Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review
Abstract
1. Introduction
2. Functional Relevance of Carotenoids
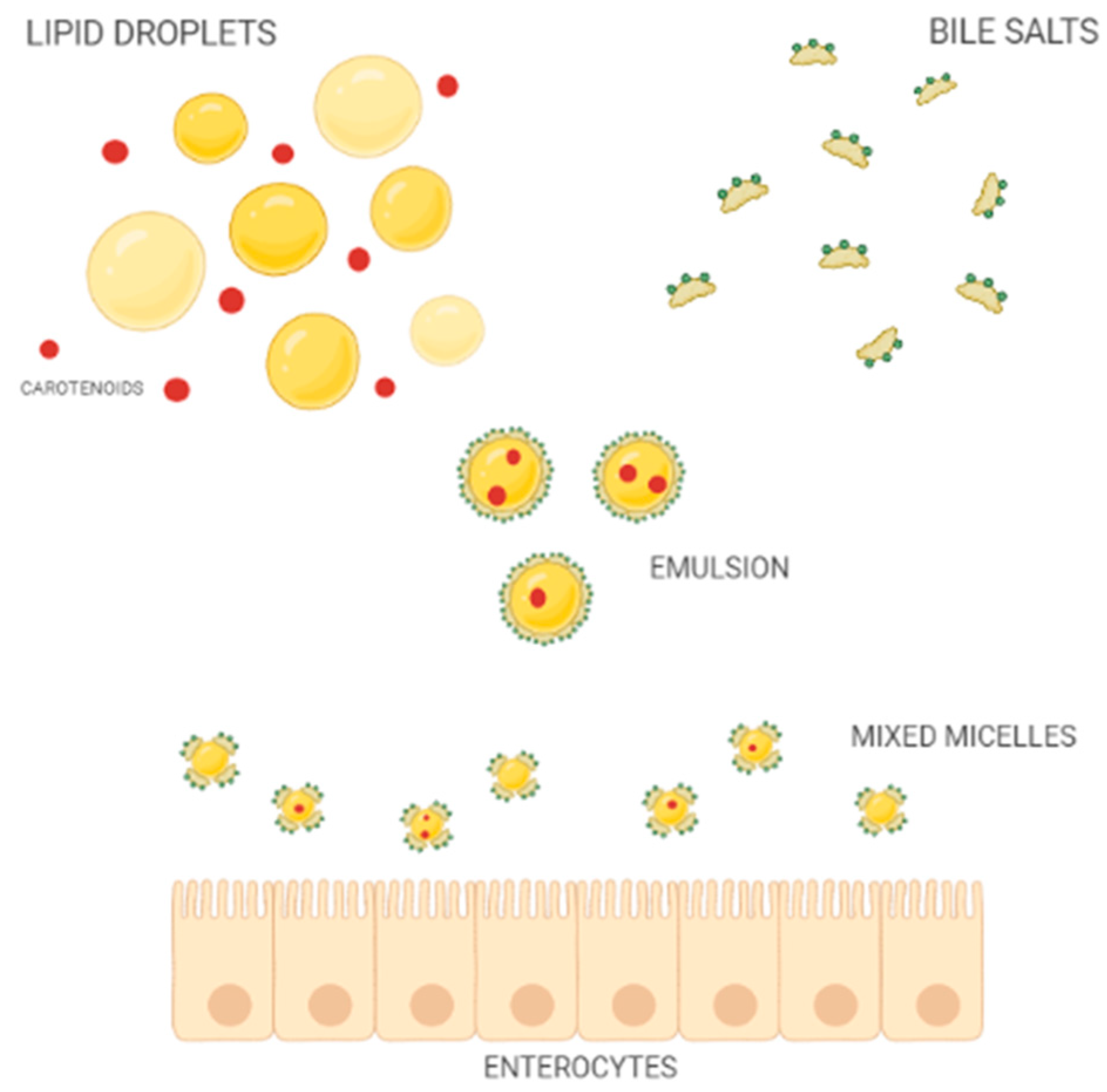
3. Bioaccessibility and Bioavailability of Carotenoids
4. Factors Affecting the Bioaccessibility and Bioavailability of Carotenoids
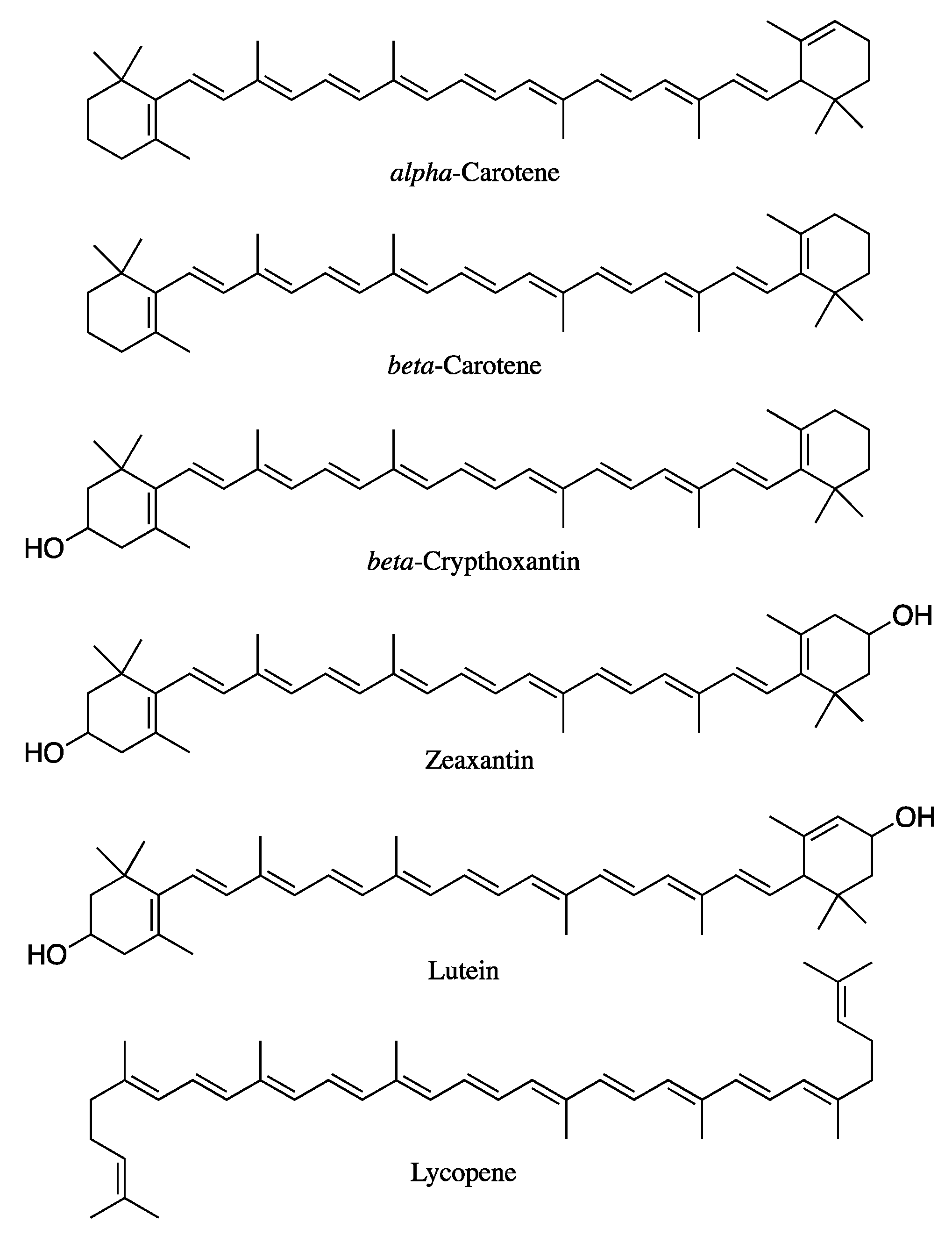
4.1. Molecular Structure of Carotenoids
4.2. Esterified Carotenoids
4.3. Amount of Carotenoids Consumed
4.4. Food Matrix and Carotenoid Location
4.5. Food Heating and Processing
4.6. Dietary Fiber Intake
4.7. Dietary Minerals Intake
4.8. Dietary Fat Intake
4.9. Dietary Protein Intake
5. Formulation of Nanostructured Delivery Systems
- Loading capacity (LC): the relation between the amount of encapsulated material and mass of carrier material; ideally, a delivery system should have a high LC; hence, it should be able to encapsulate as much material as possible.
- Loading efficiency (LE): reflects the ability of a delivery system to retain encapsulated molecules over time. During the production, storage, and transport steps, part of the carried material can be released from the delivery systems; therefore, ideally, the loading efficiency should be high.
- Delivery efficiency (DE): assesses the ability of a delivery system to carry the encapsulated compound to a specific site of action. Even in this case, delivery efficiency should be high.
- Delivery mechanism: once at the site of action, the material can be released either gradually or in response to specific environmental triggers.
- Protection against chemical degradation: chemical degradation may occur under different forms, such as oxidation, hydrolyzation, and isomerization, which can eventually lead to a loss in bioactivity. Chemical degradation can be induced and accelerated by factors, such as heat, light, oxygen, and pH variations, and can be managed through the encapsulation of the compound of interest.
- Bioaccessibility/bioavailability: delivery systems should enhance the encapsulated compound’s bioaccessibility and bioavailability.
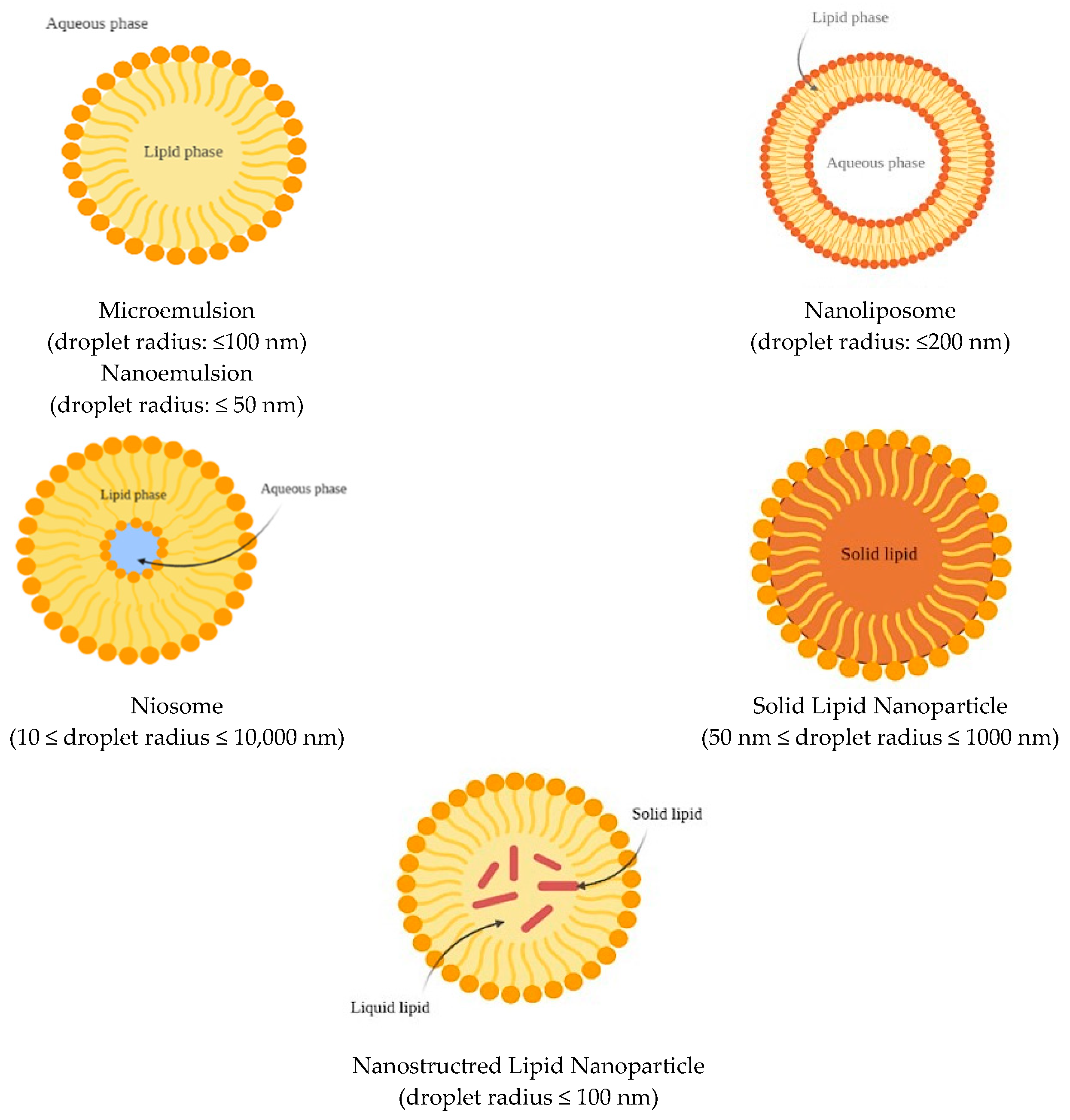
6. Lipid-Based Nanocarriers
6.1. Nanoemulsions and Microemulsions
6.2. Nanoliposomes
6.3. Niosomes
6.4. Solid Lipid Nanoparticles (SLNs)
6.5. Nanostructured Lipid Carriers (NLCs)
| Delivery System | Loaded Compound | Emulsifier and Additives | Lipid Phase | Study Outcomes | Reference |
|---|---|---|---|---|---|
| Nanoemulsion | β-carotene | Whey protein isolate | Palm oil/ coconut oil/ fish oil | NPs with palm oil were the smallest and had the highest bioaccessibility. After 42 days of storage, β-carotene was seen to be more prone to degradation in unsaturated oils. Palm oil was the most suitable carrier. | Zhou et al. [93] |
| Nanoemulsion | β-carotene | Sodium caseinate | Corn oil/ olive oil/ canola oil/ palm oil/ coconut oil/ MCTs | Amount of beta-carotene included is positively proportional to the length of the fatty acids. Oils rich in unsaturated fatty acids enhanced β-carotene micellization. | Yi et al. [94] |
| Nanoemulsion | Astaxanthin | Sodium caseinate + phosphate buffer | Olive oil/ flaxseed oil/ corn oil | Bioaccessibility depended on the unsaturation and chain length. | Liu et al. [95] |
| Nanoemulsion | β-carotene | Peanut protein isolate (PPI)/ soy protein isolate (SPI)/ rice bran protein isolate (RBPI)/ whey protein isolate (WPI) | Corn oil | All four NPs achieved high encapsulation levels. PPI-emulsified nanoemulsion had the highest lipolysis rates, bioaccessibility, smallest droplet size, and highest stability during storage. | Liu et al. [96] |
| Nanoemulsion | Lutein | Tween 20/ Tween 40/ Tween 60/ Tween 80/ Tween 85 | MCT oil | Nanoemulsion stabilized with Tween 80 was he most stable and bioavailable. | Surh et al. [97] |
| Nanoemulsion | β-carotene | Whey protein isolate/ soybean soluble polysaccharides/ decaglyceromonolaurate | MCT oil | The emulsifier had a considerable impact on the release process and micellization rates of β-carotene in emulsions stabilized with whey protein isolate, decaglycerolmonolaurate, and soybean soluble polysaccharides were, respectively, 34.0%, 24.1%, and 21.8%. | Hou et al. [98] |
| Liposome | Lutein/ β-carotene/ lycopene/ canthaxanthin | Egg yolk phospholipid + Tween 80 | Bioaccessibility observed: lutein > β-carotene > lycopene > canthaxanthin. Bioaccessibility was connected to the inclusion ability of the carotenoid into the lipid bilayer, the concentration of the molecule in the vehicle, and the nature of the delivery system. | Tan C. et al. [103] | |
