Abstract
The pandemic of COVID-19 was caused by a novel coronavirus termed as SARS-CoV2 and is still ongoing with high morbidity and mortality rates in the whole world. The pathogenesis of COVID-19 is highly linked with over-active immune and inflammatory responses, leading to activated cytokine storm, which contribute to ARDS with worsen outcome. Currently, there is no effective therapeutic drug for the treatment of COVID-19. Zinc is known to act as an immune modulator, which plays an important role in immune defense system. Recently, zinc has been widely considered as an anti-inflammatory and anti-oxidant agent. Accumulating numbers of studies have revealed that zinc plays an important role in antiviral immunity in several viral infections. Several early clinical trials clearly indicate that zinc treatment remarkably decreased the severity of the upper respiratory infection of rhinovirus in humans. Currently, zinc has been used for the therapeutic intervention of COVID-19 in many different clinical trials. Several clinical studies reveal that zinc treatment using a combination of HCQ and zinc pronouncedly reduced symptom score and the rates of hospital admission and mortality in COVID-19 patients. These data support that zinc might act as an anti-viral agent in the addition to its anti-inflammatory and anti-oxidant properties for the adjuvant therapeutic intervention of COVID-19.
1. Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19) was caused by a novel coronavirus, officially defined as severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV2) by the World Health Organism (WHO) in February 2020 [1]. In December 2019, the first cases of SARS-CoV2 infection were reported in Wuhan City, China [2,3,4] and were rapidly spreading through the whole world by asymptomatic COVID-19 patients who traveled [1]. By February 21, 2022, more than 423 million cases of COVID-19 have been diagnosed, and more than 5.8 million of COVID-19-related deaths have been documented worldwide by WHO (URL Link: https://covid19.who.int; 21 February 2022). This pandemic is still ongoing without good control of the virus, which is due to (1) the epidemiological features of SARS-CoV2 with a very long incubation time of up to 14 days or more, (2) extended survival time of virus on inanimate material surfaces, (3) viral spread by asymptomatic patients, (4) person-to-person spread via respiratory droplets from or closely contact with COVID-19 patients [5,6,7,8,9,10], and (5) no effective or unproven therapeutic drugs available. Several kinds of vaccines have been reported to reduce on morbidity and mortality. Currently, new cases of COVID-19 patients are still increasing every day and have already caused tremendous burdens to health, social, and economic systems [11].
This pandemic is an unprecedented disaster unlike any we have ever faced in the past several decades, and it presents a significant challenge for us to explore effective interventional strategies for the treatment/prevention of SARS-CoV2 infection. Although approximately more than 20 different therapeutic drugs have been investigated for use in the treatment of COVID-19 in current clinical practices [1,12,13,14], there is no proven, effective therapeutic treatment available. However, all these therapeutic treatments are still under assessment.
2. SARS-CoV2 Virus and Its Pathogenesis
SARS-CoV2 is a novel β-coronavirus, which was discovered in COVID-19 patients in Wuhan, China, in December 2019 [4]. The SARS-CoV2 virus belongs to the sub-family Coronavirrinase in the family of Coronaviridase [15,16] and it is a large enveloped virus with non-segmented, single-strand, and positive-sense RNA genome [15,16]. It has been found that SARS-CoV2 virus has approximately 30,000 nucleotides, encoding about 29 proteins, namely 4 structural proteins (spike glycoprotein, membrane, envelope, and nucleocapsid), 16 non-structural proteins including RNA-dependent RNA polymerase, 3 chymotrypsin-like protease, hemagglutinin-esterase essential for viral replication life cycle, and 9 accessory proteins [12,15,16]. Similar to other novel coronaviruses, such as SARS-CoV discovered in China, 2003 and Middle East respiratory syndrome coronavirus (MERS-CoV) discovered in 2012 [17,18], SARS-CoV2 acts as a primary respiratory pathogen and causes acute respiratory distress syndrome (ARDS). It has been reported that SARS-CoV2 has 70–80% similarity of RNA sequence homology to SARS-CoV and 50–70% similarity to MERS-CoV [13,19,20,21]. All these novel coronaviruses are generally transmitted in humans and animals, and result in ARDS with a high mortality rate.
Similar to SARS-CoV, MERS-CoV, and other coronaviruses, SARS-CoV2 enters host cells through receptor-mediated endocytosis [22,23,24,25,26,27]. Once it releases from the endosome to the cytoplasm, the virus replicates rapidly in the cytoplasm as virions, which are distributed from contaminated cells to other cells by exocytosis, leading to the death of infected cells [22,23,24,25,26,27]. Currently, it has been discovered that SARS-CoV2 virus primarily enters into host cells with direct binding of the viral structural trans-membrane spike (S) glycoprotein to the peptide domain of angiotensin-converting enzyme 2 (ACE2) in cells such as airway epithelial cells [2,3,23]. Human ACE2 protein has been considered as the cellular receptor for the SARS-CoV and SARS-CoV2 viruses [1,27]. Moreover, the host cluster of differentiation 26 (CD26) has been also considered as a potential receptor for SARS-CoV2 virus, as discussed recently [28].
It has been noted that these novel coronaviruses may inhibit the early immune defense system with anti-viral innate cytokines in the body through a viral immune evasion mechanism by the downregulation of MHC molecule expression and inhibition of MHC molecule recognition. This would induce unlimited virus replication cycle and infection amplification, which entails an over-active immune response and the delayed and substantially sustained activation of inflammatory cytokine/chemokine responses at the later stage, eventually causing hyper-inflammation, cytokine storm, and viral sepsis in severe or critical conditions of the patients with novel coronavirus infections [13,29,30,31]. Early and sufficient control of viral replication and pathogen elimination by an innate and adaptive immune system in the body would be a potential target of therapeutic intervention for the prevention/treatment of COVID-19 infection. Zinc is a known immune modulator and can act as an anti-inflammatory and anti-oxidant agent in humans. Several clinical trials indicate that zinc treatment reduces the symptoms and duration of acute respiratory infections in humans. These data suggest that zinc may have a great implication in COVID-19. We will discuss more details of zinc and COVID-19 later in Section 5, Section 6, Section 7 and Section 8.
3. Clinical Manifestation, Activated Cytokine Storm, and Treatment
COVID-19 is typically transmitted by air droplets expelled from patients including asymptomatic patients via close person-to-person contact. Typically, the incubation time of this disease typically ranges from 2–14 days, with an average of 5 days even though some cases have longer than 14 days of incubation [1,32,33]. COVID-19 has a wide range of clinical manifestations from asymptomatic, mild, moderate, severe, or critically ill such as acute lung damage, ARDS, viral sepsis, and even death resulting from respiratory failure or multi-organ failures including cardiovascular, renal, and liver failures. The severity of this disease is associated with high risk for patients over the age of 60 and those with a history of obesity, diabetes mellitus, hypertension, cancers, immune compromised conditions [13,19,28,34,35].
Clinical manifestations of COVID-19 patients have been reported in many different clinical studies, as summarized in Table 1 [12,36,37,38]. The degree of medical conditions from mild to severe or critical illness in COVID-19 patients has been reportedly linked with the excessive systemic immune and inflammatory response that entails an activated cytokine storm with a significant increase in inflammatory cytokines/chemokines and molecules, that eventually lead to acute lung damage, ARDS, respiratory failure, multiple organ dysfunctions, or even death [39]. It has been known that this hyper-inflammatory syndrome causes life-threatening ARDS in COVID-19 patients. ARDS patients have a hyper-inflammatory response that is characteristic of lymphopenia, elevated levels of C-reactive protein (CRP), inerleukine-6 (IL-6), fibrinogen, ferritin, and other inflammatory cytokine/chemokines and molecules [39].

