Antioxidant and Immune Stimulating Effects of Allium hookeri Extracts in the RAW 264.7 Cells and Immune-Depressed C57BL/6 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Its Responsible Component
2.1.1. Sample Preparation for the Experiment
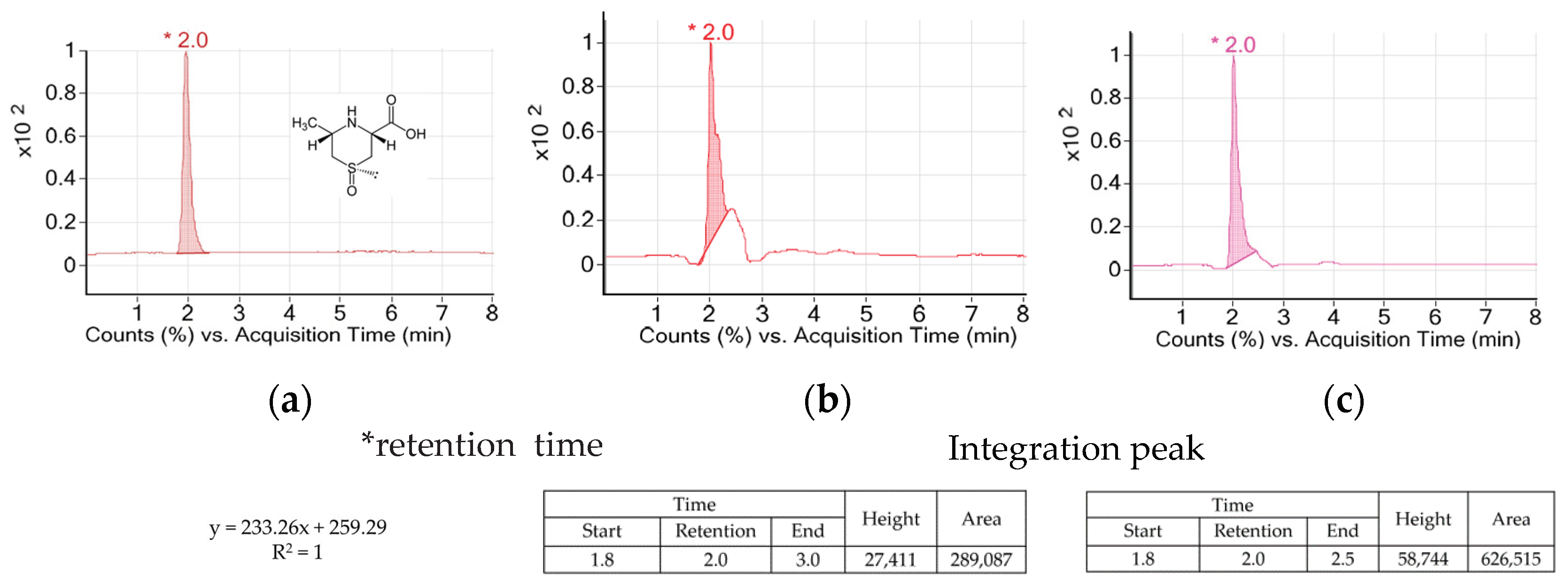
2.1.2. Measuring Cycloalliin Concentration
2.2. Evaluation of Antioxidant Activity
2.2.1. Total Phenolic Content
2.2.2. DPPH Radical Scavenging Activity
2.2.3. ATBS Radical Scavenging Activity
2.3. Cell Experiments for Evaluations of Antioxidant and Immunomodulatory Effects
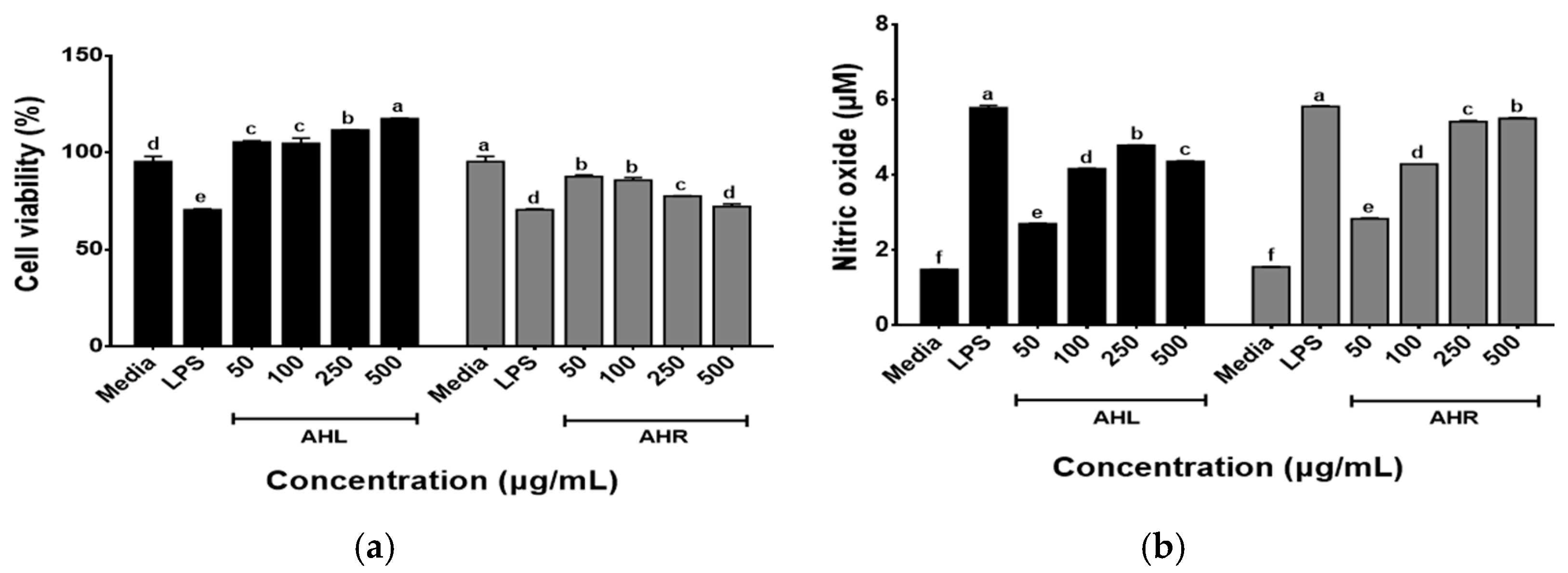
2.3.1. Measuring Cell Viability
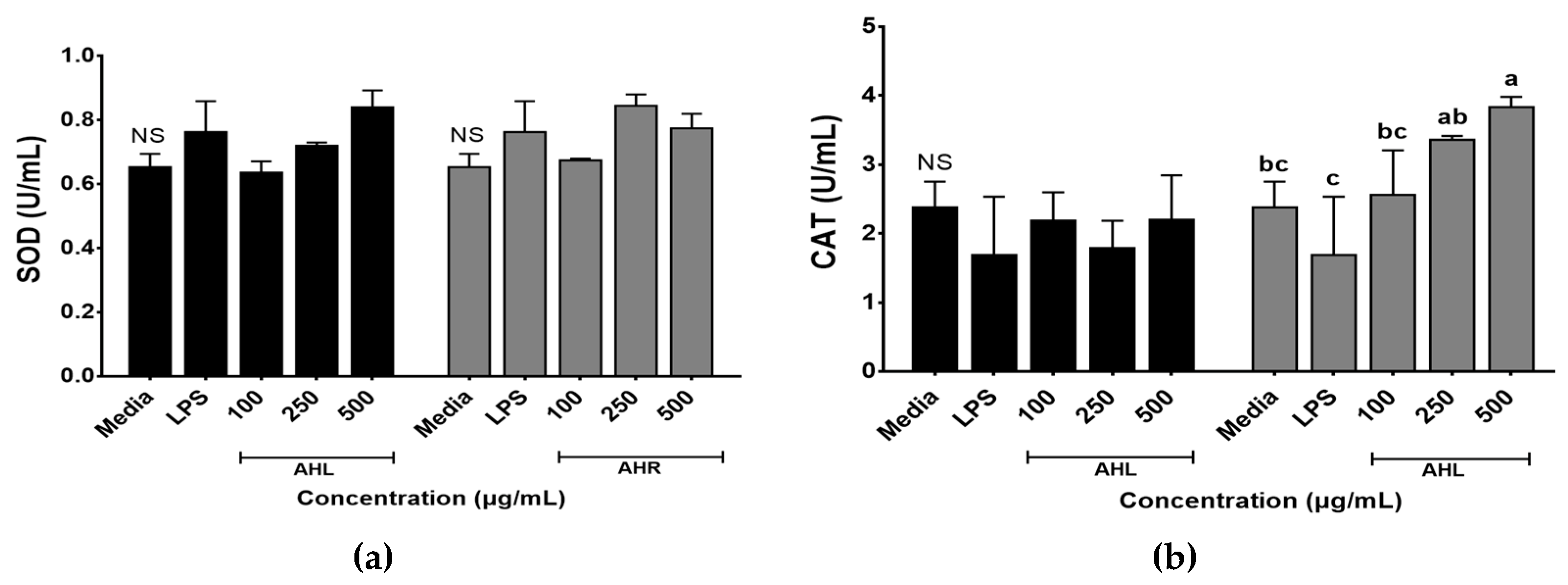
2.3.2. Superoxide Dismutase Activity
2.3.3. Catalase Activity
2.3.4. Nitric Oxide Concentration
2.3.5. Cytokine Concentrations Produced by RAW 264.7 Cells
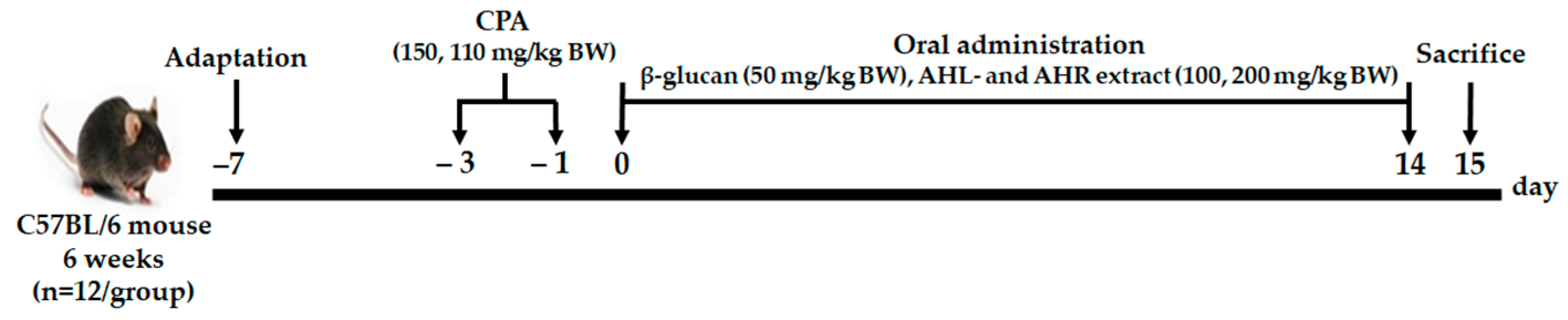
2.4. Animal Experiment
2.4.1. Experimental Design
- Group 1: CON (normal control, distilled water (DW)) (n = 10);
- Group 2: NC (negative control, CPA, DW) (n = 10);
- Group 3: PC (positive control, CPA, β-glucan 50 mg/kg BW) (n = 10);
- Group 4: AHL100 (CPA, AHL extract 100 mg/kg BW) (n = 10);
- Group 5: AHL200 (CPA, AHL extract 200 mg/kg BW) (n = 10);
- Group 6: AHR100 (CPA, AHR extract 100 mg/kg BW) (n = 10);
- Group 7: AHR200 (CPA, AHR extract 200 mg/kg BW) (n = 10).
2.4.2. Collecting Blood and Organs
2.4.3. Immunoglobulin Concentration in Serum
2.4.4. Cytokine Concentration in Serum
2.4.5. Splenocyte Proliferation
2.4.6. NK Cell Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Concentration of Cycloalliin
3.2. Total Phenolic Content and Antioxidant Activities of AHL and AHR Extracts
3.3. Enzymatic Antioxidant Effects of AHL and AHR Extracts on RAW 264.7 Cells
3.4. Effects of AHL and AHR Extracts on Cell Viability of and NO Production by RAW 264.7 Cells
3.5. Effects of AHL and AHR Extracts on Cytokine Productions by RAW 264.7 Cells
3.6. Effects of AHL and AHR Extracts on Body and Organ Weights
3.7. Effects of AHL and AHR Extracts on Immunoglobulin Concentration
3.8. Effects of AHL and AHR Extracts on Cytokine Levels in Serum