| Liposome | Lutein/ β-carotene/ lycopene/ canthaxanthin | Encapsulation of carotenoids into liposomes enhanced their antioxidant activity. The strongest activity followed the order: lutein > β-carotene > lycopene > canthaxanthin. Lutein and β-carotene also protected lipids from pro-oxidant elements. | Tan C. et al. [104] | ||
| Nanoliposome | β-carotene | Marine phospholipids/ egg phospholipids | Marine phospholipids were seen to be more suitable for the creation of β-carotene-loaded nanoliposomes because of their lower mean size and polydispersity index, as well as better capacity inhibiting lipid peroxidation and better stability during storage. | Hamadou A.H. et al. [105] | |
| Niosome | β-carotene | Span 40/ Span 60/ Span 80 + Tween 20/ Tween 40/ Tween 60 + cholesterol | The resulting systems showed high resistance to sunlight, high temperature, and induced oxidative stress. β-carotene was seen to be stable in culture medium up to 96 h and it was effectively taken up by cultured cells at concentrations covering the range of physiological levels (0.1–2 µM). | Palozza P. et al. [109] | |
| Niosome | Lycopene | Span 60 + cholesterol | Lycopene showed resistance to oxidative stress. In vitro release was gradual and prolonged. Bioavailability was enhanced producing rise in blood plasma levels of 297.19%. | Sharma P.K. et al. [110] | |
| SLN | β-carotene | Tween 80 | Blends of MCT + glyceryl stearate/partially hydrogenated palm oil | SLNs prepared using glyceryl stearate were completely digested. SLNs fabricated with HPO had higher β-carotene bioaccessibility, associated with the higher amounts of monounsaturated fatty acids in the micelle fraction. | de Abreu-Martins H. H. et al. [118] |
| SLN | β-carotene | Whey protein isolate (WPI) | Palmitic acid + corn oil | Palmitic acid was seen to form a shell around β-carotene. The use of whey protein isolate was seen to improve the stability of SLN as well as β-carotene’s oxidative stability. | Mehrad B. et al. [119] |
| SLN | β-carotene/vitamin A/ω-3 fish oil | Quillaja extract/ Quillaja extract + low-melting lecithin/ Quillaja extract + high-melting lecithin | The main impact on the structural arrangement and chemical stability of the encapsulated molecules was attributed to the solubility of the functional lipids in the aqueous phase and to the crystallization temperature in relation to that of the carrier lipid. | Salminen H. et al. [120] | |
| SLN, LLN | β-carotene | Tween 80 | Cocoa butter + or/hydrogenated palm oil | LLNs had better stability to droplet aggregation, while SLN exhibited considerable increment in particle diameter. β-carotene rate degradation was seen to be higher in SLNs. | Qian C. et al. [116] |
| NLC | β-carotene | Tween 80/ Tween 60/ Tween 20/ | Squalene + grapeseed oil + glyceryl stearate + n-hexadecyl palmitate | The smallest droplets were obtained using Tween 20 as the main surfactant. NLCs produced containing Sq and GSO were seen to enhance the antioxidant properties of the system, whereas NLCs produced with GSO and Tween 80 as the main surfactant manifested the greatest antioxidant activity towards free oxygen radicals. β-carotene-loaded NLCs revealed antibacterial activity against Escherichia coli, also showing a correlation with the concentration of β-carotene and of the liquid lipid, rather than the particle size. | Lacatusu I et al. [122] |
| NLC | Lycopene | Span 80 + Planrasens® HE20 | Cocoa butter + grapeseed oil | NLCs maintained lycopene’s stability when stored at 4 °C for 3 months. | Sirikhet J. et al. [123] |
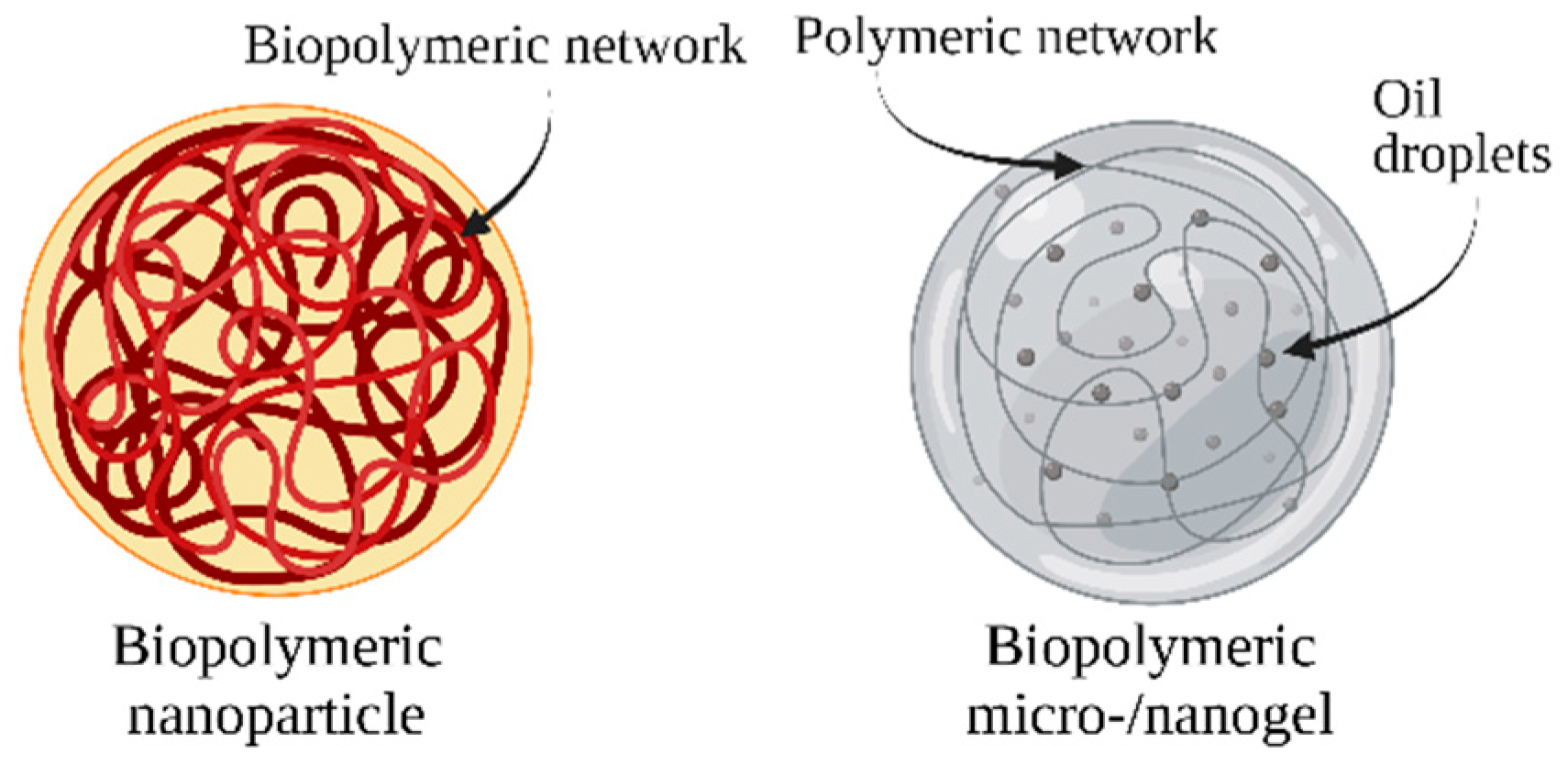
7. Biopolymeric Nanocarriers
7.1. Polysaccharide-Based Nanocarriers
7.2. Protein-Based Nanocarriers
7.3. Biopolymeric Microgels and Nanogels
| Delivery System | Loaded Compound | Biopolymer | Study Outcomes | Reference |
|---|---|---|---|---|
| Polysaccharide-based nanocarrier | Lutein | Chitosan | Lutein bioavailability was enhanced by 27.7%. Moreover, postprandial lutein levels in blood plasma (54.5%), liver (53.9%), and eyes (62.8%) in mice were much higher than the control. | Arunkumar R. et al. [130] |