Table 1.
Characteristics of clinical manifestations in COVID-19 patients.
It has been noted that the leading mortality cause of COVID-19 is acute respiratory failure resulting from ARDS, which is highly linked with the activated cytokine storm due to aberrant inflammatory response by a positive feedback loop induction pattern in the body [1,40]. The activated cytokine storm has been most commonly found in patients due to the hyper-inflammatory response to the acute infection of certain types of microbial pathogens including SARS-CoV, MERS-CoV, and SARS-CoV2 viruses [1,40]. Excessive immune and inflammatory responses are widely believed to be the main driver of pathogenesis of ARDS in SARS-CoV, MERS-CoV, and SARS-CoV2 infections [41,42,43].
The characteristics of an activated cytokine storm include abnormal levels of pro-inflammatory cytokines/chemokines and molecules such as IL-1, IL-2, IL-6, IL-7, IL-8, IL-10, IL-12, MCP-1, macrophage inflammatory protein-1 α (MIP-1A), HGF, granulocyte- macrophage colony stimulating factor (GM-CSF), interferon inducible protein-10 (IP-10), CRP, and TNF-α [40,43]. Specifically, evidence shows that ARDS-related deaths have been linked with a significant increase in levels of IL-6 and CRP [40]. Moreover, recent observation studies demonstrate that COVID-19 patients with severe illness had significantly increased levels of inflammatory cytokines/chemokines such as IL-1, IL-2, IL-6, IL-8, IL-10, MCP-1, and TNF-α [1,40,43,44]. Among all the cytokine/chemokines and molecules, IL-1, IL-6, CRP, and TNF-α are commonly considered key inflammatory markers in the acute phase response of infections.
Importantly, suppressed cellular immune functions such as hypoalbuminemia, lymphopenia, neutropenia, spleen atrophy, and lymph node necrosis have been observed in COVID-19 patients. Moreover, sustained and substantial reduction of subsets of T lymphocytes such as CD4+ helper and CD8+ cytotoxic T lymphocytes have also been found in COVID-19 patients [43]. It has been noted that the reduction of CD4+ and CD8+ T cells is related to pro-inflammatory cytokine/chemokine production [21,42,45]. A significant reduction of CD8+ cytotoxic T lymphocytes, one subset of the key viral killer cells, suggests that COVID-19 patients have dysfunction in viral clearance in the body. Therefore, aberrant immune response, excessive inflammatory response/activated cytokine storm, and suppressed cellular immune function contribute to the pathogenesis of COVID-19 [1,43,44]. However, the pathogenesis of COVID-19 is not fully understood yet.
As mentioned earlier, the severity of COVID-19 is highly linked with super-production of inflammatory cytokines and chemokines, resulting from exacerbation of the cytokine/chemokine cascade in a positive feedback pattern. The aberrant immune response and hyper-inflammation leads to the activated cytokine storm as well as the suppression of cellular immune functions, which leads to uncontrolled viral replication via a viral evasion mechanism in the body, and eventually contributes to severe lung and other tissue damage [1,14,46]. Inhibition of the virus and inflammation as well as the recovery of normal immune function seems more important. Unfortunately, there is no adequate, target-specific therapeutic drugs, which have been approved to be effective for the treatment of COVID 19. Most patients recover within 1–2 weeks without any treatment, but patients with mild symptoms are provided with supportive care or adjunctive treatment. For patients with moderate, severe, or critical illness such as ARDS, a wide variety of repurposed and experimental therapeutic treatments of antiviral drugs such as remdesivir, favipiravir, ribavirin, lopinavir, ritonavir; hydroxychloroquine, and azithromycin; anti-inflammatory drugs such as corticosteroids and cytokine inhibitors; ACE2/angiotensin receptor inhibitors, or convalescent plasma therapy in addition to supportive care and symptomatic therapy are recommended in current clinical practices [1,12,13,14,46]. Other adjuvant treatment including antioxidants and vitamins are also recommended for these patients [1,12,13,14,47]. It has been estimated that more than 15 different anti-viral drugs, and more than 10 different anti-inflammatory drugs have been used in clinical trials, as reviewed recently [1,12,13,14,47]. However, the efficacy of all the currently used drugs for COVID-19 is still under investigation.
4. Zinc and Immunity
Zinc is an essential trace mineral element in the body and widely considered as an immune modulator. Evidence shows that zinc plays a wide range of biochemical functions by the involvement of many different biological processes including cell proliferation and differentiation, cell cycle, reactive oxygen species (ROS) homeostasis, and immune response [48,49]. Zinc has been identified to be required for the stabilization of 3D structures of more than 2000 different zinc finger proteins including transcription factors, steroid receptors, hormones, and enzymes in humans [48,49]. Cysteine 2-histidine 2 (C2H2) rich zinc finger domains are involved in regulating expressions of a wide variety of genes encoding growth factors, receptors, immune response mediators, pro-inflammatory cytokines/chemokines, and other proteins by binding DNA, RNAs, and proteins [48,49]. It is noted that viral zinc homeostasis has an important role in viral replications, depending on the context of the viruses. The passive release of bound zinc from the viral zinc finger or zinc containing proteins, termed zinc ejection, has been reported to be associated with the modulation of virial activities. Zinc ejection is caused by the binding of some compounds to viral zinc finger proteins due to redox reactions, resulting in destabilization of these zinc finger proteins, eventually contributing to the inhibition of viral activity. For example, in vitro studies show that zinc ejectors such as disulfide benzamide compounds can bind to HIV nucleocapsid protein (NCp7, a zinc finger protein essential for viral replication), and cause zinc ejection, resulting in the structural destabilizations of this protein, eventually contributing to the inhibition of HIV viral activity [50,51]. Similar results of zinc ejection have been found to be associated with other benzamide compounds by targeting other zinc finger protein in HPV and HCV viruses [52,53]. The addition of zinc may reverse these effects [54]. The phenomenon of zinc ejection has not been reported in novel coronaviruses, which may have different kinds of viral zinc homeostasis. Evidence shows that zinc ionophore compounds such as HCQ can increase the binding of zinc to viral enzymes such as RdRp and 3CL, which contain zinc binding sites, resulting in the inhibition of viral activity in novel coronaviruses. The details of how zinc modulates RdRp and 3CL in novel coronaviruses including COVID-19 virus will be discussed in Section 8.1 and Section 8.2.
In the 1960′s, Prasad and his team were the first to report zinc deficiency syndrome in the Middle East. The clinical manifestations of this syndrome included growth retardation, anemia, hypogonadism in male patients, and immune response dysfunctions such as increased infections [55]. It was found that this syndrome was caused by diets rich in organic phosphate compounds called as phytates, which impairs the bioavailability of zinc in the body. Iron (Fe) treatment only corrected anemia, while zinc treatment corrected all these symptoms [55]. These early findings strongly suggest that zinc had potential as an essential nutrient in the human body. Subsequently, a large number of experimental and human studies have provided solid evidence to support the essentiality of zinc as a trace mineral nutrient in the human body [56,57,58].