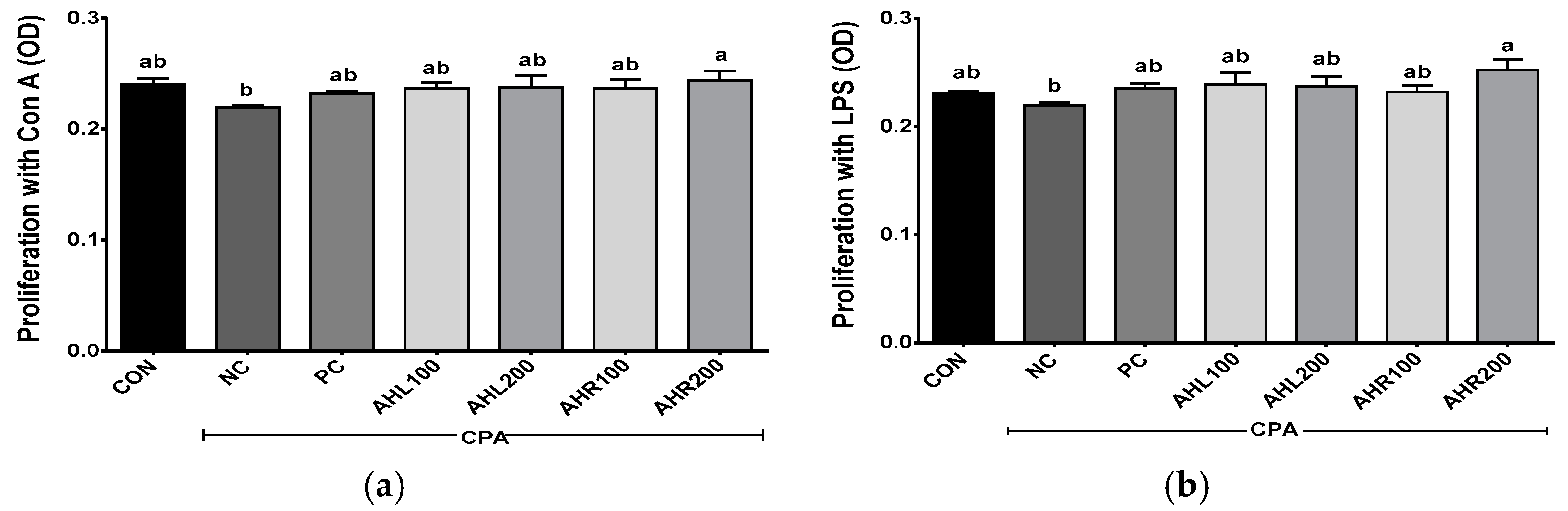
3.9. Effects of AHL and AHR Extracts on the Proliferation of Mice Splenocytes
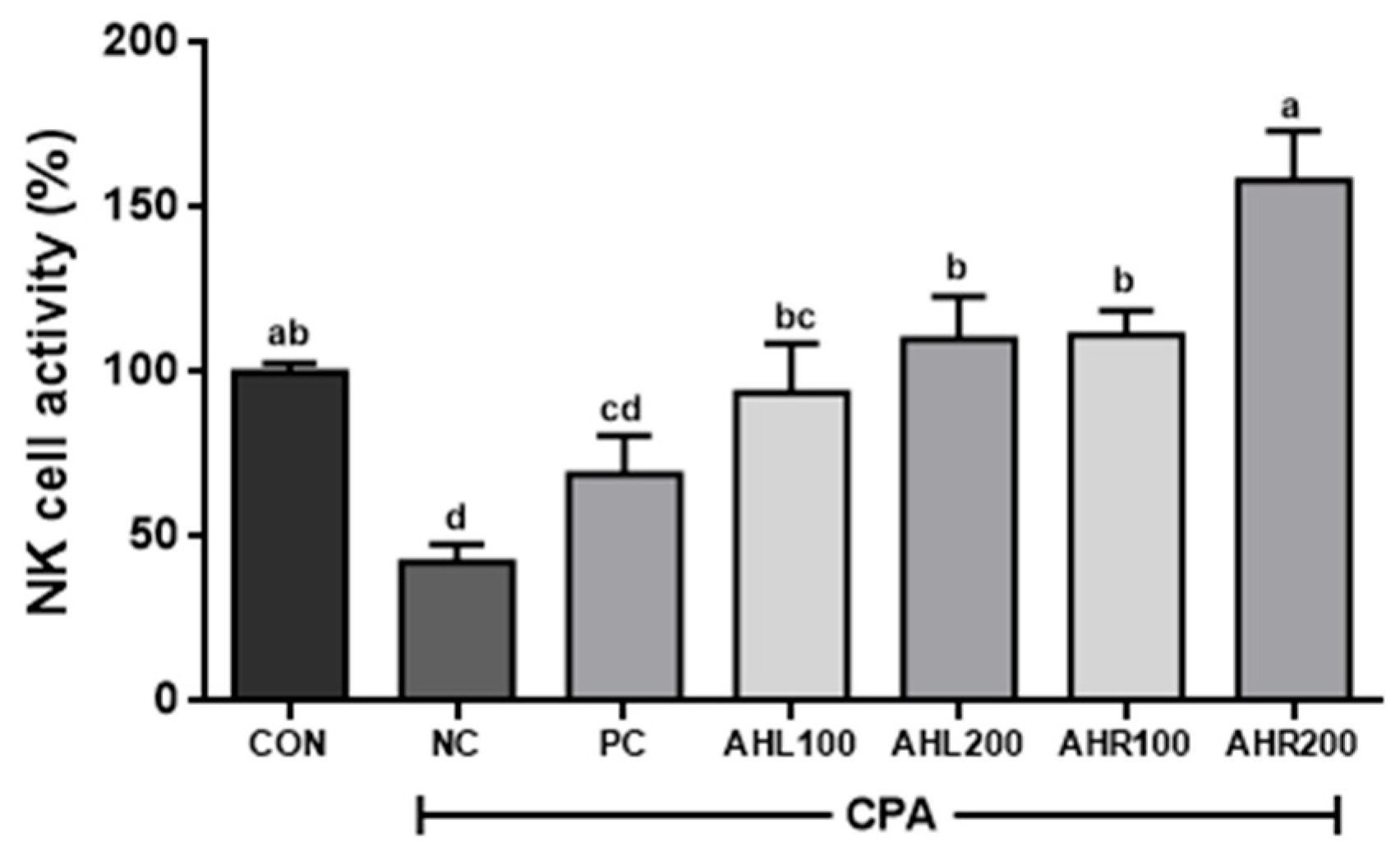
3.10. Effects of AHL and AHR Extracts on the NK Cell Activity in Immunosuppressed Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.B.; Lee, S.; Park, J.; Shin, D.; Yoon, M. Profiling of organosulphur compounds using HPLC-PDA and GC/MS system and antioxidant activities in hooker chive (Allium hookeri). Nat. Prod. Res. 2016, 30, 2798–2804. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, K.J.; Choi, S.Y.; Koh, E.J.; Park, J.D.; Lee, B.Y. Korean ginseng extract ameliorates abnormal immune response through the regulation of inflammatory constituents in Sprague Dawley rat subjected to environmental heat stress. J. Ginseng. Res. 2019, 43, 252–260. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Serrador, J.M. Nitric oxide signaling in Tcell-mediated immunity. Trends Mol. Med. 2018, 24, 412–427. [Google Scholar] [CrossRef]
- Shreshtha, S.; Sharma, P.; Kumar, P.; Shara, R.; Singh, S. Nitric oxide: It’s role in immunity. J. Clin. Diagn. Res. 2018, 12, BE01–BE05. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Aslani, B.A.; Ghobadi, S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016, 146, 163–173. [Google Scholar] [CrossRef]
- Deka, B.; Manna, P.; Borah, J.C.; Talukdar, N.C. A review on phytochemical, pharmacological attributes and therapeutic uses of Allium hookeri. Phytomed. Plus 2022, 2, 100262. [Google Scholar] [CrossRef]
- Jun, H.I.; Yang, J.H.; Choi, J.Y.; Lee, S.H.; Song, G.S.; Kim, K.S.; Kim, Y.S. Changes in volatile flavor compounds in steam-dried Allium hookeri root. Food Sci. Biotechnol. 2016, 25, 1327–1331. [Google Scholar] [CrossRef]
- Ren, F.; Wang, L.; Zhuo, W.; Chen, D.; Huang, H.; Zhang, L. Complete chloroplast genome of the rare medicinal vegetable Allium hookeri. Mitochondrial DNA B Resour. 2022, 7, 6–7. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, M.J.; You, B.R.; Jin, J.S.; Lee, S.H.; Yun, Y.R.; Kim, H.J. Allium hookeri root extract exerts anti-inflammatory effects by nuclear factor-κB down-regulation in lipopolysaccharide-induced RAW264.7 cells. BMC Complement. Altern. Med. 2017, 17, 126. [Google Scholar]