| Polysaccharide-based nanocarrier | Astaxanthin | Poly(ethylene oxide)-4-methoxycinnamoylphthaloyl-chitosan (PCPLC)/ poly(vinylalcohol-co-vinyl-4-methoxycinnamate) (PB4)/ ethylcellulose (EC) | Encapsulation into PCPLC showed the best results, with high encapsulation efficiency (98%), loading (40%), and high stability to heat. On the contrary, encapsulation into PB4 and EC did not produce positive results. | Tachaprutinun A. et al. [131] |
| Polysaccharide-based nanocarrier | β-carotene | Chitosan + sodium tripolyphosphate/ chitosan + carboxymethylcellulose | The chitosan and sodium tripolyphosphate carrier showed considerable β-carotene release in aqueous media and gastric fluid, and adequate release in intestinal fluids. The chitosan and carboxymethylcellulose carrier showed an optimal release behavior in aqueous media and gastric fluid; however, the release percentage in the intestinal fluid was small. In both cases, β-carotene release was enhanced when included in food systems. | Rutz J. et al. [132] |
| Protein-based nanocarrier | β-carotene | Casein | The nanocarrier successfully protected β-carotene during sterilization, pasteurization, high hydrostatic pressure, and baking. | Sáiz-Abajo M.J. et al. [133] |
| Protein-based nanocarrier | β-carotene | Native β-casein | Micelles were optimally primed at pH 5.5 with a temperature of 2 °C for 5 min, and successfully loaded with β-carotene. | Moeller H. et al. [135] |
| Protein-based nanocarrier | β-carotene | Ferritin | The resulting nanocages became highly water-soluble and the thermal stability of β-carotene was improved. | Chen L. et al. [136] |
| Protein-based nanocarrier | Lycopene (dissolved in soybean oil) | Whey protein/ carbohydrate-based matrices (dextran/chitosan) | The whey protein nanocarrier showed a higher encapsulation efficiency compared with the carbohydrate-based ones, as well as better protection against moisture and thermal degradation. | Pérez-Masiá et al. [137] |
| Protein-based nanocarrier | Lutein | Zein | The stability was tested in an in vitro gastrointestinal model, and it was seen to be enhanced by 58%; however, micellization efficiency decreased by 42%. | Cheng C. J. et al. [138] |
| Hydrogel beads | β-carotene | Alginate | β-carotene was encapsulated into hydrogel beads formed by using 0.5% alginate, 1% alginate, or into nanoemulsions. The hydrogel beads were generally seen to better prevent the compound from chemical degradation, in particular hydrogels with 1% alginate provided the best protection. However, gastrointestinal studies showed that nanoemulsions were more accessible than the hydrogel beads. | Zhang Z. et al. [142] |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed]
- Scarmo, S.; Cartmel, B.; Lin, H.; Leffell, D.J.; Welch, E.; Bhosale, P.; Bernstein, P.S.; Mayne, S.T. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch. Biochem. Biophys. 2010, 504, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Debelo, H.; Novotny, J.A.; Ferruzzi, M.G. Vitamin A. Adv. Nutr. 2017, 8, 992–994. [Google Scholar] [CrossRef]
- Hughes, D.A. Dietary carotenoids and human immune function. Nutrition 2001, 17, 823–827. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef]
- Milani, A.; Marzieh, B.; Sepideh, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Sosa Fernandez Del Campo, S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Harari, A.; Melnikov, N.; Kandel Kfir, M.; Kamari, Y.; Mahler, L.; Ben-Amotz, A.; Harats, D.; Cohen, H.; Shaish, A. Dietary β-carotene rescues vitamin A deficiency and inhibits atherogenesis in apolipoprotein E-deficient mice. Nutrients 2020, 12, 1625. [Google Scholar] [CrossRef]
- Relevy, N.Z.; Harats, D.; Harari, A.; Ben-Amotz, A.; Bitzur, R.; Rühl, R.; Shaish, A. Vitamin A-deficient diet accelerated atherogenesis in apolipoprotein E(-/-) mice and dietary β-carotene prevents this consequence. Biomed. Res. Int. 2015, 2015, 758723. [Google Scholar] [CrossRef]
