| Index | Residue | AA | Distance H-A | Distance D-A | Donor Angle | Protein Donor | Side Chain | Donor Atom | Acceptor Atom |
| 1 | 25A | ASN | 1.83 | 2.69 | 144.42 | ✔ | ☓ | 199 [Nam] | 1543 [O3] |
| 2 | 25B | ASN | 2.50 | 3.27 | 134.51 | ☓ | ☓ | 955 [Nam] | 1543 [O3] |
| 3 | 27B | GLY | 2.65 | 3.40 | 134.26 | ☓ | ☓ | 1543 [O3] | 966 [O2] |
| 4 | 27B | GLY | 2.17 | 3.07 | 152.14 | ✔ | ☓ | 1538 [Nam] | 966 [O2] |
| 5 | 29B | ASP | 3.27 | 3.72 | 109.53 | ✔ | ✔ | 972 [Nam] | 1546 [O3] |
| 6 | 30A | ASP | 2.63 | 3.44 | 139.54 | ✔ | ☓ | 224 [Nam] | 1540 [Npl] |
| 7 | 30A | ASP | 2.95 | 3.75 | 139.88 | ☓ | ☓ | 1540 [Npl] | 227 [O2] |
| 8 | 30B | ASP | 2.49 | 3.41 | 156.75 | ✔ | ✔ | 980 [Nam] | 1546 [O3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadaun, P.; Seniya, C.; Pal, S.K.; Kumar, S.; Kumar, P.; Nema, V.; Kulkarni, S.S.; Mukherjee, A. Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through In Silico and In Vitro Approaches. Antioxidants 2022, 11, 1942. https://doi.org/10.3390/antiox11101942
Jadaun P, Seniya C, Pal SK, Kumar S, Kumar P, Nema V, Kulkarni SS, Mukherjee A. Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through In Silico and In Vitro Approaches. Antioxidants. 2022; 11(10):1942. https://doi.org/10.3390/antiox11101942
Chicago/Turabian StyleJadaun, Pratiksha, Chandrabhan Seniya, Sudhir Kumar Pal, Sanjit Kumar, Pramod Kumar, Vijay Nema, Smita S Kulkarni, and Anupam Mukherjee. 2022. "Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through In Silico and In Vitro Approaches" Antioxidants 11, no. 10: 1942. https://doi.org/10.3390/antiox11101942
APA StyleJadaun, P., Seniya, C., Pal, S. K., Kumar, S., Kumar, P., Nema, V., Kulkarni, S. S., & Mukherjee, A. (2022). Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through In Silico and In Vitro Approaches. Antioxidants, 11(10), 1942. https://doi.org/10.3390/antiox11101942