Zinc is generally recognized as an essential immune mediator to maintain normal immune defense mechanism against microbial infection in the body. Zinc deficiency has been found to increase infections of a variety of micro-organisms such as viruses, bacterial fungus, and parasites [48,58,59,60]. A large number of experimental and human studies have provided clear evidence to support that zinc plays an essential role through modulation of cellular immune responses including natural killer cells, T lymphocytes, and B lymphocytes [48,57,58,59,60]. For example, zinc can increase NK cell activity, T cell activation, mitogenesis, and antibody synthesis. Our early experimental studies revealed that dietary restriction-induced zinc depletion reduced the relative level of CD8+/CD73+ T lymphocytes in volunteer subjects [61,62]. This subset of T cells is considered as the main precursor of cytolytic T lymphocytes. CD73+ cell surface protein is required in antigen recognition on these cells [57,62]. Furthermore, zinc deficiency causes thymic atrophy along with the down-regulation of thymulin, a zinc sensitive thymic hormone for the regulation of T cell proliferation and differentiation as well as NK cell activation [57,63]. Early experimental studies also revealed that zinc deficient condition (1 μM zinc) suppressed the expressions of IL-2, IL-2 receptor (IL-2R), and IFN-γ in HUT-78 cells (a malignant lymphoid leukemia cell), as compared to zinc sufficient condition (15 μM zinc) [64,65,66,67]. All these findings suggest zinc’s important role in cellular immunity. However, it has been noted that high levels or toxic levels of zinc impair immunity, which is similar to the effect of zinc deficiency on immunity. For example, zinc deficient (1 μM) and zinc toxic (100 μM) conditions decreased the expressions of IL-2, IL-2R-α, and TNF-α in HUT-78 cells, as compared to zinc sufficient or physiological conditions (15 μM). Other documents also support this scenario, in which toxic level of zinc impairs normal immunity [68]. Therefore, zinc homeostasis seems be critical for the maintenance of normal immunity in the body.
Additionally, emerging numbers of studies have indicated that zinc reduces inflammatory cytokines/chemokines such as TNF-α, IL-1β, IL-6, MCP-1, vascular cell adhesion molecule (VCAM), and CRP as well as oxidative stress markers such as lipid peroxidation products (MDA + HAE) and DNA intermittent oxidation product (8-OHdG) [64,69]. Zinc supplementation reduces the production of inflammatory cytokines/chemokines and oxidative stress markers along with the reduction of infection incidence in children, elderly subjects, and sickle cell anemia (SCD) patients [69,70,71,72,73], which clearly suggest that zinc may act as a potent anti-oxidant and anti-inflammatory agent, potentially through the regulation of multiple molecular and cellular mechanisms including A20, NF-κB, MT, zinc transporters, TTP, and PPAR, as reviewed recently [56,74].
As discussed earlier, COVID-19 pathogenesis is closely linked with the dysregulation of immune-inflammatory response in the body. Any candidate drug or agent that relieves patient symptoms using anti-viral drugs may prolong the survival time of patients and may provide a chance for the recovery of the immune defense system leading to the eradication of the virus in patients. Since zinc is non-toxic, non-expansive, readily available, and plays an important role as an immune modulator, its application in COVID-19 may provide a clinical, cost-effective benefit to facilitate a meaningful recovery with a better prognosis.
5. Zinc Deficiency in Acute Respiratory Infection
As mentioned earlier, the alteration of zinc homeostasis or zinc deficiency in the body is highly linked with infections of micro-organisms such as bacteria, funguses, and viruses including respiratory virus infections [49,56]. Zinc deficiency increases infection incidence while zinc supplementation corrects this adverse effect [49,56]. For example, a new study reports that zinc deficiency increased incidence rate of acute upper and lower respiratory infection and mean duration of disease in children, compared to non-deficient groups. Zinc supplementation (20 mg zinc daily; 2 weeks) to these zinc deficient children significantly reversed these adverse effects during a 6-month follow-up period [75], which suggests that zinc may play a protective role in acute virus infection.
6. Zinc Homeostasis in COVID-19 Infection
It is interesting that zinc status in the body is also linked with the severity of COVID-19 infection. It has been reported that pregnant female patients with COVID-19 infection have a lower level of serum zinc during pregnancy, when compared to the non-infected pregnant control women. Zinc level is negatively linked with acute phase markers including IL-6 [76]. Another prospective study reveals that COVID-19 patients have a significant lower level of zinc when compared to the non-infected control group (serum zinc: 74.5 μg/dL vs. 105.8 μg/dL) [77]. A level of 89 μg/dL of serum zinc or less has been considered as zinc deficiency while 90 μg/dL or more considered as zinc sufficiency or normal range in the body [71,78]. Moreover, zinc deficiency has been found to be correlated to the severity of COVID-19 infection such as higher rates of complications, ARDS, steroid therapy, prolong stay of hospitalization, and death [77]. These findings may explain why elderly populations, who are susceptible to being zinc deficient, have a significantly higher risk of COVID-19 morbidity and mortality, as reviewed recently [79]. However, the detailed role of zinc homeostasis in the pathogenesis of COVID-19 is not fully elucidated.
7. Zinc Treatment/Supplementation for Virus Infections in COVID-19
It has been widely known that the application of zinc has displayed a clinical benefit in human health and diseases, including in children and elderly subject, and for SCD, cancers, and diabetes [70,80,81,82,83,84]. Accumulating numbers of experimental and clinical studies have provided clear evidence that zinc plays an important role in anti-viral immunity by inhibiting viral replication cycle, RNA synthesis, and protein processes in several viral infections including human immunodeficiency virus-1 (HIV-1), herpes simples virus, human papilloma virus, hepatitis C virus, coronavirus, and varicella-zoster virus, which has been reviewed recently [85], suggesting that zinc may be used for COVID-19 treatment. For example, our early clinical trial study revealed that zinc treatment (13 mg zinc as gluconate zinc for every 2–3 h daily) significantly reduced the overall duration of rhinovirus upper respiratory infection (common cold) (4.0 vs. 7.1 days) in human adults, compared to the placebo-controlled patients. The severity of the disease was significantly decreased in the zinc treatment group [86,87]. Similar findings were observed by other groups [85,88,89,90]. We also observed the mean change in plasma levels of soluble IL-1 receptor agonist (sIL-1ra) in the zinc treatment group whereas there was no change found in the placebo-controlled group. The mean change in plasma level of sICAM showed a significant reduction in the zinc treatment group [87]. One recent meta-analysis including three randomized placebo-controlled trial studies with 199 common cold patients confirmed that the high dose of zinc treatment displays a therapeutic benefit for the treatment of rhinovirus infection in humans [91]. These findings suggest that zinc treatment reduces the severity of rhinovirus infection along with the up-regulation of sIL-1ra, an endogenous IL-1 receptor inhibitor and inhibition of sICAM, an inflammatory cytokine acting as a viral entry receptor.
Moreover, one clinical trial demonstrated that the combined treatment of HCQ, azithromycin, and zinc decreased the rates of hospital admission (2.8% vs. 15.4%) and death (0.7% vs. 3.4%) of COVID-19 patients, in comparison to untreated control patients [92,93]. It has been reported that the administration of HCQ and azithromycin did not show a rapid anti-viral clearance and any clinical benefit in COVID-19 patients with severe illness [94] even though both drugs have been considered as anti-viral agents for COVID-19 treatment. It is interesting that CQ has been found to act as a zinc-specific ionophore, which enhances cellular zinc bio-availability in vitro. Meanwhile, zinc can increase CQ-induced cytotoxicity, leading to apoptosis of cells in vitro [95]. Therefore, these findings from such triple administrations with zinc suggest that zinc might provide an important role in anti-viral immunity for COVID-19 treatment. According to personal experience, 10 days of treatment of 200 mg HCQ plus 50 mg zinc twice a day can cure symptomatic COVID-19 patients who are over the age of 90, which suggests an important role of zinc in COVID-19 patients who receive HCQ therapy. Moreover, several more clinical trial studies have revealed that zinc or the combination of HCQ with zinc have positive clinical outcomes in the treatment of COVID-19, as summarized in Table 2 [92,93,96,97,98,99,100,101,102],. These data support the positive role of zinc in COVID-19 treatment. The exact mechanism of synergic effect of zinc with HCQ/CQ for COVID-19 treatment is still not fully understood. Currently, more than 18 clinical trials with different doses (ranging from 6 mg to 440 mg) of zinc and treatment periods of time (from 5 days to 2 months) have been documented for the treatment/prevention of COVID-19 as recently reviewed [103,104]. The effectiveness of zinc for COVID-19 is still under investigation.