- Bechor, S.; Relevy, N.Z.; Harari, A.; Almog, T.; Kamari, Y.; Ben-Amotz, A.; Harats, D.; Shaish, A. 9-Cis β-carotene increased cholesterol efflux to HDL in macrophages. Nutrients 2016, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, X.; Pinos, I.; Abraham, B.M.; Barrett, T.J.; von Lintig, J.; Fisher, E.A.; Amengual, J. β-Carotene conversion to vitamin A delays atherosclerosis progression by decreasing hepatic lipid secretion in mice. J. Lipid Res. 2020, 61, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Coronel, J.; Marques, C.; Aradillas-García, C.; Vargas Morales, J.M.; Andrade, F.C.D.; Erdman, J.W.; Teran-Garcia, M. β-Carotene Oxygenase 1 activity modulates circulating cholesterol concentrations in mice and humans. J. Nutr. 2020, 150, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Laukkanen, J.A.; Mäkikallio, T.H.; Ronkainen, K.; Kurl, S. Serum β-carotene and the risk of sudden cardiac death in men: A population-based follow-up study. Atherosclerosis 2013, 226, 172–177. [Google Scholar] [CrossRef]
- Westphal, A.; Böhm, V. Carotenoids. Properties, distribution, bioavailability, metabolism and health effects. Ernahr. Umsch. 2015, 62, 196–207. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum beta carotene and overall and cause-specific mortality. Circ Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Fathalipour, M.; Fathalipour, H.; Safa, O.; Nowrouzi-Sohrabi, P.; Mirkhani, H.; Hassanipour, S. The therapeutic role of carotenoids in diabetic retinopathy: A systematic review. Diabetes Metab. Syndr. Obes. 2020, 13, 2347–2358. [Google Scholar] [CrossRef]
- Mares, J. Lutein and zeaxanthin isomers in eye health and disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin carotenoids in public health and nutricosmetics: The Emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef]
- Goodman, G.E.; Omenn, G.S.; Thornquist, M.D.; Lund, B.; Metch, B.; Gylys-Colwell, I. The Carotene and Retinol Efficacy Trial (CARET) to prevent lung cancer in high-risk populations: Pilot study with cigarette smokers. Cancer Epidemiol. Biomark. Prev. 1993, 2, 389–396. [Google Scholar]
- The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. Ann. Epidemiol. 1994, 4, 1–10. [Google Scholar] [CrossRef]
- Duffield-Lillico, A.J.; Begg, C.B. Reflections on the landmark studies of beta-carotene supplementation. J. Natl. Cancer Inst. 2004, 96, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: The Women’s Health Study. J. Natl. Cancer Inst. 1999, 91, 2102–2106. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Stukel, T.A.; Stevens, M.M.; Mandel, J.S.; Spencer, S.K.; Elias, P.M.; Lowe, N.; Nierenberg, D.W.; Bayrd, G.; et al. and the Skin Cancer Prevention Study Group. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. N. Engl. J. Med. 1990, 323, 789–795. [Google Scholar] [CrossRef]
- Choi, R.Y.; Chortkoff, S.C.; Gorusupudi, A.; Bernstein, P.S. Crystalline maculopathy associated with high-dose lutein supplementation. JAMA Ophthalmol. 2016, 134, 1445–1448. [Google Scholar] [CrossRef]
- Beaulieu, R.A.; Warwar, R.E.; Buerk, B.M. Canthaxanthin retinopathy with visual loss: A case report and review. Case Rep. Ophthalmol. Med. 2013, 2013, 140901. [Google Scholar] [CrossRef]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W.; Johnson, E.J. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, F.; Gonz, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and bioconversion of carotenoids. Ann. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Van Het Hof, K.H.; Tijburg, L.B.M.; Pietrzik, K.; Weststrate, J.A. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Br. J. Nutr. 1999, 82, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Widjaja-Adhi, M.A.K.; Golczak, M. The molecular aspects of absorption and metabolism of carotenoids and retinoids in vertebrates. Biochim. Biophys. Acta 2020, 1865, 158571. [Google Scholar] [CrossRef] [PubMed]
- Tyssandier, V.; Reboul, E.; Dumas, J.F.; Bouteloup-Demange, C.; Armand, M.; Marcand, J.; Sallas, M.; Borel, P. Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am. J. Physiol. 2003, 284, G913–G923. [Google Scholar] [CrossRef]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Mercadante, A.Z. The bioaccessibility of carotenoids impacts the design of functional foods. Curr. Opin. Food Sci. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- McClements, D.J.; Peng, S.F. Current status in our understanding of physicochemical basis of bioaccessibility. Curr. Opin. Food Sci. 2020, 31, 57–62. [Google Scholar] [CrossRef]
- Nagao, A.; Kotake-Nara, E.; Hase, M. Effects of fats and oils on the bioaccessibility of carotenoids and vitamin E in vegetables. Biosci. Biotech. Biochem. 2014, 77, 1055–1060. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the relationship between food structure, processing, and bioavailability. Crit. Rev. Food Sci. Nut. 2014, 54, 902–909. [Google Scholar] [CrossRef]
