Combined Treatment with Herbal Medicine and Drug Ameliorates Inflammation and Metabolic Abnormalities in the Liver of an Amyotrophic Lateral Sclerosis Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
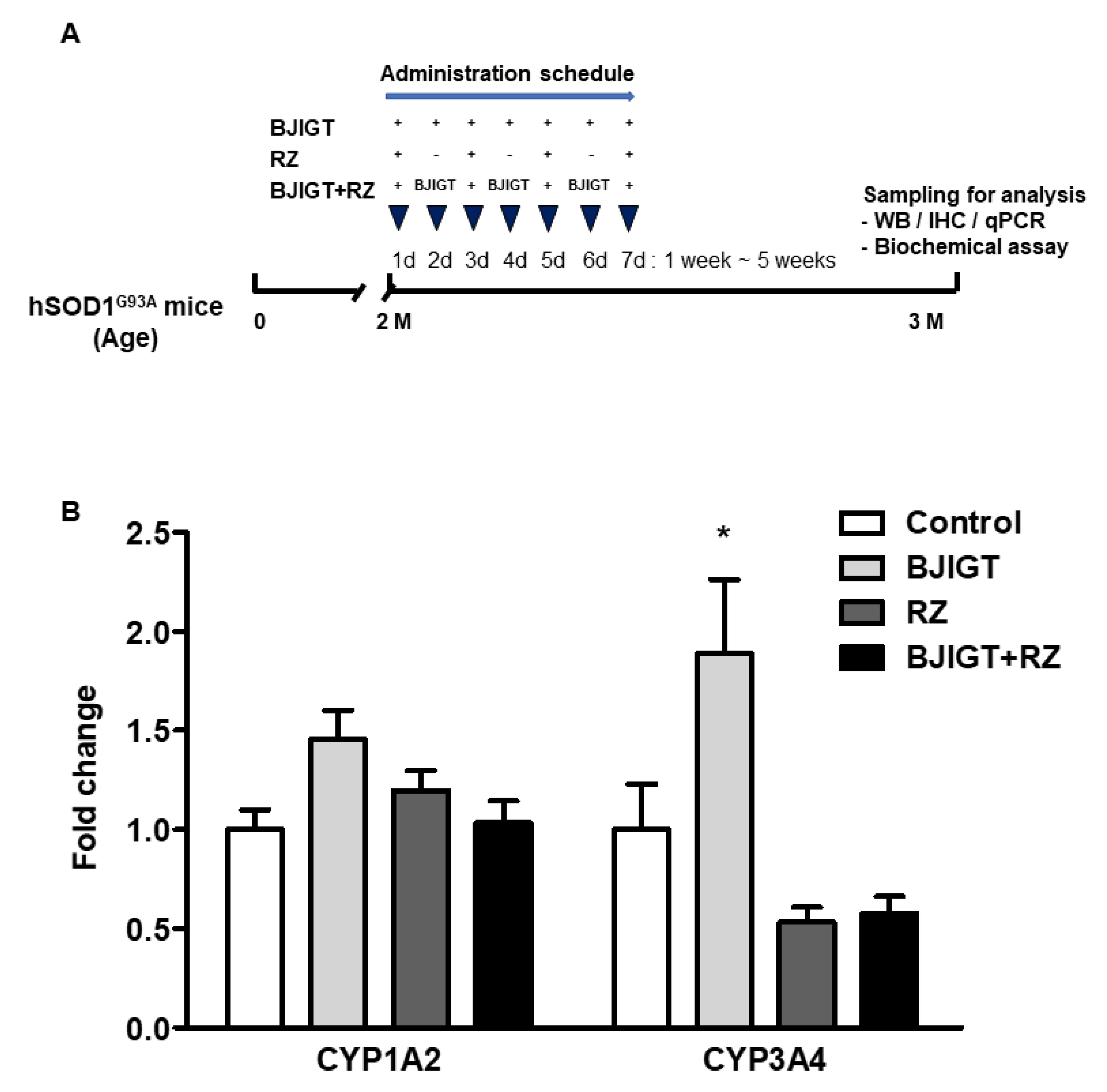
2.2. Drug Administration
2.3. Tissue Preparation
2.4. Western Blot Analysis