Table 2.
Clinical outcomes or benefits of zinc treatment in COVID-19.
The beneficial role of zinc in the prophylaxis or prevention of COVID-19 infection has also received a lot of attention. A case-control study in primary care centers was conducted to examine the effectiveness of zinc supplementation on COVID-19 prophylaxis and treatment. A total of 104 adult volunteers received 15–50 mg zinc for at least 4 weeks as the experimental group while 96 adult volunteers received placebo as the control. The results indicate that zinc supplemented group had significantly lower incidence of symptomatic COVID-19 infection, compared to the placebo group [103]. One prospective, randomized, double-blind study reports that supplementation of doxycycline with zinc (15 mg zinc, 6 weeks) significantly reduces the incidence of COVID-19 infection in health care workers [104]. Another randomized study (open-label) reports that 42 days of supplementation of zinc (80 mg) and vitamin C (500 mg) reduces the incidence of COVID-19 in young healthy subjects (mean age 33 years), compared to control subjects who only received vitamin C (47.3% vs. 70%) [105]. These findings strongly suggest that zinc may play a beneficial role in prophylaxis. However, a well-designed study is required to investigate the detailed role of zinc in prophylaxis of COVID-19 infection.
9. Adverse Effect of Zinc Treatment/Supplementation
Generally, zinc is nontoxic, which is similar to other trace mineral nutrients in the body. The main source of zinc intake by humans is from foods such as meats, fish, eggs, seeds, seafood, certain vegetables, whole cereal, and beans [182,183]. The recommended daily allowance (RDA) of zinc is 11 mg for men, and 8 mg for women in the US [184,185]. The median lethal dose (LD50) is estimated to be 27 g for humans based on animal studies [68], which is remarkably lower than the LD50 value of zinc. Between 15–50 mg of zinc supplementation for over-extended periods of time has been used in different populations including elderly subjects and SCD patients without any adverse effect observed [69,71,78,186].
It has been identified that high dose of zinc intake may cause adverse gastrointestinal effects such as abdominal pain, nausea, and vomiting, which is often tolerated. For example, in our early human subject studies, we did not observe any adverse effects in elderly subjects and SCD patients who followed 45 mg of oral zinc intake daily for 6–12 months [71,78]. In our zinc clinical trials, we also did not observe any adverse effects in patients with the common cold who followed high dose of zinc treatment (13 mg zinc, 7 times daily, equivalent to 91 mg zinc daily) for more than 11 days, compared to the placebo-control patients [86,87], which is consistent with the findings from other reports [89,90]. However, one clinical trial shows minor side effects of nausea and bad-taste reaction following higher dose of zinc treatment (13.3 mg zinc every 2 h while awake daily) for therapy of the common cold. Furthermore, high dose of zinc-induced copper deficiency syndrome has received a great amount of attention [68,187,188].
Several animal reports indicate that high dose of zinc intake over an extended period of time is frequently linked with high risk of copper deficiency in rats and mice [189,190], which is induced by a competitive absorption between zinc and copper in the intestinal mucosa [189,190,191,192]. Several human case reports confirmed these findings [193,194,195]. For example, one SCD patient took therapeutic dose of zinc (150 mg daily) during hospitalization followed by 110 mg of zinc treatment for more than one year, which later led to the development of copper deficiency syndrome like lower levels of serum copper and anemia. This zinc-induced copper deficiency was easily corrected by the treatment of 1 mg copper daily for 2 weeks [195]. Similar findings were reported in another case of the Hallervorden-Spatz syndrome patient who took a therapeutic dose of zinc (100 mg) daily for more than five years [194]. Conversely, one human subject study demonstrated that 26 of 47 healthy volunteers had symptoms of abdominal cramp, nausea, and vomiting following the supplementation of 150 mg zinc daily for six weeks [196]. Plasma copper level was not significantly altered [196]. After 12 weeks of zinc supplementation, only female volunteers had a 14% reduction of serum ceruloplasmin (from 13.0 to 11.3 U/mL) [197]. These adverse effects were related to lower body weight [197]. These data suggest that zinc is safe when 50 mg or less daily is used for long term supplementation while 50–150 mg daily for a short term is safe for therapeutic purpose.
In one early AREDS study supported by the National Institute of Health/the National Eye Institute in the USA, which examined the effect of antioxidants and zinc on age-related macular degeneration (AMD) in the elderly subjects, 2 mg of copper daily was added to the zinc supplemented group (80 mg zinc daily) in order to prevent copper deficiency development. This study was performed for more than 6 years without any adverse effects observed in the zinc supplemented group [198]. These findings suggest that copper deficiency induced by high dose of zinc administration can be prevented by the co-supplementation of 2 mg copper with zinc. The copper status in the body may be required for monitoring based on the dose of zinc and the period of time when it is given. Generally, zinc is nontoxic, and its common adverse effects usually include gastric discomfort, nausea, and vomiting. The detrimental effect of copper deficiency induced by a large amount of zinc supplementation/treatment would be preventable by the co-administration of 2 mg of copper.
10. Conclusive Remark and Future Prospective
In summary, the COVID-19 pandemic was caused by a novel coronavirus SARS-CoV2, which is still ongoing with a high morbidity and mortality rate. The pathogenesis of COVID-19 is highly linked with suppressed cellular immune function, aberrant immune response, and hyper-inflammation, which leads to an activated cytokine storm and results in ARDS. The higher levels of key inflammatory cytokines/chemokines including IL-1, IL-6, TNF-α, MCP-1, and CRP are highly linked with severity of the disease. Dysregulations of T cells including CD4+ and CD8+ T cells are reported in COVID-19 and are linked with the high risk of severity of this disease.
To date, there is no proven and effective therapeutic treatment for COVID-19, which provides a significant challenge in exploring a better therapeutic strategy for the treatment of this disease. It is known that zinc is an essential trace mineral element in human health by the involvement of a wide variety of biological processes like the innate and adaptive immune responses. The application of zinc as an anti-inflammatory and anti-oxidant agent has been on the rise for use in human health and disease. Early clinical data showed that the application of zinc can decrease the severity of viral infections including rhinovirus infection (the common cold) in human adults and the incidence of infection in children and elderly subjects through down-regulation of inflammatory cytokines/chemokines and oxidative stress markers.
New evidence reveals that the triple combination of HQC, azithromycin, and zinc reduces rates of hospital admission and death of COVID-19. These findings suggest that zinc may have anti-viral activity that could be applied for treatment of viral infections including COVID-19 but its detailed mechanism is not fully understood. Despite this, the results from increasing numbers of studies suggest that zinc may have an inhibitory effect on some viral infections including SARS-CoV2, potentially through the regulation of viral RdRp and 3CL protease proteins as well as host IFNs, IL-6, ICAM cytokines, NK cells, and CD4+/CD8+ T cells as shown in Figure 1. Currently, it has been reported that more than 18 clinical trials using zinc with a wide range of doses and treatment periods are registered in current clinical practice. Therefore, the application of zinc as a potent anti-viral agent along with its anti-inflammatory and anti-oxidant properties may provide meaningful clinical benefit for the treatment of COVID-19. Well-designed clinical trials are required to further understand the clinical benefit of zinc as an anti-viral agent for the treatment and/or prevention of COVID-19 infection in the future.