- Lemmens, L.; Colle, I.; Van Buggenhout, S.; Palmero, P.; Van Loey, A.; Hendrickx, M. Carotenoid bioaccessibility in fruit- and vegetable-based food products as affected by product (micro)structural characteristics and the presence of lipids: A review. Trends Food Sci. Technol. 2014, 38, 125–135. [Google Scholar] [CrossRef]
- Widjaja-Adhi, M.A.K.; Lobo, G.P.; Golczak, M.; Von Lintig, J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum. Mol. Genet. 2015, 24, 3206–3219. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; von Lintig, J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta, beta-carotene absorption and vitamin A production. FASEB J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Ordóñez, T.; Carle, R.; Schweiggert, R. Bioaccessibility of carotenoids from plant and animal foods. J. Sci. Food Agric. 2019, 99, 3220–3239. [Google Scholar] [CrossRef]
- Priyadarshani, A.M.B. A review on factors influencing bioaccessibility and bioefficacy of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1710–1717. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Kopec, R.E.; Villalobos-Gutierrez, M.G.; Högel, J.; Quesada, S.; Esquivel, P.; Schwartz, S.J.; Carle, R. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: A randomised cross-over study. Br. J. Nutr. 2014, 111, 490–498. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 2018, 648, 36–43. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Xanthophyll esterification accompanying carotenoid overaccumulation in chromoplast of Capsicum annuum ripening fruits is a constitutive process and useful for ripeness index. J. Agric. Food Chem. 2000, 48, 1617–1622. [Google Scholar] [CrossRef]
- Brevik, A.; Andersen, L.F.; Karlsen, A.; Trygg, K.U.; Blomhoff, R.; Drevon, C.A. Six carotenoids in plasma used to assess recommended intake of fruits and vegetables in a controlled feeding study. Eur. J. Clin. Nutr. 2004, 58, 1166–1173. [Google Scholar] [CrossRef]
- Olmedilla, B.; Granado, F.; Southon, S.; Wright, A.J.A.; Blanco, I.; Gil-Martinez, E.; Berg, H.; Corridan, B.; Roussel, A.-M.; Chopra, M.; et al. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br. J. Nutr. 2001, 85, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; van Kappel, A.L.; Ferrari, P.; Slimani, N.; Steghens, J.-P.; Bingham, S.; Johansson, I.; Wallström, P.; Overvad, K.; Tjønneland, A.; et al. Plasma levels of six carotenoids in nine European countries: Report from the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2004, 7, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Rauma, A.L.M.; Mykkänen, H. Antioxidant status in vegetarians versus omnivores. Nutrition 2000, 16, 111–119. [Google Scholar] [CrossRef]
- Prince, M.R.; Frisoli, J.K. Beta-carotene accumulation in serum and skin. Am. J. Clin. Nutr. 1993, 57, 175–181. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E.; Linssen, J.P.H.; Van Het Hof, K.H.; Voragen, A.G.J. The food matrix of spinach is a limiting factor in determining the bioavailability of β-carotene and to a lesser extent of lutein in humans. J. Nutr. 1999, 129, 349–355. [Google Scholar] [CrossRef]
- Palmero, P.; Lemmens, L.; Ribas-Agustí, A.; Sosa, C.; Met, K.; De Dieu Umutoni, J.; Hendrickx, M.; Van Loey, A. Novel targeted approach to better understand how natural structural barriers govern carotenoid in vitro bioaccessibility in vegetable-based systems. Food Chem. 2013, 141, 2036–2043. [Google Scholar] [CrossRef]
- Jeffery, J.; Holzenburg, A.; King, S. Physical barriers to carotenoid bioaccessibility. Ultrastructure survey of chromoplast and cell wall morphology in nine carotenoid-containing fruits and vegetables. J. Sci. Food Agric. 2012, 92, 2594–2602. [Google Scholar] [CrossRef]
- Hedrén, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef]
- Stinco, C.M.; Fernández-Vázquez, R.; Escudero-Gilete, M.L.; Heredia, F.J.; Meléndez-Martínez, A.J.; Vicario, I.M. Effect of orange juices processing on the color, particle size, and bioaccessibility of carotenoids. J. Agric. Food Chem. 2012, 60, 1447–1455. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Günther, W. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Verrijssen, T.A.J.; Christiaens, S.; Verkempinck, S.H.E.; Boeve, J.; Grauwet, T.; Van Loey, A.M.; Salvia-Trujillo, L.; Hendrickx, M.E. In vitro β-carotene bioaccessibility and lipid digestion in emulsions: Influence of pectin type and degree of methyl-esterification. J. Food Sci. 2016, 81, C2327–C2336. [Google Scholar] [CrossRef] [PubMed]