2.5. Quantitative Reverse Transcription PCR
2.6. Hematoxylin and Eosin (H&E) Staining
2.7. Statistical Analysis
3. Results
3.1. Combination Treatment Did Not Affect Gene Expression Levels of Cytochrome P450 Enzymes in the Liver of hSOD1G93A Mice
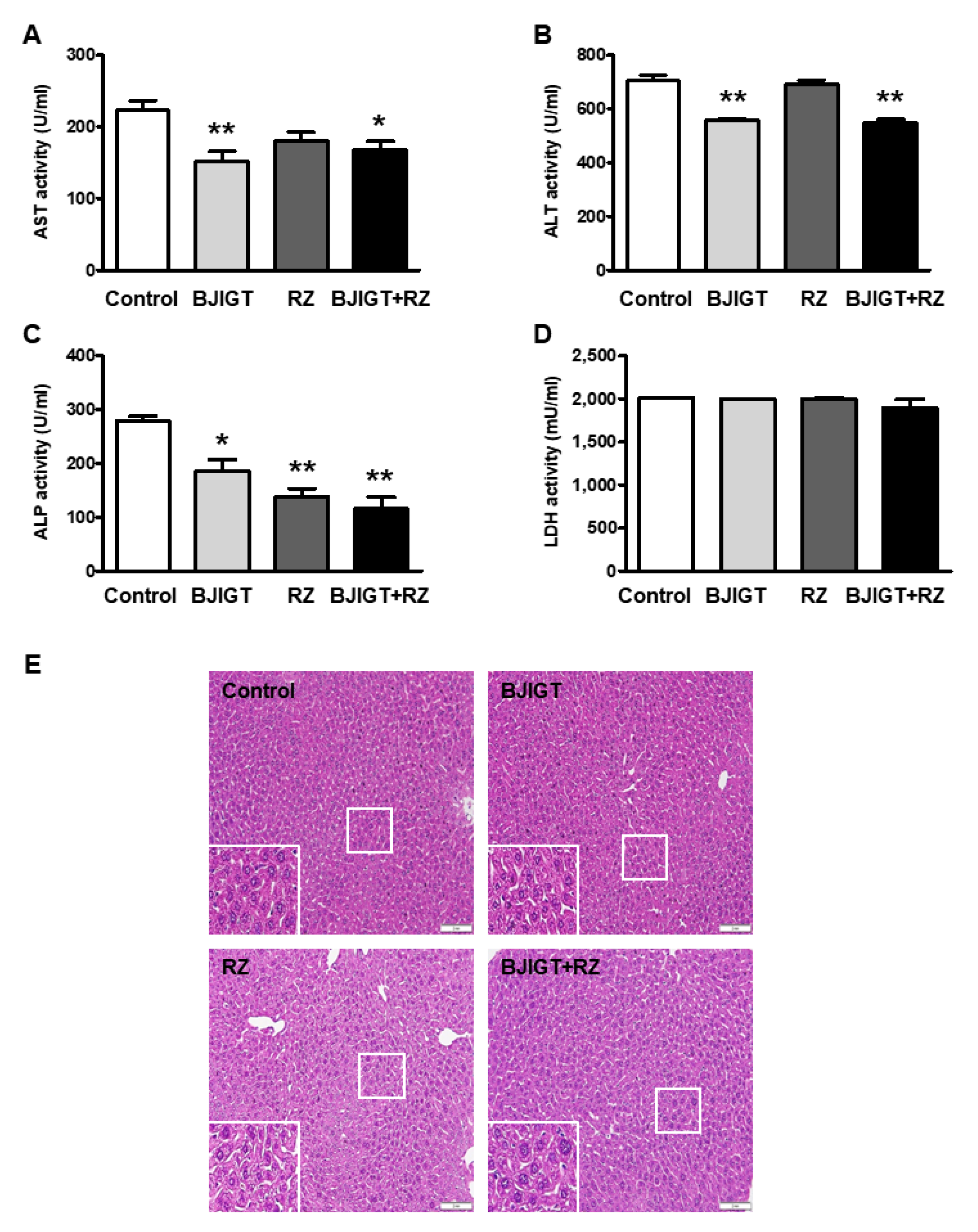
3.2. Combined Administration of BJIGT and RZ Attenuated Abnormal Liver Function in hSOD1G93A Mice