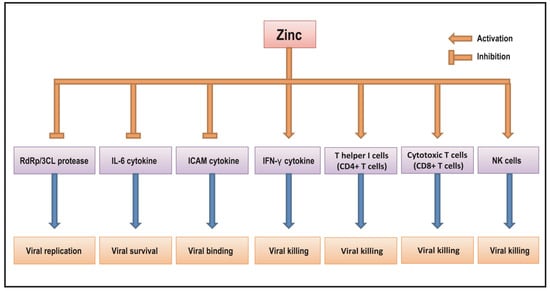
Figure 1.
The putative mechanism of zinc action as an anti-viral agent in COVID-19.
Author Contributions
Conceptualization, A.S.P.; writing—original draft preparation, B.B.; writing—review and editing, A.M., G.B. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Ananda S. Prasad contributed to concept designs; Agnes Malysa and Bin Bao contributed to preparation of manuscript draft; Gerold Bepler and Andrew Fribley contributed to manuscript editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020, 92, 441–447. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Monpara, J.D.; Sodha, S.J.; Gupta, P.K. COVID-19 associated complications and potential therapeutic targets. Eur. J. Pharm. 2020, 886, 173548. [Google Scholar] [CrossRef]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Kar, S.; Aliter, K.F. Potential Anti-COVID-19 Therapeutics that Block the Early Stage of the Viral Life Cycle: Structures, Mechanisms, and Clinical Trials. Int. J. Mol. Sci. 2020, 21, 5224. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin. Immunol. 2020, 215, 108448. [Google Scholar] [CrossRef]
- Di, F.S.; Alfieri, A.; Petrou, S.; Damiani, G.; Passavanti, M.B.; Pace, M.C.; Leone, S.; Fiore, M. Current status of COVID-19 treatment: An opinion review. World J. Virol. 2020, 9, 27–37. [Google Scholar]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020, 19, 100682. [Google Scholar] [CrossRef]
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Chan, J.F.; Lau, S.K.; Woo, P.C. The emerging novel Middle East respiratory syndrome coronavirus: The “knowns” and “unknowns”. J. Formos Med. Assoc. 2013, 112, 372–381. [Google Scholar] [CrossRef]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Farahani, F.; Khodadadi, I.; Tayebinia, H. Diagnosis and treatment of coronavirus disease 2019 (COVID-19): Laboratory, PCR, and chest CT imaging findings. Int. J. Surg. 2020, 79, 143–153. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Haji, A.M.; Moradi-Kalbolandi, S.; Zarei, M.; Bose, D.; Majidzadeh, A.; Farahmand, L. Potential role of interferons in treating COVID-19 patients. Int. Immunopharmacol. 2021, 90, 107171. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Kao, R.Y.; Zhou, Y.; He, Y.; Zhao, G.; Wong, C.; Jiang, S.; Yuen, K.Y.; Jin, D.Y.; Zheng, B.J. Cleavage of spike protein of SARS coronavirus by protease factor Xa is associated with viral infectivity. Biochem. Biophys Res. Commun. 2007, 359, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Li, W.; Choe, H.; Farzan, M. Angiotensin-converting enzyme 2: A functional receptor for SARS coronavirus. Cell Mol. Life Sci. 2004, 61, 2738–2743. [Google Scholar] [CrossRef]
- Simmons, G.; Reeves, J.D.; Rennekamp, A.J.; Amberg, S.M.; Piefer, A.J.; Bates, P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. USA 2004, 101, 4240–4245. [Google Scholar] [CrossRef]
- Millet, J.K.; Whittaker, G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA 2014, 111, 15214–15219. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Zhang, F.; Zhang, L.; Li, L. COVID-19 in Elderly Adults: Clinical Features, Molecular Mechanisms, and Proposed Strategies. Aging Dis. 2020, 11, 1481–1495. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Cameron, M.J.; Bermejo-Martin, J.F.; Danesh, A.; Muller, M.P.; Kelvin, D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 2008, 133, 13–19. [Google Scholar] [CrossRef]
- Min, C.K.; Cheon, S.; Ha, N.Y.; Sohn, K.M.; Kim, Y.; Aigerim, A.; Shin, H.M.; Choi, J.Y.; Inn, K.S.; Kim, J.H.; et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016, 6, 25359. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Chen, P.L.; Lee, N.Y.; Cia, C.T.; Ko, W.C.; Hsueh, P.R. A Review of Treatment of Coronavirus Disease 2019 (COVID-19): Therapeutic Repurposing and Unmet Clinical Needs. Front. Pharmacol. 2020, 11, 584956. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Inter. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Liu, L.; Zhao, X.A.; Zhang, Z.; Xue, L.; Yan, X.; Huang, S.; Li, Y.; Cheng, J.; et al. Overweight and Obesity are Risk Factors of Severe Illness in Patients with COVID-19. Obesity 2020, 28, 2049–2055. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Wang, Z.; Liu, C.; Long, F.; Jin, P. Relation between blood glucose and the prognosis of severe coronavirus disease 2019. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 530–535. [Google Scholar]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Ohadian, M.S. A Review on Currently Available Potential Therapeutic Options for COVID-19. Int. J. Gen. Med. 2020, 13, 443–467. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical, biochemical, and pharmacological role of zinc. Annu. Rev. Pharmacol Toxicol. 1979, 19, 393–426. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc deficiency in human subjects. Prog. Clin. Biol. Res. 1983, 129, 1–33. [Google Scholar]
- Loo, J.A.; Holler, T.P.; Sanchez, J.; Gogliotti, R.; Maloney, L.; Reily, M.D. Biophysical characterization of zinc ejection from HIV nucleocapsid protein by anti-HIV 2,2′-dithiobis [benzamides] and benzisothiazolones. J. Med. Chem. 1996, 39, 4313–4320. [Google Scholar] [CrossRef] [PubMed]
- Tummino, P.J.; Scholten, J.D.; Harvey, P.J.; Holler, T.P.; Maloney, L.; Gogliotti, R.; Domagala, J.; Hupe, D. The in vitro ejection of zinc from human immunodeficiency virus (HIV) type 1 nucleocapsid protein by disulfide benzamides with cellular anti-HIV activity. Proc. Natl. Acad. Sci. USA 1996, 93, 969–973. [Google Scholar] [CrossRef]
- Rehman, A.U.; Zhen, G.; Zhong, B.; Ni, D.; Li, J.; Nasir, A.; Gabr, M.T.; Rafiq, H.; Wadood, A.; Lu, S.; et al. Mechanism of zinc ejection by disulfiram in nonstructural protein 5A. Phys. Chem. Chem. Phys. 2021, 23, 12204–12215. [Google Scholar] [CrossRef]
- Beerheide, W.; Bernard, H.U.; Tan, Y.J.; Ganesan, A.; Rice, A.W.; Ting, A.E. Potential Drugs Against Cervical Cancer: Zinc-Ejecting Inhibitors of the Human Papillomavirus Type 16 E6 Oncoprotein. J. Natl. Cancer Inst. 1999, 91, 1211–1220. [Google Scholar] [CrossRef]
- Shiah, S.G.; Kao, Y.R.; Wu, F.Y.H.; Wu, C.W. Inhibition of Invasion and Angiogenesis by Zinc-Chelating Agent Disulfiram. Mol. Pharmac. 2003, 64, 1076–1084. [Google Scholar] [CrossRef]
- Prasad, A.S.; Miale, A., Jr.; Farid, Z.; Sandstead, H.H.; Schulert, A.R. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J. Lab Clin. Med. 1963, 61, 537–549. [Google Scholar]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133 (Suppl. S1), 1452S–1456S. [Google Scholar] [CrossRef]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc and immunity. Mol. Cell Biochem. 1998, 188, 63–69. [Google Scholar] [CrossRef]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll Nutr. 2009, 28, 257–265. [Google Scholar] [CrossRef]
- Beck, F.W.; Prasad, A.S.; Kaplan, J.; Fitzgerald, J.T.; Brewer, G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997, 272, E1002–E1007. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.W.; Kaplan, J.; Fine, N.; Handschu, W.; Prasad, A.S. Decreased expression of CD73 (ecto-5′-nucleotidase) in the CD8+ subset is associated with zinc deficiency in human patients. J. Lab. Clin. Med. 1997, 130, 147–156. [Google Scholar] [CrossRef]
