Inhibitory Effect on Nitric Oxide Release in LPS-Stimulated Macrophages and Free Radical Scavenging Activity of Croton linearis Jacq. Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Samples
2.3. Free Radical Scavenging Activity
2.4. Cell Viability Assay
2.5. Nitrite Determination
2.6. UPLC-QTOF-MS Analysis
2.7. Data Processing
2.8. Statistical Analysis
3. Results
3.1. Free Radical Scavenging Activity
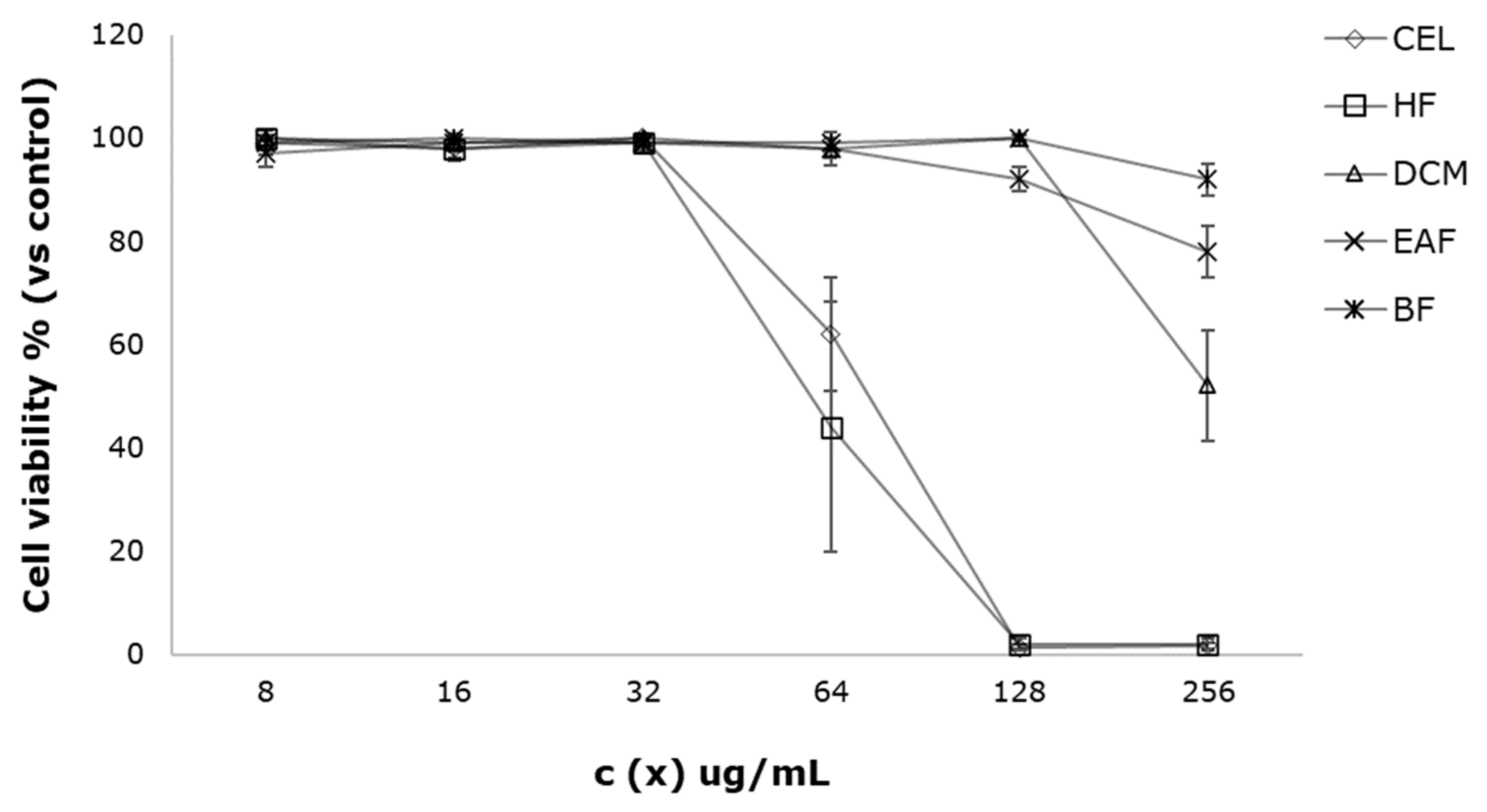
3.2. Cell Viability Assay
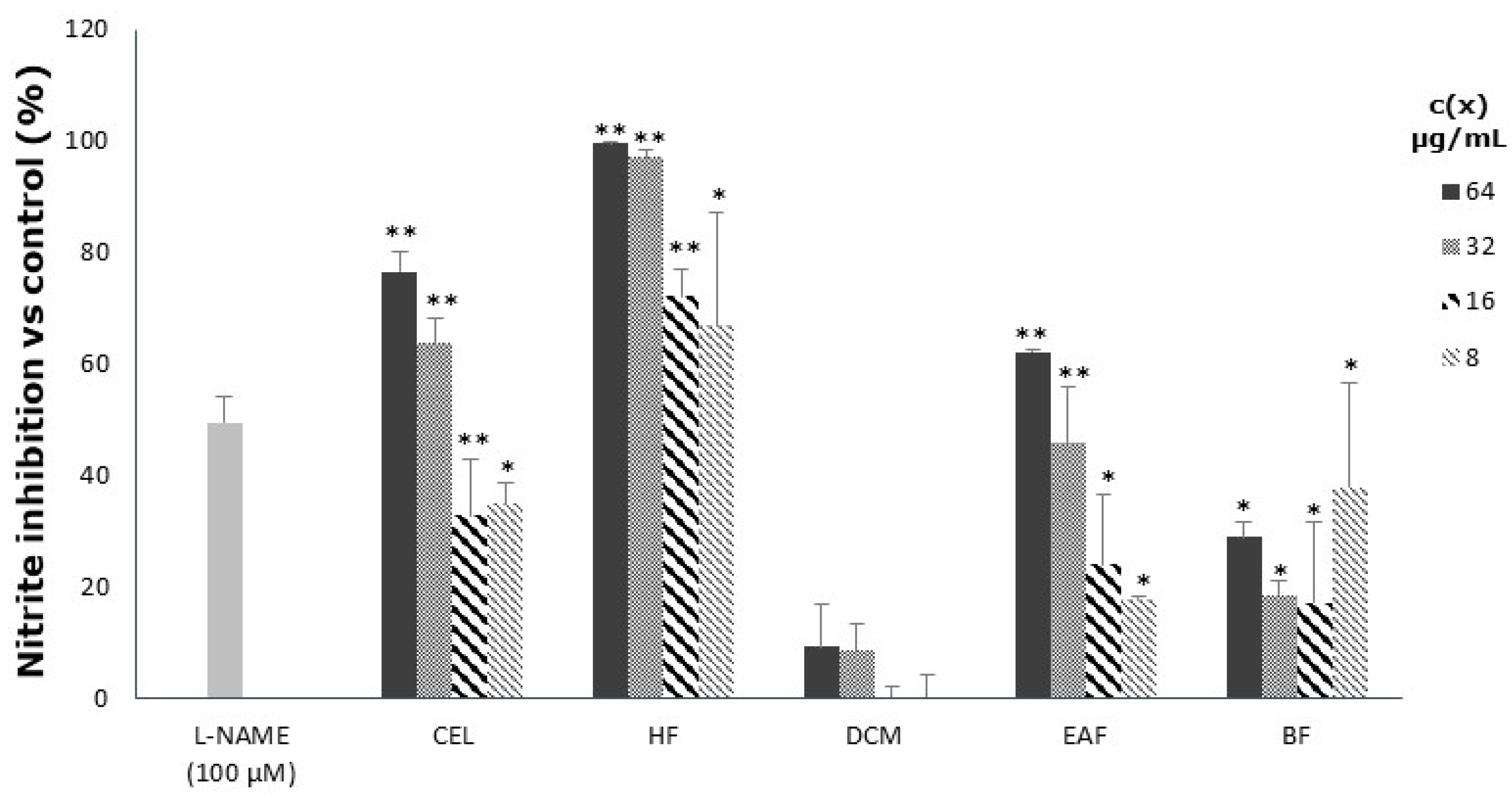
3.3. Inhibitory Effect on Nitric Oxide Release in LPS-Stimulated Macrophages
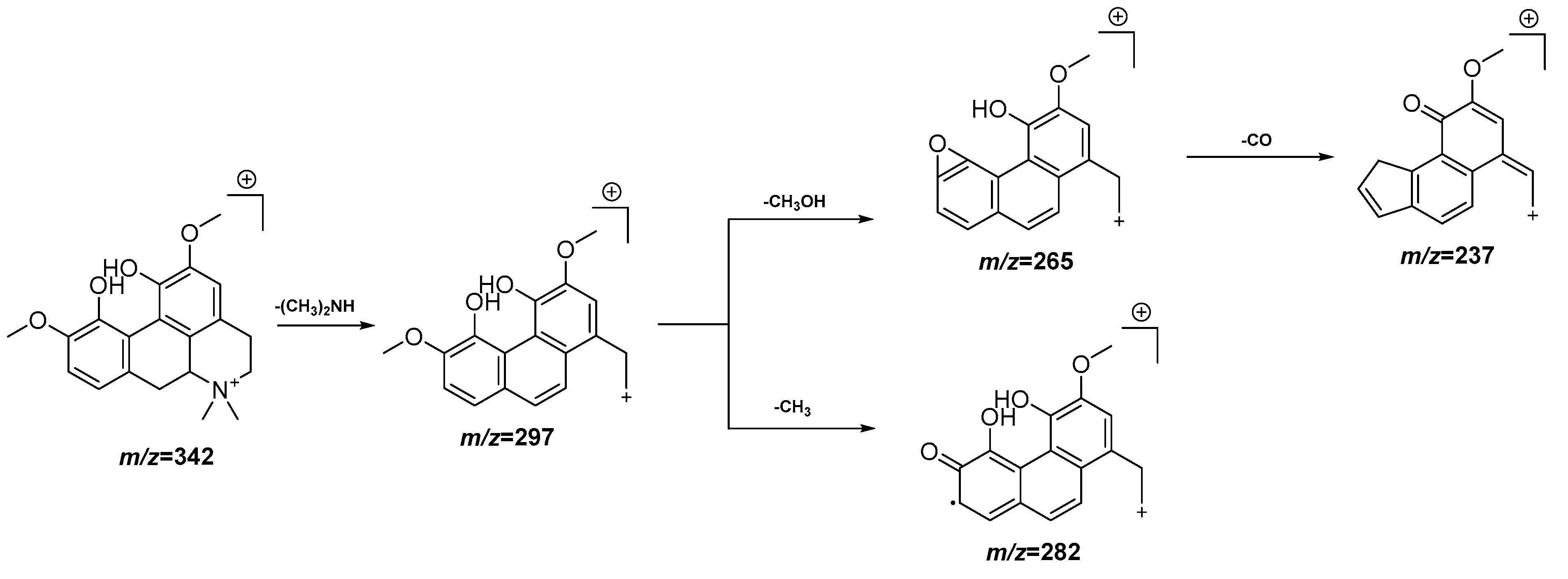
3.4. UPLC-QTOF-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants, 1st ed.; Shalaby, E., Brzozowski, T., Eds.; IntechOpen: London, UK, 2019; Volume 10, pp. 1–29. [Google Scholar] [CrossRef]
- Niki, E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Rai, D.K.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Prinzo, F.; Milella, L. Antioxidant activity and phytochemical characterization of Senecio clivicolus Wedd. Molecules 2018, 23, 2497. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Methods to measure the antioxidant activity of phytochemicals and plant extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2019, 9, 19. [Google Scholar] [CrossRef]
- Holt-McCormack, J.K.; Maier, P.B. Wallens Bush Medicine of the Bahamas: A Cross-Cultural Perspective from San Salvador Island, Including Pharmacology and Oral Histories Hardcover, 1st ed.; J H M Designs Publications: Charlottesville, VA, USA, 2011; pp. 73–74. [Google Scholar]
- Fong, A.; Maceira, D.; Alverson, W.S.; Shopland, J.M. (Eds.) Rapid Biological Inventories; Report 10. Cuba: SiboneyJuticí; Field Museum Of Natural History: Chicago, HL, USA, 2005; p. 112. ISBN 0914868586. [Google Scholar]