- Lee, Y.; Lee, S.H.; Jeong, M.S.; Kim, J.B.; Jang, H.H.; Jeong, S.C.; Lillehoj, H.S. In vitro analysis of the immunomodulating effects of Allium hookeri on lymphocytes, macrophages, and tumour cells. J. Poult. Sci. 2017, 54, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.H.; Lee, S.J.; Gadde, U.D.; Oh, S.T.; Han, H.; Lillehoj, H.S. Effects of dietary Allium hookeri root on growth performance and antioxidant activity in young broiler chickens. Res. Vet. Sci. 2018, 118, 345–350. [Google Scholar] [CrossRef]
- Bhat, R. Bioactive Compounds of Allium species. In Bioactive Compounds in Underutilized Vegetables and Legumes; Reference Series in Phytochemistry; Murthy, H.N., Paek, K.Y., Eds.; Springer: Cham, Swizerland, 2020; pp. 1–20. [Google Scholar]
- Kim, N.S.; Choi, B.K.; Lee, S.H.; Jang, H.H.; Kim, J.B.; Kim, H.R.; Choe, J.S.; Cho, Y.S.; Kim, Y.S.; Yang, J.H.; et al. Effects of Allium hookeri extracts on glucose metabolism in type II diabetic mice. Korean J. Pharm. 2016, 47, 158–164. [Google Scholar]
- Galavi, A.; Hosseinzadeh, H.; Razavi, B.M. The effects of Allium cepa L. (onion) and its active constituents on metabolic syndrome: A review. Iran. J. Basic Med. Sci. 2021, 24, 3–16. [Google Scholar] [PubMed]
- Kim, H.J.; Lee, M.J.; Jang, J.Y.; Lee, S.H. Allium hookeri root extract inhibits adipogenesis by promoting lipolysis in high fat diet-induced obese mice. Nutrients 2019, 11, 2262. [Google Scholar] [CrossRef]
- Kovarovič, J.; Bystrická, J.; Vollmannová, A.; Tóth, T.; Brindza, J. Biologically valuable substances in garlic (Allium sativum L.)—A review. J. Cen. Eur. Agric. 2019, 20, 292–304. [Google Scholar] [CrossRef]
- Park, S.H.; Bae, U.J.; Choi, E.K.; Jung, S.J.; Lee, S.H.; Yang, J.H.; Kim, Y.S.; Jeong, D.Y.; Kim, H.J.; Park, B.H.; et al. A randomized, double-blind, placebo-controlled crossover clinical trial to evaluate the anti-diabetic effects of Allium hookeri extract in the subjects with prediabetes. BMC Complement. Med. Ther. 2020, 20, 211. [Google Scholar] [CrossRef] [PubMed]