- Prasad, A.S.; Dardenne, M.; Abdallah, J.; Meftah, S.; Brewer, G.J.; Bach, J.F. Serum thymulin and zinc deficiency in humans. Trans. Assoc. Am. Physicians 1987, 100, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Godmere, M. Zinc modulates mRNA levels of cytokines. Am. J. Physiol. Endocrinol Metab. 2003, 285, E1095–E1102. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Bao, G.W.; Singh, T.; Ali, S.; Sarkar, F.H. Intracellular free zinc up-regulates IFN-gamma and T-bet essential for Th(1) differentiation in Con-A stimulated HUT-78 cells. Biochem. Biophys Res. Commun. 2011, 407, 703–707. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Sarkar, F.H. Zinc activates NF-kappaB in HUT-78 cells. J. Lab. Clin. Med. 2001, 138, 250–256. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Sarkar, F.H. Zinc enhances the expression of interleukin-2 and interleukin-2 receptors in HUT-78 cells by way of NF-kappaB activation. J. Lab. Clin. Med. 2002, 140, 272–289. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essentia.l toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Snell, D.; Suneja, A.; Sarkar, F.H.; Doshi, N.; Fitzgerald, J.T.; Swerdlow, P. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl. Res. 2008, 152, 67–80. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Kucuk, O.; Sarkar, F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004, 37, 1182–1190. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Bhan, M.K.; Bhandari, N.; Sinha, A.; Jalla, S. Zinc supplementation in young children with acute diarrhea in India. N. Engl. J. Med. 1995, 333, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Sazawal, S.; Black, R.E.; Jalla, S.; Mazumdar, S.; Sinha, A.; Bhan, M.K. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: A double-blind, controlled trial. Pediatrics 1998, 102, 1–5. [Google Scholar] [CrossRef]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Khera, D.; Singh, S.; Purohit, P.; Sharma, P.; Singh, K. Prevalence of Zinc Deficiency and the Effect of Zinc Supplementation on the Prevention of Acute Respiratory Infections. Turk. Thorac. J. 2020, 21, 371–376. [Google Scholar] [CrossRef]
- Anuk, A.T.; Polat, N.; Akdas, S.; Erol, S.A.; Tanacan, A.; Biriken, D.; Keskin, H.L.; Moraloglu, T.O.; Yazihan, N.; Sahin, D. The Relation between Trace Element Status (Zinc, Copper, Magnesium) and Clinical Outcomes in COVID-19 Infection During Pregnancy. Biol. Trace Elem. Res. 2020, 199, 3608–3617. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Fitzgerald, J.T.; Snell, D.; Bao, G.W.; Singh, T.; Cardozo, L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.; de Oliveira, A.R.; Marreiro, D.N. Antioxidant role of zinc in diabetes mellitus. World J. Diabetes 2015, 6, 333–337. [Google Scholar] [CrossRef]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130 (Suppl. S5), 1447S–1454S. [Google Scholar] [CrossRef]
- Prasad, A.S.; Abbasi, A.A.; Rabbani, P.; DuMouchelle, E. Effect of zinc supplementation on serum testosterone level in adult male sickle cell anemia subjects. Am. J. Hematol. 1981, 10, 119–127. [Google Scholar] [CrossRef]
- Prasad, A.S.; Kaplan, J.; Beck, F.W.; Penny, H.S.; Shamsa, F.H.; Salwen, W.A.; Marks, S.C.; Mathog, R.H. Trace elements in head and neck cancer patients: Zinc status and immunologic functions. Otolaryngol. Head Neck Surg. 1997, 116, 624–629. [Google Scholar] [CrossRef]
- Prasad, A.S.; Kucuk, O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Prasad, A.S.; Fitzgerald, J.T.; Bao, B.; Beck, F.W.; Chandrasekar, P.H. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann. Inter. Med. 2000, 133, 245–252. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Snell, D.; Fitzgerald, J.T. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J. Infect. Dis. 2008, 197, 795–802. [Google Scholar] [CrossRef]
- Mossad, S.B.; Macknin, M.L.; Medendorp, S.V.; Mason, P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann. Inter. Med. 1996, 125, 81–88. [Google Scholar] [CrossRef]
- Godfrey, J.C.; Conant, S.B.; Smith, D.S.; Turco, J.H.; Mercer, N.; Godfrey, N.J. Zinc gluconate and the common cold: A controlled clinical study. J. Int. Med. Res. 1992, 20, 234–246. [Google Scholar] [CrossRef]
- Eby, G.A.; Davis, D.R.; Halcomb, W.W. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob. Agents Chemother. 1984, 25, 20–24. [Google Scholar] [CrossRef]
- Hemilä, H.; Fitzgerald, J.T.; Petrus, E.J.; Prasad, A. Zinc Acetate Lozenges May Improve the Recovery Rate of Common Cold Patients: An Individual Patient Data Meta-Analysis. Open Forum Infect. Dis. 2017, 4, ofx059. [Google Scholar] [CrossRef] [PubMed]
- Derwand, R.; Scholz, M.; Zelenko, V. COVID-19 outpatients: Early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: A retrospective case series study. Int. J. Antimicrob. Agents 2020, 56, 106214. [Google Scholar] [CrossRef] [PubMed]
- Derwand, R.; Scholz, M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med. Hypotheses 2020, 142, 109815. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Delaugerre, C.; Le, G.J.; Mela-Lima, B.; Ponscarme, D.; Goldwirt, L.; de Castro, N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020, 50, 384. [Google Scholar] [CrossRef]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.Q. Chloroquine is a zinc ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, S.; Soliman, S.; Esmail, E.S.; Khalaf, M.; Mostafa, E.F.; Medhat, M.A.; Ahmed, O.A.; El Ghafar, M.S.A.; Alboraie, M.; Hassany, S.M. Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine?: A Randomized, Multicenter Trial. Biol. Trace Elem. Res. 2021, 199, 3642–3646. [Google Scholar] [CrossRef]
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020, 69, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Elalfy, H.; Besheer, T.; El-Mesery, A.; El-Gilany, A.H.; Soliman, M.A.; Alhawarey, A.; Alegezy, M.; Elhadidy, T.; Hewidy, A.A.; Zaghloul, H.; et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J. Med. Virol. 2021, 93, 3176–3183. [Google Scholar] [CrossRef]
- Finzi, E.; Harrington, A. Zinc treatment of outpatient COVID-19: A retrospective review of 28 consecutive patients. J. Med. Virol. 2021, 93, 2588–2590. [Google Scholar] [CrossRef]
- Frontera, J.A.; Rahimian, J.O.; Yaghi, S.; Liu, M.; Lewis, A.; de Havenon, A.; Mainali, S.; Huang, J.; Scher, E.; Wisniewski, T.; et al. Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Patel, O.; Chinni, V.; El-Khoury, J.; Perera, M.; Neto, A.S.; McDonald, C.; See, E.; Jones, D.; Bolton, D.; Bellomo, R.; et al. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J. Med. Virol. 2021, 93, 3261–3267. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Hardigan, P.C. A Case-Control Study for the Effectiveness of Oral Zinc in the Prevention and Mitigation of COVID-19. Front. Med. 2021, 8, 756707. [Google Scholar] [CrossRef]