- Acevedo-Rodríguez, P.; Strong, M.T. (Eds.) Catalogue of Seed Plants of the West Indies, 3rd ed.; Smithsonian Institution: Washington, DC, USA, 2012; pp. 323–330. [Google Scholar] [CrossRef]
- Roig, J.T. Plantas Medicinales, Aromáticas o Venenosas de Cuba, 1st ed.; Editorial Científico-Técnica: La Habana, Cuba, 1974; p. 714. [Google Scholar]
- Marcelino, V.; Fechine, J.; Guedes, J.R.; Toscano, V.; Filgueiras, P.; Leitao, E.V.; Barbosa-Filho, J.M.; Sobral, M. Phytochemistry of the genus Croton. Nat. Prod. Res. Rev. 2012, 1, 221–370. [Google Scholar]
- Garcia, J.; Tuenter, E.; Escalona-Arranz, J.C.; Llaurado, G.; Cos, P.; Pieters, L. Antimicrobial activity of leaf extracts and isolated constituents of Croton linearis. J. Ethnopharmacol. 2019, 236, 250–257. [Google Scholar] [CrossRef]
- Alexander, I.C.; Pascoe, K.O.; Manchard, P.; Williams, L.A.D. An insecticidal diterpene from Croton linearis. Phytochemistry 1991, 30, 1801–1803. [Google Scholar] [CrossRef]
- García-Díaz, J.; Escalona-Arranz, J.C.; do Carvallo, M.G.; Rojas-Vargas, J.; Machado-García, R.; Acosta, J.D. Aislamiento y caracterización de metabolitos de hojas de Croton linearis Jacq. Rev. Cub. Quim. 2015, 27, 289–301. [Google Scholar]
- Garcia Diaz, J.; Escalona Arranz, J.C.; Jaen Batista, D.D.G.; Monzote Fidalgo, L.; de la Vega Acosta, J.; Bidart de Macedo, M.; Cos, P. Antileishmanial potentialities of Croton linearis Jacq. leaves essential oil. Nat. Prod. Commun. 2018, 13, 629–634. [Google Scholar] [CrossRef]
- Garcia, J.; Tuenter, E.; Escalona-Arranz, J.C.; Llaurado, G.; Cos, P.; Pieters, L. Antiplasmodial activity of alkaloids from Croton linearis leaves. Exp. Parasitol. 2022, 236, 108254. [Google Scholar] [CrossRef]
- Rodriguez-Amado, J.R.; Lafourcade-Prada, A.; Garcia-Diaz, J.; Souto, R.N.P.; Escalona-Arranz, J.C.; de Souza, T.P. Development, larvicide activity, and toxicity in nontarget species of the Croton linearis Jacq essential oil nanoemulsion. Environ. Sci. Pollut. Res. 2020, 27, 9410–9423. [Google Scholar] [CrossRef]