- Biehler, E.; Hoffmann, L.; Krause, E.; Bohn, T. Divalent minerals decrease micellarization and uptake of carotenoids and digestion products into Caco-2 cells. J. Nutr. 2011, 141, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Corte-Real, J.; Iddir, M.; Soukoulis, C.; Richling, E.; Hoffmann, L.; Bohn, T. Effect of divalent minerals on the bioaccessibility of pure carotenoids and on physical properties of gastro-intestinal fluids. Food Chem. 2016, 197, 546–553. [Google Scholar] [CrossRef]
- Corte-Real, J.; Desmarchelier, C.; Borel, P.; Richling, E.; Hoffmann, L.; Bohn, T. Magnesium affects spinach carotenoid bioaccessibility in vitro depending on intestinal bile and pancreatic enzyme concentrations. Food Chem. 2018, 239, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Corte-Real, J.; Bertucci, M.; Soukoulis, C.; Desmarchelier, C.; Borel, P.; Richling, E.; Hoffmann, L.; Bohn, T. Negative effects of divalent mineral cations on the bioaccessibility of carotenoids from plant food matrices and related physical properties of gastro-intestinal fluids. Food Funct. 2017, 8, 1008–1019. [Google Scholar] [CrossRef]
- Corte-Real, J.; Guignard, C.; Gantenbein, M.; Weber, B.; Burgard, K.; Hoffmann, L.; Richling, E.; Bohn, T. No influence of supplemental dietary calcium intake on the bioavailability of spinach carotenoids in humans. Br. J. Nutr. 2017, 117, 1560–1569. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, 21–32. [Google Scholar] [CrossRef]
- Van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D. Influence of dietary fat on β-carotene absorption and bioconversion into vitamin A. Nutr. Rev. 2002, 60, 104–110. [Google Scholar] [CrossRef]
- Liu, X.; Bi, J.; Xiao, H.; McClements, D.J. Increasing carotenoid bioaccessibility from yellow peppers using excipient emulsions: Impact of lipid type and thermal processing. J. Agric. Food Chem. 2015, 63, 8534–8543. [Google Scholar] [CrossRef] [PubMed]
- Kopec, R.E.; Cooperstone, J.L.; Schweiggert, R.M.; Young, G.S.; Harrison, E.H.; Francis, D.M.; Clinton, S.K.; Schwartz, S.J. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high-β-carotene tomato sauce and from carrots. J. Nutr. 2014, 144, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. In vitro bioaccessibility of colored carotenoids in tomato derivatives as affected by ripeness stage and the addition of different types of oil. J. Food Sci. 2018, 83, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Vahid, F.; Merten, D.; Larondelle, Y.; Bohn, T. Influence of proteins on the absorption of lipophilic vitamins, carotenoids and curcumin—A Review. Mol. Nutr. Food Res. 2022, 66, e2200076. [Google Scholar] [CrossRef]
- Iddir, M.; Dingeo, G.; Porras Yaruro, J.F.; Hammaz, F.; Borel, P.; Schleeh, T.; Desmarchelier, C.; Larondelle, Y.; Bohn, T. Influence of soy and whey protein, gelatin and sodium caseinate on carotenoid bioaccessibility. Food Funct. 2020, 11, 5446–5459. [Google Scholar] [CrossRef]
- Iddir, M.; Degerli, C.; Dingeo, G.; Desmarchelier, C.; Schleeh, T.; Borel, P.; Larondelle, Y.; Bohn, T. Whey protein isolate modulates beta-carotene bioaccessibility depending on gastro-intestinal digestion conditions. Food Chem. 2019, 291, 157–166. [Google Scholar] [CrossRef]
- Iddir, M.; Porras Yaruro, J.F.; Cocco, E.; Hardy, E.M.; Appenzeller, B.M.R.; Guignard, C.; Larondelle, Y.; Bohn, T. Impact of protein-enriched plant food items on the bioaccessibility and cellular uptake of carotenoids. Antioxidants 2021, 10, 1005. [Google Scholar] [CrossRef]
- Iddir, M.; Pittois, D.; Guignard, C.; Weber, B.; Gantenbei, M.; Larondelle, Y.; Bohn, T. Whey- and soy protein isolates added to a carrot-tomato juice alter carotenoid bioavailability in healthy adults. Antioxidants 2021, 10, 1748. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotech. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control Rel. 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotech. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Salem, M.A.; Ezzat, S.M. Nanoemulsions in Food Chemistry; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-grade nanoemulsions: Preparation, stability and application in encapsulation of bioactive compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Komaiko, J.S.; Mcclements, D.J. Formation of food-grade nanoemulsions using low-energy preparation methods: A review of available methods. Comp. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.M. Nanotechnology: Current uses and future applications in the food industry. 3 Biotech 2018, 8, 74. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Wang, C.; Zhao, C.; Peng, Q.; Zhang, T.; Zhao, C. Stability and in vitro digestibility of beta-carotene in nanoemulsions fabricated with different carrier oils. Food Sci. Nutr. 2018, 6, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhong, F.; Zhang, Y.; Yokoyama, W.; Zhao, L. Effects of lipids on in vitro release and cellular uptake of β-carotene in nanoemulsion-based delivery systems. J. Agric. Food Chem. 2015, 63, 10831–10837. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; McClements, D.J.; Li, F.; Liu, H.; Cao, Y.; Xiao, H. Nanoemulsion-based delivery systems for nutraceuticals: Influence of long-chain triglyceride (LCT) type on in vitro digestion and astaxanthin bioaccessibility. Food Biophys. 2018, 13, 412–421. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zhang, S.; Li, J.; Zheng, H.; Jin, H.; Xu, J. Comparison of different protein emulsifiers on physicochemical properties of β-carotene-loaded nanoemulsion: Effect on formation, stability, and in vitro digestion. Nanomaterials 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Decker, E.A.; McClements, D.J. Utilisation of spontaneous emulsification to fabricate lutein-loaded nanoemulsion-based delivery systems: Factors influencing particle size and colour. Int. J. Food Sci. Technol. 2017, 52, 1408–1416. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, Y.; Lei, F.; Gao, Y. Investigation into the in vitro release properties of β-carotene in emulsions stabilized by different emulsifiers. Food Sci. Technol. 2014, 59, 867–873. [Google Scholar] [CrossRef]
- Zhang, L.; Hayes, D.G.; Chen, G.; Zhong, Q. Transparent dispersions of milk-fat-based nanostructured lipid carriers for delivery of β-carotene. J. Agric. Food Chem. 2013, 61, 9435–9443. [Google Scholar] [CrossRef]
- Lopes, N.A.; Brandelli, A. Nanostructures for delivery of natural antimicrobials in food. Crit. Rev. Food Sci. Nutr. 2018, 58, 2202–2212. [Google Scholar] [CrossRef]
- Shade, C.W. Liposomes as advanced delivery systems for nutraceuticals. Integr. Med. 2016, 15, 33–36. [Google Scholar]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloid Surf. B 2014, 123, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposome as a delivery system for carotenoids: Comparative antioxidant activity of carotenoids as measured by ferric reducing antioxidant power, DPPH assay and lipid peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Hamadou, A.H.; Huang, W.C.; Xue, C.; Mao, X. Comparison of β-carotene loaded marine and egg phospholipids nanoliposomes. J. Food Eng. 2020, 283, 110055. [Google Scholar] [CrossRef]
- Masjedi, M.; Montahaei, T. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102234. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Nutraceutical nanodelivery; an insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit. Rev. Food Sci. Nutr. 2021, 61, 3031–3065. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Palozza, P.; Muzzalupo, R.; Trombino, S.; Valdannini, A.; Picci, N. Solubilization and stabilization of β-carotene in niosomes: Delivery to cultured cells. Chem. Phys. Lipids. 2006, 139, 32–42. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Tekade, K.R.; Balasubramaniam, A. Novel encapsulation of lycopene in niosomes and assessment of its anticancer activity. J. Bioequiv. Bioavailab. 2016, 8, 224–232. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Da Silva Santos, V.; Badan Ribeiro, A.P.; Andrade Santana, M.H. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.H.; Diep, T.T.; Le, T.T.T.; Nguyen, V. Advances in colloidal dispersions: A review. J. Dispers. Sci. Technol. 2020, 41, 479–494. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Impact of lipid nanoparticle physical state on particle aggregation and β-carotene degradation: Potential limitations of solid lipid nanoparticles. Food Res. Int. 2013, 52, 342–349. [Google Scholar] [CrossRef]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- De Abreu-Martins, H.H.; Artiga-Artigas, M.; Hilsdorf Piccoli, R.; Martín-Belloso, O.; Salvia-Trujillo, L. The lipid type affects the in vitro digestibility and β-carotene bioaccessibility of liquid or solid lipid nanoparticles. Food Chem. 2020, 311, 126024. [Google Scholar] [CrossRef]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef]