- Stambouli, N.; Driss, A.; Gargouri, F.; Bahrini, K.; Arfaoui, B.; Abid, R.; Taamallah, K.; Hannachi, S.; Boughariou, S.; Rebai, A.; et al. COVID-19 prophylaxis with doxycycline and zinc in health care workers: A prospective, randomized, double-blind clinical trial. Int. J. Infect. Dis. 2022, 122, 553–558. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Quek, A.M.L.; Ooi, D.S.Q.; Sengupta, S.; Lakshminarasappa, S.R.; Koo, C.Y.; So, J.B.Y.; Goh, B.C.; Loh, K.S.; Fisher, D.; et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial. Int. J. Infect. Dis. 2021, 106, 314–322. [Google Scholar] [CrossRef]
- Arentz, S.; Hunter, J.; Yang, G.; Goldenberg, J.; Beardsley, J.; Myers, S.P.; Mertz, D.; Leeder, S. Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: A rapid review. Adv. Integr. Med. 2020, 7, 252–260. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2020, 199, 2882–2892. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.J.; Harman, R.J.; Bunnell, B.A.; Schreiber, M.A.; Xiang, C.; Wang, F.S.; Santidrian, A.F.; Minev, B.R. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J. Transl. Med. 2020, 18, 203–223. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Monych, N.K.; Turner, R.J. Zinc and SARS-CoV-2: A molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2021, 47, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Shao, Y.M.; Yang, W.B.; Peng, H.P.; Hsu, M.F.; Tsai, K.C.; Kuo, T.H.; Wang, A.H.; Liang, P.H.; Lin, C.H.; Yang, A.S.; et al. Structure-based design and synthesis of highly potent SARS-CoV 3CL protease inhibitors. Chembiochem 2007, 8, 1654–1657. [Google Scholar] [CrossRef]
- Hsu, J.T.; Kuo, C.J.; Hsieh, H.P.; Wang, Y.C.; Huang, K.K.; Lin, C.P.; Huang, P.F.; Chen, X.; Liang, P.H. Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett. 2004, 574, 116–120. [Google Scholar] [CrossRef]
- Weiss, S.R.; Navas-Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef]
- Lee, C.C.; Kuo, C.J.; Hsu, M.F.; Liang, P.H.; Fang, J.M.; Shie, J.J.; Wang, A.H. Structural basis of mercury- and zinc-conjugated complexes as SARS-CoV 3C-like protease inhibitors. FEBS Lett. 2007, 581, 5454–5458. [Google Scholar] [CrossRef]
- Kim, U.J.; Won, E.J.; Kee, S.J.; Jung, S.I.; Jang, H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir. Ther. 2016, 21, 455–459. [Google Scholar] [CrossRef]
- Lim, J.; Jeon, S.; Shin, H.Y.; Kim, M.J.; Seong, Y.M.; Lee, W.J.; Choe, K.W.; Kang, Y.M.; Lee, B.; Park, S.J. Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: The Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J. Korean Med. Sci. 2020, 35, e79. [Google Scholar] [CrossRef]
- Liu, F.; Xu, A.; Zhang, Y.; Xuan, W.; Yan, T.; Pan, K.; Yu, W.; Zhang, J. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020, 95, 183–191. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Samuel, C.E. Antiviral Actions of Interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef] [PubMed]
- Marié, I.; Durbin, J.E.; Levy, D.E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998, 17, 6660–6669. [Google Scholar] [CrossRef]
- De Weerd, N.A.; Samarajiwa, S.A.; Hertzog, P.J. Type I interferon receptors: Biochemistry and biological functions. J. Biol. Chem. 2007, 282, 20053–20057. [Google Scholar] [CrossRef]
- Liu, Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef]
- Hermant, P.; Michiels, T. Interferon-λ in the context of viral infections: Production, response and therapeutic implications. J. Innate Immun. 2014, 6, 563–574. [Google Scholar] [CrossRef]
- Hooks, J.J.; Wang, Y.; Detrick, B. The critical role of IFN-gamma in experimental coronavirus retinopathy. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3402–3408. [Google Scholar] [CrossRef]
- Zhou, F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int. Rev. Immunol. 2009, 28, 239–260. [Google Scholar] [CrossRef]
- Hu, Z.J.; Xu, J.; Yin, J.M.; Li, L.; Hou, W.; Zhang, L.L.; Zhou, Z.; Yu, Y.Z.; Li, H.J.; Feng, Y.M.; et al. Lower Circulating Interferon-Gamma Is a Risk Factor for Lung Fibrosis in COVID-19 Patients. Front. Immunol. 2020, 11, 585647. [Google Scholar] [CrossRef]
- Brandstadter, J.D.; Yang, Y. Natural killer cell responses to viral infection. J. Innate Immun. 2011, 3, 274–279. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, B.; Hashimoto, K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020, 87, 59–73. [Google Scholar] [CrossRef]
- Prasad, A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000, 182 (Suppl. S1), S62–S68. [Google Scholar] [CrossRef] [PubMed]
- Sahebnasagh, A.; Saghafi, F.; Avan, R.; Khoshi, A.; Khataminia, M.; Safdari, M.; Habtemariam, S.; Ghaleno, H.R.; Nabavi, S.M. The prophylaxis and treatment potential of supplements for COVID-19. Eur. J. Pharmacol. 2020, 887, 173530. [Google Scholar] [CrossRef]
- Ferguson-Smith, A.C.; Chen, Y.F.; Newman, M.S.; May, L.T.; Sehgal, P.B.; Ruddle, F.H. Regional localization of the interferon-beta 2/B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21. Genomics 1988, 2, 203–208. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. The biology and medical implications of interleukin-6. Cancer Immunol. Res. 2014, 2, 288–294. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Taga, T.; Hibi, M.; Hirata, Y.; Yamasaki, K.; Yasukawa, K.; Matsuda, T.; Hirano, T.; Kishimoto, T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell 1989, 58, 573–581. [Google Scholar] [CrossRef]
- Kallen, K.J.; zum Büschenfelde, K.H.; Rose-John, S. The therapeutic potential of interleukin-6 hyperagonists and antagonists. Expert Opin. Investig. Drugs 1997, 6, 237–266. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef]
- Thammathiwat, T.; Tungsanga, S.; Tiankanon, K.; Torvorapanit, P.; Chumpangern, W.; Udomkarnjananun, S.; Avihingsanon, Y.; Sriprasart, T.; Srisawat, N.; Jutivorakool, K.; et al. A case of successful treatment of severe COVID-19 pneumonia with favipiravir and tocilizumab in post-kidney transplant recipient. Transpl. Infect. Dis. 2020, 23, e13388. [Google Scholar] [CrossRef]
- Voraberger, G.; Schäfer, R.; Stratowa, C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5′-regulatory region. Induction by cytokines and phorbol ester. J. Immunol. 1991, 147, 2777–2786. [Google Scholar] [PubMed]
- Ramos, T.N.; Bullard, D.C.; Barnum, S.R. ICAM-1: Isoforms and phenotypes. J. Immunol. 2014, 192, 4469–4474. [Google Scholar] [CrossRef]
- Whiteman, S.C.; Bianco, A.; Knight, R.A.; Spiteri, M.A. Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J. Biol. Chem. 2003, 278, 11954–11961. [Google Scholar] [CrossRef]
- Bounou, S.; Giguere, J.F.; Cantin, R.; Gilbert, C.; Imbeault, M.; Martin, G.; Tremblay, M.J. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 2004, 18, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Winther, B.; Arruda, E.; Witek, T.J.; Marlin, S.D.; Tsianco, M.M.; Innes, D.J.; Hayden, F.G. Expression of ICAM-1 in nasal epithelium and levels of soluble ICAM-1 in nasal lavage fluid during human experimental rhinovirus infection. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 131–136. [Google Scholar] [CrossRef]