- Maury, G.; Rodríguez, D.; Hendrix, S.; Arranz, J.; Boix, Y.; Pacheco, A.; Díaz, J.; Morris-Quevedo, H.; Dubois, A.; Aleman, E.; et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef]
- Berenguer-Rivas, C.A.; Mas-Ortiz, M.; Batista-Corbal, P.L.; Costa-Acosta, J.; Escalona-Arranz, J.C. Chemical composition and in-vitro antioxidant activity of extracts of Adelia ricinella L. Rev. Cub. Quim. 2018, 30, 191–210. [Google Scholar]
- Arró-Díaz, D.J.; Castelnaux-Ochoa, N.; Ochoa-Pacheco, A.; Do-Nascimento, Y.M. Antioxidant activity of bioactive compounds isolated from leaves and bark of Gymnanthes lucida Sw. Rev. Cub. Quim. 2021, 33, 22–39. [Google Scholar]
- Maury, G.L.; Morris-Quevedo, H.; Heykers, A.; Lanckacker, E.; Cappoen, D.; Delputte, P.; Berghe, W.V.; Salgueiro, Z.; Cos, P. Differential Induction Pattern Towards Classically Activated Macrophages in Response to an Immunomodulatory Extract from Pleurotus ostreatus Mycelium. J. Fungi 2021, 11, 206. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vandergheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Jiao, Q.-S.; Xu, L.-L.; Zhang, J.-Y.; Wang, Z.-J.; Jiang, Y.-Y.; Liu, B. Rapid Characterization and Identification of Non-Diterpenoid Constituents in Tinospora sinensis by HPLC-LTQ-Orbitrap MSn. Molecules 2018, 23, 274. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Pang, H.; Guo, Y.; Xiao, P.; Chu, C.; Guo, L.; Liu, E.-H. Rapid profiling of alkaloid analogues in Sinomenii Caulis by an integrated characterization strategy and quantitative analysis. J. Pharm. Biomed. Anal. 2019, 174, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Panighel, A.; De Rosso, M.; Dalla Vedova, A.; Flamini, R. Putative identification of new p-coumaroyl glycoside flavonoids in grape by ultra-high performance liquid chromatography/high-resolution mass spectrometry. Rapid. Commun. Mass Spectrom. 