- Salminen, H.; Gömmel, C.; Leuenberger, B.H.; Weiss, J. Influence of encapsulated functional lipids on crystal structure and chemical stability in solid lipid nanoparticles: Towards bioactive-based design of delivery systems. Food Chem. 2016, 190, 928–937. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Ovidiu, O.; Bojin, D.; Meghea, A. Highly antioxidant carotene-lipid nanocarriers: Synthesis and antibacterial activity. J. Nanopart. Res. 2012, 14, 902. [Google Scholar] [CrossRef]
- Sirikhet, J.; Chanmahasathien, W.; Raiwa, A.; Kiattisin, K. Stability enhancement of lycopene in Citrullus lanatus extract via nanostructured lipid carriers. Food Sci. Nutr. 2021, 9, 1750–1760. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutrit. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Nowak, E.; Livney, Y.D.; Niu, Z.; Singh, H. Delivery of bioactives in food for optimal efficacy: What inspirations and insights can be gained from pharmaceutics? Trends Food Sci. Technol. 2019, 91, 557–573. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in applications of nanotechnology in global food industry. Food Chem. 2020, 342, 128318. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, A.; McClemments, J.D. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Arunkumar, R.; Prashanth, K.V.H.; Baskaran, V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: Characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013, 141, 327–337. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Da Rosa, C.G.; Da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Sáiz-Abajo, M.J.; González-Ferrero, C.; Moreno-Ruiz, A.; Romo-Hualde, A.; González-Navarro, C.J. Thermal protection of β-carotene in re-assembled casein micelles during different processing technologies applied in food industry. Food Chem. 2013, 138, 1581–1587. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Dave, A.; Singh, H. Nature-assembled structures for delivery of bioactive compounds and their potential in functional foods. Front. Chem. 2020, 8, 564021. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P.C. Native casein micelles as nanocarriers for β-carotene: pH-and temperature-induced opening of the micellar structure. Int. J. Food Sci. Technol. 2017, 52, 1122–1130. [Google Scholar] [CrossRef]
- Chen, L.; Bai, G.; Yang, R.; Zang, J.; Zhou, T.; Zhao, G. Encapsulation of β-carotene within ferritin nanocages greatly increases its water-solubility and thermal stability. Food Chem. 2014, 149, 307–312. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; Lagaron, J.M.; Lopez-Rubio, A. Morphology and stability of edible lycopene-containing micro- and nanocapsules produced through electrospraying and spray drying. Food Bioprocess Technol. 2015, 8, 459–470. [Google Scholar] [CrossRef]
- Cheng, C.J.; Ferruzzi, M.; Jones, O.G. Fate of lutein-containing zein nanoparticles following simulated gastric and intestinal digestion. Food Hydrocoll. 2019, 87, 229–236. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Matalanis, A.; McClements, D.J. Hydrogel microspheres for encapsulation of lipophilic components: Optimization of fabrication and performance. Food Hydrocoll. 2013, 31, 15–25. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- McClements, D.J. Edible lipid nanoparticles: Digestion, absorption, and potential toxicity. Prog. Lipid Res. 2013, 52, 409–423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molteni, C.; La Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931. https://doi.org/10.3390/antiox11101931
Molteni C, La Motta C, Valoppi F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants. 2022; 11(10):1931. https://doi.org/10.3390/antiox11101931
Chicago/Turabian StyleMolteni, Camilla, Concettina La Motta, and Fabio Valoppi. 2022. "Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review" Antioxidants 11, no. 10: 1931. https://doi.org/10.3390/antiox11101931
APA StyleMolteni, C., La Motta, C., & Valoppi, F. (2022). Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants, 11(10), 1931. https://doi.org/10.3390/antiox11101931