- Bakhouche, I.; Aliat, T.; Boubellouta, T.; Gali, L.; Şen, A.; Bellik, Y. Phenolic contents and in vitro antioxidant, anti-tyrosinase, and anti-inflammatory effects of leaves and roots extracts of the halophyte Limonium delicatulum. S. Afr. J. Bot. 2021, 139, 42–49. [Google Scholar] [CrossRef]
- Senguttuvan, J.; Paulsamy, S.; Karthika, K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac. J. Trop. Biomed. 2014, 4, S359–S367. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, R.; Sarikurkcu, C.; Mutlu, M.; Tepe, B. Sophora alopecuroides var. alopecuroides: Phytochemical composition, antioxidant and enzyme inhibitory activity of the methanolic extract of aerial parts, flowers, leaves, roots, and stem. S. Afr. J. Bot 2021, 143, 282–290. [Google Scholar] [CrossRef]
- Wang, F.; Long, S.; Zhang, J.; Yu, J.; Xiong, Y.; Zhou, W.; Qiu, J.; Jiang, H. Antioxidant activities and anti-proliferative effects of Moringa oleifera L. extracts with head and neck cancer. Food Biosci. 2020, 37, 100691. [Google Scholar] [CrossRef]
- Arumugam, R.; Elanchezhian, B.; Samidurai, J.; Amirthaganesan, K. Comparative antioxidant, antibacterial and phytochemical analysis of roots, stems, leaves and seeds from Cleome rutidosperma DC. Natr. Resour. Hum. Health 2022, 2, 479–484. [Google Scholar] [CrossRef]
- Boligon, A.A.; Machado, M.M.; Athayde, M.L. Technical evaluation of antioxidant activity. Med. Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef]
- Ojha, S.; Raj, A.; Roy, A.; Roy, S. Extraction of total phenolics, flavonoids and tannins from Paederia foetida L. leaves and their relation with antioxidant activity. Pharmacogn. J. 2018, 10, 541–547. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.I.; Jang, H.; Ahn, D.; Kim, D.K.; Yang, J.H.; Yun, B.S.; Kim, Y.S. Isolation and characterization of phenolic compound from Allium hookeri root for potential use as antioxidant in foods. Food Sci. Biotechnol. 2015, 24, 2031–2034. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, C.; Zhu, G. Chemical composition and biological activities of essential oils from the leaves, stems, and roots of Kadsura coccinea. Molecules 2021, 26, 6259. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.; Kumari, K.; Samant, S.S.; Paul, S.; Dutt, S. Population status, distribution, antioxidant properties and antibacterial activity of threatened herb Gentiana kurroo Royle. J. Biol. Chem. Chron. 2019, 5, 18–27. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of solvent polarity on the ultrasound assisted extraction and antioxidant activity of phenolic compounds from habanero pepper leaves (Capsicum chinense) and its identification by UPLC-PDA-ESI-MS/MS. Ultrason. Sonochem. 2021, 76, 105658. [Google Scholar] [CrossRef] [PubMed]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Effect of ohmic heating on the extraction yield, polyphenol content and antioxidant activity of olive mill leaves. Clean Technol. 2022, 4, 512–528. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPS): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar]
- Zheng, Q.; Chen, J.; Yuan, Y.; Zhang, X.; Li, L.; Zhai, Y.; Gong, X.; Li, B. Structural characterization, antioxidant, anti-inflammatory activity of polysaccharides from Plumula nelumbinis. Int. J. Biol. Macromol. 2022, 212, 111–122. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, Y.S.; Kim, S.M.; Jung, A.J.; Yoo, J.H.; Lee, S.H.; Jeong, H.C.; Lee, H.J. Immune-enhancing effects of red Platycodon grandifloras root extract via p38 MAPK-mediated NF-κB activation. Appl. Sci. 2020, 10, 5457. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cheon, J.M.; Choi, E.O.; Jeong, J.W.; Lee, K.W.; Kin, K.Y.; Kim, S.G.; Kim, S.; Hong, S.H.; Park, C.; et al. The immunomodulatory activity of Mori folium, the leaf of Morus alba L., in RAW264.7 macrophages in vitro. J. Cancer Prev. 2016, 21, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.B.; Ko, M.N.; Hyun, C.G. Carica papaya leaf water extract promotes innate immune response via MAPK signaling pathways. J. Appl. Biol. Chem. 2021, 64, 277–284. [Google Scholar] [CrossRef]