2015, 29, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Haynes, L.J.; Husbands, G.E.M.; Stuart, K.L. Alkaloids from Croton Species. Part VllI. Morphinandienone Derivatives from Croton linearis Jacq. J. Chem. Soc. 1968, 8, 951–957. [Google Scholar]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Pizzorno, J.E.; Murray, M.T. Textbook of Natural Medicine-E-Book, 15th ed.; Elsevier Health Sciences; Churchill Livingstone publisher: London, UK, 2020; pp. 1–51. [Google Scholar]
- Salehi, B.; Martorell, M.; Arbiser, J.; Sureda, A.; Martins, N.; Maurya, P.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or negative actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacol 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Ondua, M.; Njoya, E.M.; Abdalla, M.A.; McGaw, L.J. Anti-inflammatory and antioxidant properties of leaf extracts of eleven South African medicinal plants used traditionally to treat inflammation. J. Ethnopharm. 2019, 234, 27–35. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, S.J. Effect of quercetin on the production of nitric oxide in murine macrophages stimulated with lipopolysaccharide from Prevotella intermedia. J. Periodontal. Implant. Sci. 2013, 43, 191–197. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Kang, C.W.; Kim, G.D. Kaempferol-3-O-β-rutinoside suppresses the inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. Int. J. Mol. Med. 2019, 44, 2321–2328. [Google Scholar] [CrossRef]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Njoya, E.M.; Eloff, J.N.; Mcgaw, L.J. Croton gratissimus leaf extracts inhibit cancer cell growth by inducing caspase 3/7 activation with additional anti-inflammatory and anti-oxidants activities. BMC Compl. Altern. Med. 