- Müller, A.M.; Cronen, C.; Müller, K.M.; Kirkpatrick, C.J. Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J. Pathol. 2002, 198, 270–275. [Google Scholar] [CrossRef]
- Hussman, J.P. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front. Pharmacol. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Jiang, Y.; Xia, D.; Xiong, Y.; Zheng, Q.; Chen, F.; Zou, L.; Xiao, W.; Zhu, Y. Elevated Expression of Serum Endothelial Cell Adhesion Molecules in COVID-19 Patients. J. Infect. Dis. 2020, 222, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal. Transduct Target. Ther. 2020, 5, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.M.; Khakoo, S.I.; Biron, C.A. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr. Opin. Virol. 2011, 1, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, S.N.; Reighard, S.D.; Gyurova, I.E.; Cranert, S.A.; Mahl, S.E.; Karmele, E.P.; McNally, J.P.; Moran, M.T.; Brooks, T.R.; Yaqoob, F.; et al. Roles of natural killer cells in antiviral immunity. Curr. Opin. Virol. 2016, 16, 15–23. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, X.; Guan, J.; Qin, S.; Wang, Z.; Lu, H.; Qian, J.; Wu, L.; Chen, Y.; Chen, Y.; et al. COVID-19 pneumonia: CD8+ T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin. Immunol. 2020, 218, 108516. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef]
- Song, J.W.; Zhang, C.; Fan, X.; Meng, F.P.; Xu, Z.; Xia, P.; Cao, W.J.; Yang, T.; Dai, X.P.; Wang, S.Y.; et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020, 11, 3410. [Google Scholar] [CrossRef]
- Li, M.; Guo, W.; Dong, Y.; Wang, X.; Dai, D.; Liu, X.; Wu, Y.; Li, M.; Zhang, W.; Zhou, H.; et al. Elevated Exhaustion Levels of NK and CD8+ T Cells as Indicators for Progression and Prognosis of COVID-19 Disease. Front. Immunol. 2020, 11, 580237. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.; Chandra, R.K. Effect of vitamin and trace element supplementation on immune indices in healthy elderly. Int. J. Vitam. Nutr. Res. 1995, 65, 117–121. [Google Scholar]
- Ravaglia, G.; Forti, P.; Maioli, F.; Bastagli, L.; Facchini, A.; Mariani, E.; Savarino, L.; Sassi, S.; Cucinotta, D.; Lenaz, G. Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged >/=90 y. Am. J. Clin. Nutr. 2000, 71, 590–598. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: Mechanisms of host defense. J. Nutr. 2007, 137, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Haase, H.; Rink, L. Zinc signals and immune function. Biofactors 2014, 40, 27–40. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Winter, C.C.; Wagtmann, N.; Long, E.O. The Ig-related killer cell inhibitory receptor binds zinc and requires zinc for recognition of HLA-C on target cells. J. Immunol. 1995, 155, 4143–4146. [Google Scholar]
- Chandra, R.K. Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet 1992, 340, 1124–1127. [Google Scholar] [CrossRef]
- Rolles, B.; Maywald, M.; Rink, L. Influence of zinc deficiency and supplementation on NK cell cytotoxicity. J. Funct. Foods 2018, 48, 322–328. [Google Scholar] [CrossRef]
- Taniuchi, I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu. Rev. Immunol. 2018, 36, 579–601. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef]
- Nakayama, T.; Hirahara, K.; Onodera, A.; Endo, Y.; Hosokawa, H.; Shinoda, K.; Tumes, D.J.; Okamoto, Y. Th2 Cells in Health and Disease. Annu. Rev. Immunol. 2017, 35, 53–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; Sun, B. TH1/TH2 cell differentiation and molecular signals. Adv. Exp. Med. Biol. 2014, 841, 15–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Zhao, J.; Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014, 59, 118–128. [Google Scholar] [CrossRef]
- Barnett, J.B.; Dao, M.C.; Hamer, D.H.; Kandel, R.; Brandeis, G.; Wu, D.; Dallal, G.E.; Jacques, P.F.; Schreiber, R.; Kong, E.; et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2016, 103, 942–951. [Google Scholar] [CrossRef]
- Fortes, C.; Forastiere, F.; Agabiti, N.; Fano, V.; Pacifici, R.; Virgili, F.; Piras, G.; Guidi, L.; Bartoloni, C.; Tricerri, A.; et al. The effect of zinc and vitamin A supplementation on immune response in an older population. J. Am. Geriatr. Soc. 1998, 46, 19–26. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Veccia, S.; Ancarani, F.; Scalise, G.; Fabris, N. Benefit of oral zinc supplementation as an adjunct to zidovudine (AZT) therapy against opportunistic infections in AIDS. Int. J. Immunopharmacol. 1995, 17, 719–727. [Google Scholar] [CrossRef]
- Santos, H.O.; Teixeira, F.J.; Schoenfeld, B.J. Dietary vs. pharmacological doses of zinc: A clinical review. Clin. Nutr. 2020, 39, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Mossink, J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr. Prev. Health 2020, 3, 111–117. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Poppema, S.; Lai, R.; Visser, L.; Yan, X.J. CD45 (leucocyte common antigen) expression in T and B lymphocyte subsets. Leuk Lymphoma 1996, 20, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Sarkar, F.H. Correction of interleukin-2 gene expression by in vitro zinc addition to mononuclear cells from zinc-deficient human subjects: A specific test for zinc deficiency in humans. Transl. Res. 2006, 148, 325–333. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Ogiso, T.; Moriyama, K.; Sasaki, S.; Ishimura, Y.; Minato, A. Inhibitory effect of high dietary zinc on copper absorption in rats. Chem. Pharm. Bull. 1974, 22, 55–60. [Google Scholar] [CrossRef]
- Ogiso, T.; Ogawa, N.; Miura, T. Inhibitory effect of high dietary zinc on copper absorption in rats. II. Binding of copper and zinc to cytosol proteins in the intestinal mucosa. Chem. Pharm. Bull. 1979, 27, 515–521. [Google Scholar] [CrossRef]
- Magee, A.C.; Matrone, G. Studies on growth, copper metabolism of rats fed high levels of zinc. J. Nutr. 1960, 72, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Van Campen, D.R. Copper interference with the intestinal absorption of zinc-65 by rats. J. Nutr. 1969, 97, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Porea, T.J.; Belmont, J.W.; Mahoney, D.H., Jr. Zinc-induced anemia and neutropenia in an adolescent. J. Pediatr. 2000, 136, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.A.; Mattman, A.; Lockitch, G.; Farrell, K.; Wadsworth, L.D. Element of caution: A case of reversible cytopenias associated with excessive zinc supplementation. CMAJ 2003, 169, 129–131. [Google Scholar] [PubMed]
- Prasad, A.S.; Brewer, G.J.; Schoomaker, E.B.; Rabbani, P. Hypocupremia induced by zinc therapy in adults. JAMA 1978, 240, 2166–2168. [Google Scholar] [CrossRef]
- Samman, S.; Roberts, D.C. The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. Med. J. Aust. 1987, 146, 246–249. [Google Scholar] [CrossRef]
- Samman, S.; Roberts, D.C. The effect of zinc supplements on lipoproteins and copper status. Atherosclerosis 1988, 70, 247–252. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).