- Rod-in, W.; Talapphet, N.; Monmai, C.; Jang, A.Y.; You, S.G.; Park, W.J. Immune enhancement effects of Korean ginseng berry polysaccharides on RAW264.7 macrophages through MAPK and NF-kB signaling pathways. Food Agric. Immunol. 2021, 32, 298–309. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.H.; Gadde, U.D.; Oh, S.T.; Lee, S.J.; Lillehoj, H.S. Dietary Allium hookeri reduces inflammatory response and increases expression of intestinal tight junction proteins in LPS-induced young broiler chicken. Res. Vet. Sci. 2017, 112, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Jing-Wen, L.; Yang, L.; Boa-Hui, L.; Yue-Yang, W.; Hui, W.; Chang-Lim, Z. A polysaccharide purified from Radix Adenophorae promotes cell activation and pro-inflammatory cytokine production in murine RAW264.7 macrophages. Chin. J. Nat. Med. 2016, 14, 370–376. [Google Scholar]
- Wei, H.; Wang, Y.; Li, W.; Qiu, Y.; Hua, C.; Zhang, Y.; Guo, Z.; Xie, Z. Immunomodulatory activity and active mechanisms of a low molecular polysaccharide isolated from Lanzhou lily bulbs in RAW264.7 macrophages. J. Funct. Foods 2022, 92, 105071. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, K.C.; Kim, J.S.; Park, H.J.; Ko, S.G.; Moon, P.D. STB (composed of Panax ginseng and Aconitum carmichaeli) and stigmasterol enhances nitric oxide production and exerts curative properties as a potential anti-oxidant and immunity-enhancing agent. Antioxidants 2022, 11, 199. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.H.; Lee, E.B.; Kim, J.S.; Jung, J.E.; Jeong, U.Y.; Lee, S.H. Anti-Diabetic Effects of Allium hookeri Extracts Prepared by Different Methods in Type 2 C57BL/J-db/db Mice. Pharmaceuticals 2022, 15, 486. [Google Scholar] [CrossRef]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, Q.; Li, A.; Yang, M.; Huang, W.; Xu, H.; Zhao, Z.; Li, S. Immuno-enhancement effects of Yifei Tongluo Granules on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2016, 194, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bang, S.; Jang, H.H.; Lee, E.B.; Kim, B.S.; Kim, S.H.; Lillehoj, H.S. Effects of Allium hookeri on gut microbiome related to growth performance in young broiler chickens. PLoS ONE 2020, 15, e0226833. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.A.; Descoteaus, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 1–12. [Google Scholar]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. BioMol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Karin, M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2007, 14, 109–119. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, E.B.; Jang, H.H.; Cha, Y.S.; Park, Y.S.; Lee, S.H. Allium Hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-neuroinflammation in Mice. Nutrients 2021, 13, 2890. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cho, S.S.; Li, Y.; Bae, C.S.; Park, K.M.; Park, D.H. Anti-inflammatory effect of Curcuma longa and Allium hookeri co-treatment via NF-κB and COX-2 pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Tran, L.; Radwan, I.; Minh, L.H.N.; Low, S.K.; Hashan, M.R.; Gomaa, M.D.; Abdelmongy, M.; Abdelaziz, A.I.; Mohamed, A.; Tawfik, G.M.; et al. Role of cytokines produced by T helper immune-modulators in dengue pathogenesis: A systematic review and meta-analysis. Acta Trop. 2021, 216, 105823. [Google Scholar] [CrossRef]
- Zheng, Y.; Guan, J.; Wang, L.; Luo, X.; Zhang, X. Comparative proteomic analysis of spleen reveals key immune-related proteins in the yak (Bos grunniens) at different growth stages. Comp. Biochem. Physiol.-D Genom. Proteom 2022, 42, 100968. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Choi, J.H.; Hwang, I.G.; Jang, H.H.; Hwang, K.A.; Park, S.Y.; Lee, S.H. Immunomodulatory Effects of Doraji (Platycodon grandiflorum) Extracts with High Platycodin D in the Immunosuppressed Mice. J. Korean Soc. Food Sci. Nutr. 2020, 49, 1161–1168. [Google Scholar] [CrossRef]



| Sample | Concentration (μg/mL) | TPC 1 (μg GAE/g) | DPPH Radical Scavenging Activity (%) | ABTS Radical Scavenging activity (%) |
|---|---|---|---|---|
| AHL extract | 50 | 0.07 ± 0.00 e | 8.58 ± 0.37 c | 12.81 ± 2.72 d |
| 100 | 0.13 ± 0.01 d | 8.56 ± 0.30 c | 13.32 ± 2.72 d | |
| 250 | 0.28 ± 0.00 c | 9.97 ± 0.59 bc | 20.87 ± 3.35 c | |
| 500 | 0.53 ± 0.01 b | 11.60 ± 0.25 b | 32.73 ± 1.07 b | |
| 1000 | 0.96 ± 0.03 a | 13.84 ± 0.30 a | 53.56 ± 1.28 a | |