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Rampa, K.M.; Van De Venter, M.; Koekemoer, T.C.; Swanepoel, B.; Venables, L.; Hattingh, A.C.; Kamatou, G.P. Exploring four South African Croton species for potential anti-inflammatory properties: In vitro activity and toxicity risk assessment. J. Ethnopharm. 2022, 282, 114596. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.S.; Nascimento, Y.M.D.; Espirito-Santo, R.F.D.; Meira, C.S.; Santos, I.P.; Brandão, R.B.; Souto, A.L.; Guedes, M.L.S.; Soares, M.B.P.; Villarreal, C.F.; et al. Phenylpropanoids from Croton velutinus with cytotoxic, trypanocidal and anti-inflammatory activities. Fitoterapia 2020, 145, 104632. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Rotilio, G.; Ciriolo, M.R. Role of Nitric Oxide Synthases in Parkinson’s Disease: A Review on the Antioxidant and Anti-inflammatory Activity of Polyphenols. Neurochem. Res. 2008, 33, 2416–2426. [Google Scholar] [CrossRef]
- Jung, Y.; Bor, H.Y.C.; Gow, C.Y. Evaluation of Antioxidant Activity and Inhibitory Effect on Nitric Oxide Production of Some Common Vegetables. J. Agric. Food Chem. 2006, 54, 1680–1686. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- da Rocha, A.R.; Sousa, H.G.; do Vale Júnior, E.P.; de Lima, F.L.; Costa, A.S.; de Araújo, A.R.; Lago, E.C. Extracts and fractions of Croton L. (Euphorbiaceae) species with antimicrobial activity and antioxidant potential. LWT 2021, 139, 110521. [Google Scholar] [CrossRef]
- Ayele, D.T.; Akele, M.L.; Melese, A.T. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef]
- Novello, C.R.; Marques, L.C.; Pires, M.E.; Kutschenco, A.P.; Nakamura, C.V.; Nocchi, S.; Sarragiotto, M.H.; Mello, J.C.P. Bioactive indole alkaloids from Croton echioides. J. Braz. Chem. Soc. 2016, 27, 2203–2209. [Google Scholar]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Yong-Bing, X.; Gui-Lin, C.; Ming-Quan, G. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D.A. Comparative study on phenolic content, antioxidant activity and anti-Inflammatory capacity of aqueous and ethanolic extracts of Sorghum in lipopolysaccharide-induced RAW 264.7 Macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef]
| Sample | ABTS IC50 (µg/mL) | DPPH IC50 (µg/mL) | Nitric Oxide Inhibitory Activity IC50 (µg/mL) | Cytotoxicity in RAW 264.7 Cells CC50 (µg/mL) |
|---|---|---|---|---|
| CEL | 105.10 ± 7.43 b | 153.76 ± 23.61 c | 21.59 ± 2.97 b | 75.30 ± 3.71 a |
| HF | 406.22 ± 25.82 d | 426.74 ± 22.00 d | 4.88 ± 2.64 a | 70.12 ± 7.21 a |
| DCM | 55.93 ± 10.75 a | 13.47 ± 3.17 a | >64 | >256 |
| EAF | 35.53 ± 3.33 a | 84.23 ± 6.95 b | 40.03 ± 2.94 c | >256 |
| BF | >500 | >500 | >64 | >256 |
| AA | 182.35 ± 8.07 c | 28.49 ± 2.91 a | ND | ND |
| Tamoxifen | ND | ND | ND | 1.41 ± 0.21 |
| Peak No. | Rt (min) | Precursor Ion (m/z) | Theoretical Mass (m/z) | Accuracy (ppm) | MS/MS Ions | Precursor Ion Formula | Tentative Identification | Extract |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.09 | 334.1655 [M + H]+ | 334.1654 | 0.3 | 318.1709, 190.6034 | C18H23NO5 | Unknown | DCM |
| 2 | 3.16 | 318.1707 [M + H]+ | 318.1705 | 0.6 | 243.1026, 211.0762, 181.0654 | C18H23NO4 | N-demethyl dihydrosinomenine | DCM |
| 3 | 3.35 | 334.1656 [M + H]+ | 334.1654 | 0.6 | 259.0967, 213.0911, 188.0712 | C18H23NO5 | Sinococuline | EAF |
| 4 | 3.73 | 342.1703 [M]+ | 342.1705 | −0.6 | 297.1128, 282.0896, 265.0868, 237.0917 | C20H24NO4+ | Magnoflorine | EAF |
| 5 | 3.84 | 332.1499 [M + H]+ | 332.1498 | 0.1 | 275.0918, 227.0708 | C18H21NO5 | N-oxide demethyl sinomenine | EAF, DCM |
| 6 | 4.04 | 476.1945 [M + H]+ | 476.1921 | 5.0 | 326.1244, 314.1393, 180.0660, 164.0711 | C24H29NO9 | Dauricoside (1) | EAF, DCM |
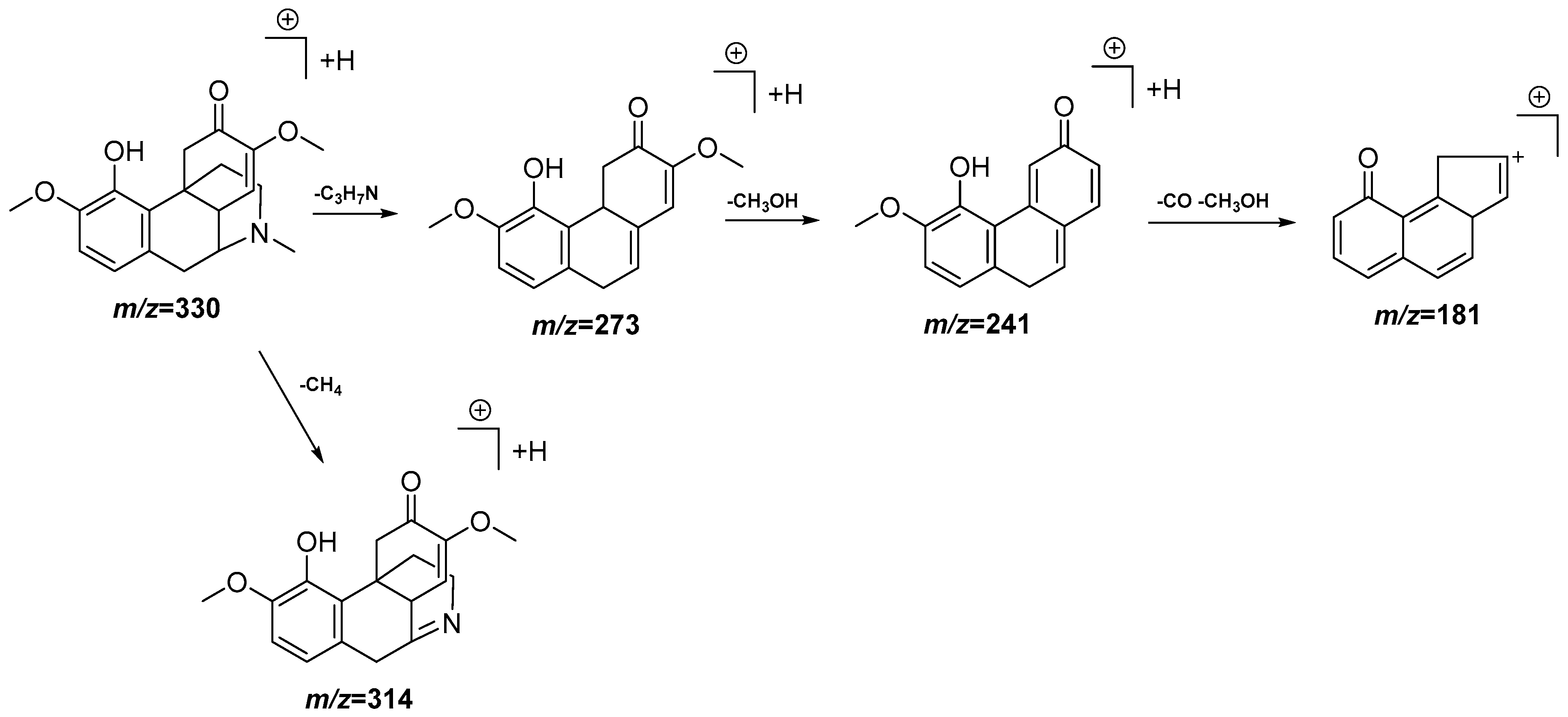
| 7 | 4.12 | 330.1717 [M + H]+ | 330.1705 | 3.6 | 314.1402, 273.1138, 241.0874, 181.0657 | C19H23NO4 | Sinomenine | DCM |