| AHR extract | 50 | 0.11 ± 0.00 d | 9.23 ± 0.35 d | 11.12 ± 0.67 e |
| 100 | 0.24 ± 0.04 d | 9.37 ± 0.26 d | 15.53 ± 0.49 d | |
| 250 | 0.51 ± 0.01 c | 11.00 ± 0.34 c | 32.87 ± 0.63 c | |
| 500 | 0.96 ± 0.04 b | 14.01 ± 0.24 b | 54.45 ± 0.21 b | |
| 1000 | 1.79 ± 0.02 a | 17.21 ± 0.35 a | 83.26 ± 0.52 a |
| CON 1 | NC | PC | AHL 100 | AHL 200 | AHR 100 | AHR 200 | |
|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 23.45 ± 0.24 NS | 23.04 ± 0.37 | 22.87 ± 0.32 | 22.99 ± 0.20 | 23.09 ± 0.32 | 22.97 ± 0.36 | 23.42 ± 0.39 |
| Final body weight (g) | 25.56 ± 0.37 a | 24.33 ± 0.31 b | 24.05 ± 0.37 b | 24.46 ± 0.30 b | 24.42 ± 0.41 b | 24.11 ± 0.40 b | 24.45 ± 0.39 b |
| Tissue weight (% of BW) | |||||||
| Spleen | 0.26 ± 0.02 NS | 0.28 ± 0.01 | 0.28 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.02 | 0.30 ± 0.01 | 0.30 ± 0.01 |
| Thymus | 0.15 ± 0.01 NS | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, U.-Y.; Jung, J.; Lee, E.-B.; Choi, J.-H.; Kim, J.-S.; Jang, H.-H.; Park, S.-Y.; Lee, S.-H. Antioxidant and Immune Stimulating Effects of Allium hookeri Extracts in the RAW 264.7 Cells and Immune-Depressed C57BL/6 Mice. Antioxidants 2022, 11, 1927. https://doi.org/10.3390/antiox11101927
Jeong U-Y, Jung J, Lee E-B, Choi J-H, Kim J-S, Jang H-H, Park S-Y, Lee S-H. Antioxidant and Immune Stimulating Effects of Allium hookeri Extracts in the RAW 264.7 Cells and Immune-Depressed C57BL/6 Mice. Antioxidants. 2022; 11(10):1927. https://doi.org/10.3390/antiox11101927
Chicago/Turabian StyleJeong, Un-Yul, Jieun Jung, Eun-Byeol Lee, Ji-Hye Choi, Ji-Su Kim, Hwan-Hee Jang, Shin-Young Park, and Sung-Hyen Lee. 2022. "Antioxidant and Immune Stimulating Effects of Allium hookeri Extracts in the RAW 264.7 Cells and Immune-Depressed C57BL/6 Mice" Antioxidants 11, no. 10: 1927. https://doi.org/10.3390/antiox11101927
APA StyleJeong, U.-Y., Jung, J., Lee, E.-B., Choi, J.-H., Kim, J.-S., Jang, H.-H., Park, S.-Y., & Lee, S.-H. (2022). Antioxidant and Immune Stimulating Effects of Allium hookeri Extracts in the RAW 264.7 Cells and Immune-Depressed C57BL/6 Mice. Antioxidants, 11(10), 1927. https://doi.org/10.3390/antiox11101927