| 8 | 4.24 | 760.2825 [M + H]+ | 760.2817 | 1.1 | 638.2453, 598.2291, 565.1902, 326.1239, 164.0710 | C37H45NO16 | Unknown | EAF |
| 9 | 4.38 | 344.1862 [M + H]+ | 344.1862 | 0.0 | 299.1291, 267.1029, 192.1027, 175.0765 | C20H25NO4 | Laudanidine | EAF, DCM |
| 10 | 4.45 | 612.2462 [M + H]+ | 612.2445 | 2.8 | 478.2087, 344.1858, 342.1710, 178.0870 | C32H37NO11 | Unknown | EAF |
| 11 | 4.68 | 741.1842 [M − H]− | 741.1878 | −4.9 | 609.1470, 477.1773, 301.0334, 300.0270 | C32H38O20 | Quercetin-O-pentosyl-pentosyl- glucuronide | EAF |
| 12 | 4.78 | 476.1929 [M + H]+ | 476.1921 | 1.7 | 358.2010, 314.1391, 175.0757 | C24H29NO9 | Dauricoside (2) | EAF |
| 13 | 4.99 | 609.1456 [M − H]− | 609.1456 | 0.0 | 301.0351, 300.0281 | C27H30O16 | Quercetin- O- pentosyl- glucuronide | EAF |
| 14 | 5.18 | 952.3251 | 952.3239 | 1.3 | 462.1924, 303.0508 | C47H53NO20 | Unknown | EAF |
| 15 | 5.26 | 593.1503 [M − H]− | 593.1506 | −0.5 | 285.0394 284.0324 | C27H30O15 | Kaempferol-O- deoxyhexosyl-hexoside (1) | EAF |
| 16 | 5.38 | 593.1500 [M − H]− | 593.1506 | −1.0 | 285.0399 284.0326 | C27H30O15 | Kaempferol- O- deoxyhexosyl-hexoside (2) | EAF |
| 17 | 5.60 | 477.1031 [M − H]− | 477.1033 | −0.4 | 447.0953, 315.0496, 314.0439, 285.0401, 284.0331, 271.0251, 255.0298, 243.0297, | C22H22O12 | Isorhamnetin 3-O-glucoside | EAF |
| 18 | 5.68 | 477.1031 [M − H]− | 477.1038 | 1.0 | 315.0496, 314.0441, 285.0410, 271.0253, 243.0297 | C22H22O12 | Isorhamnetin 7-O-glucoside | EAF |
| 19 | 5.95 | 356.1870 [M + H]+ | 356.1862 | 2.2 | 325.1547, 310.1197, 294.1272 | C21H25NO4 | Glaucine | DCM |
| 20 | 6.71 | 593.1284 [M − H]− | 593.1295 | −1.9 | 447.0956, 285.0406, 284.0329 | C30H26O13 | Tiliroside | EAF, DCM |
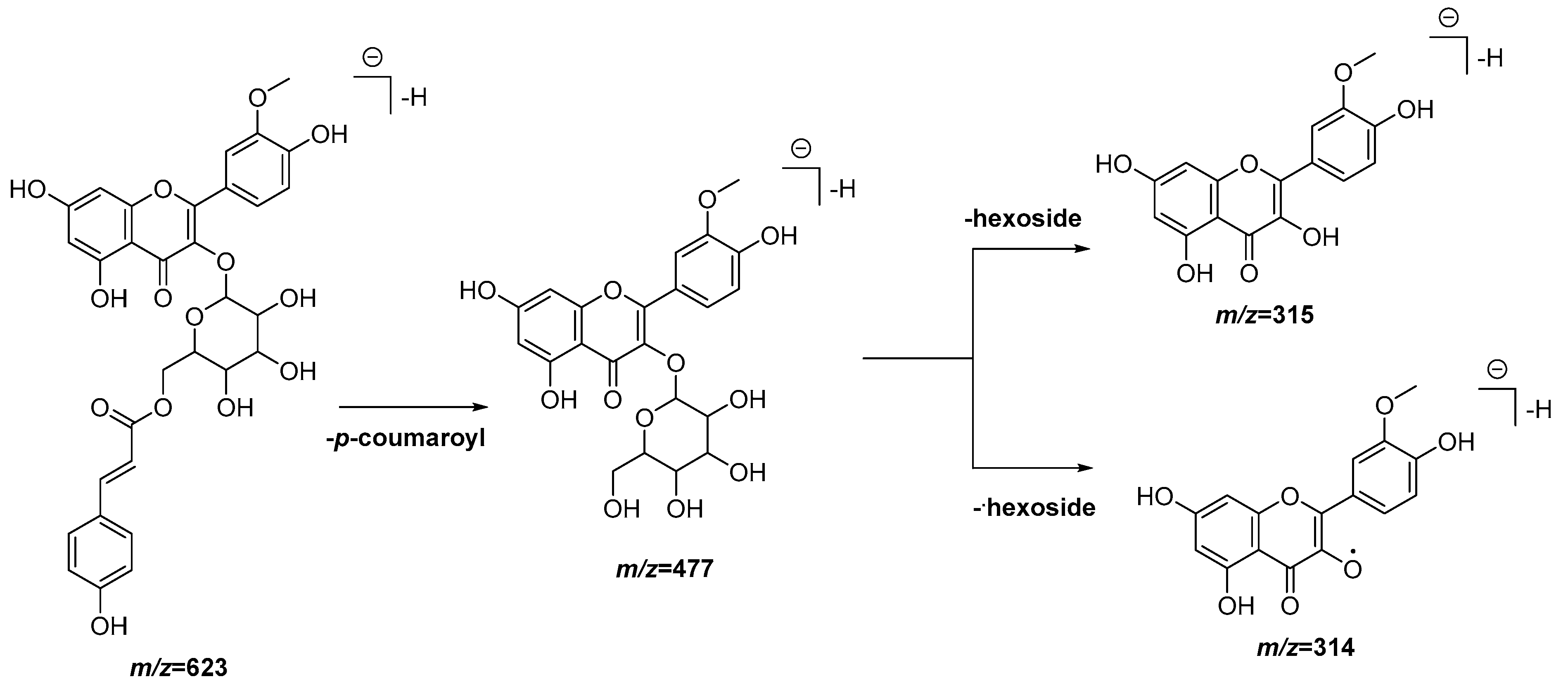
| 21 | 6.84 | 623.1404 [M − H]− | 623.1401 | 0.5 | 477.1058, 315.0513, 314.0438 | C31H28O14 | Isorhamnetin- O-p-coumaroyl hexoside (1) | EAF, DCM |
| 22 | 6.90 | 623.1393 [M − H]− | 623.1401 | −1.3 | 477.1062, 315.0517, 314.0440 | C31H28O14 | Isorhamnetin-O-p-coumaroyl hexoside (2) | EAF, DCM |
| 23 | 7.40 | 880.4304 [M + Na]+ 858.4481 [M + H]+ | 858.4487 | −1.3 | 429.7295 | C42H67NO17 | Unknown | EAF, DCM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Díaz, J.; González Fernández, R.; Escalona Arranz, J.C.; Llauradó Maury, G.; Méndez Rodríguez, D.; De Vooght, L.; Molina, E.; Tuenter, E.; Pieters, L.; Cos, P. Inhibitory Effect on Nitric Oxide Release in LPS-Stimulated Macrophages and Free Radical Scavenging Activity of Croton linearis Jacq. Leaves. Antioxidants 2022, 11, 1915. https://doi.org/10.3390/antiox11101915
García Díaz J, González Fernández R, Escalona Arranz JC, Llauradó Maury G, Méndez Rodríguez D, De Vooght L, Molina E, Tuenter E, Pieters L, Cos P. Inhibitory Effect on Nitric Oxide Release in LPS-Stimulated Macrophages and Free Radical Scavenging Activity of Croton linearis Jacq. Leaves. Antioxidants. 2022; 11(10):1915. https://doi.org/10.3390/antiox11101915
Chicago/Turabian StyleGarcía Díaz, Jesús, Rosalia González Fernández, Julio César Escalona Arranz, Gabriel Llauradó Maury, Daniel Méndez Rodríguez, Linda De Vooght, Enrique Molina, Emmy Tuenter, Luc Pieters, and Paul Cos. 2022. "Inhibitory Effect on Nitric Oxide Release in LPS-Stimulated Macrophages and Free Radical Scavenging Activity of Croton linearis Jacq. Leaves" Antioxidants 11, no. 10: 1915. https://doi.org/10.3390/antiox11101915
APA StyleGarcía Díaz, J., González Fernández, R., Escalona Arranz, J. C., Llauradó Maury, G., Méndez Rodríguez, D., De Vooght, L., Molina, E., Tuenter, E., Pieters, L., & Cos, P. (2022). Inhibitory Effect on Nitric Oxide Release in LPS-Stimulated Macrophages and Free Radical Scavenging Activity of Croton linearis Jacq. Leaves. Antioxidants, 11(10), 1915. https://doi.org/10.3390/antiox11101915