3.3. Increased Inflammatory Cytokines in the Liver of hSOD1G93A Mice Were Ameliorated by Combined Administration of BJIGT and RZ
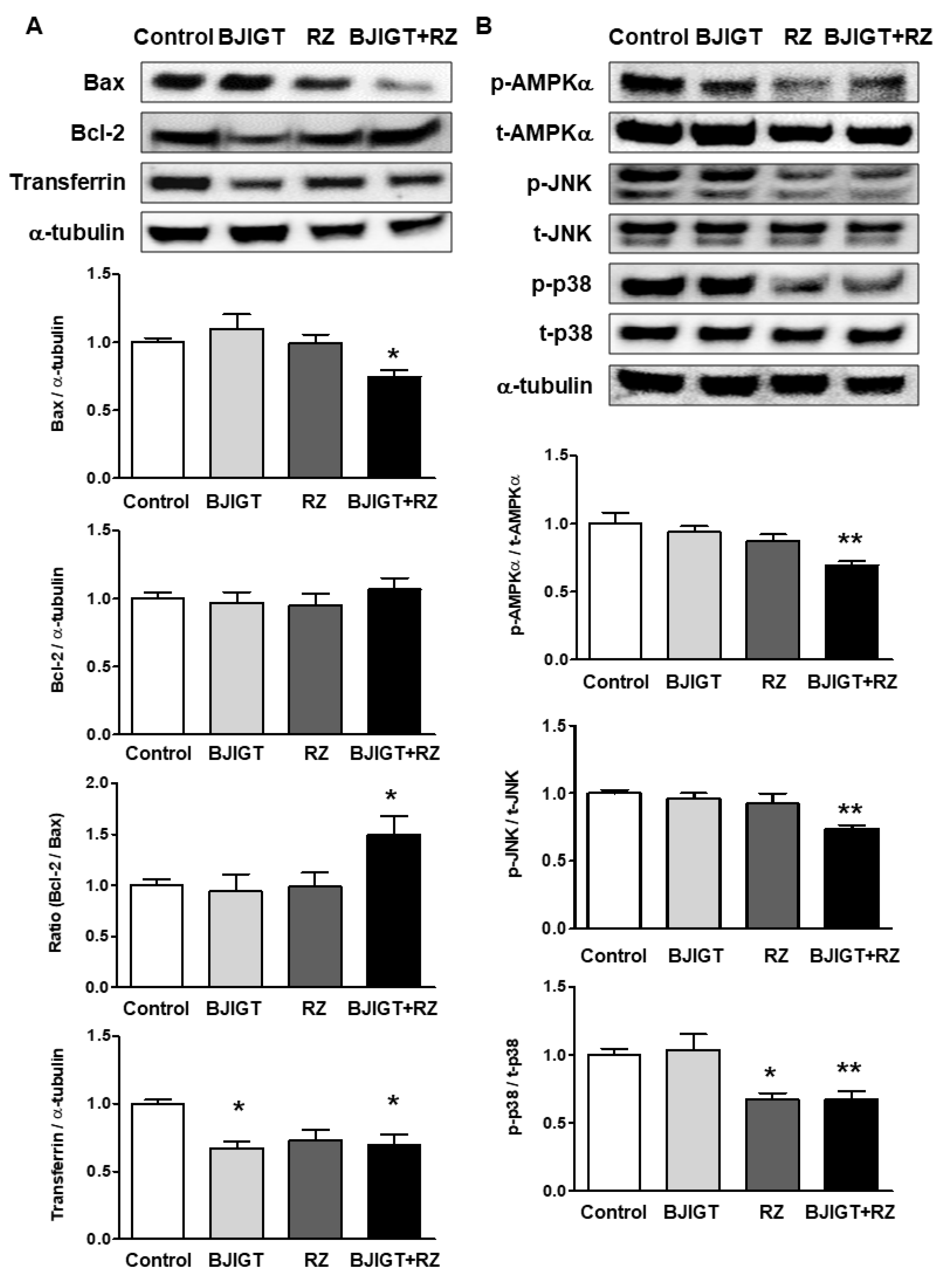
3.4. Cellular Stress-Mediated Proteins Were Attenuated by Combined Administration of BJIGT and RZ in the Liver of hSOD1G93A Mice
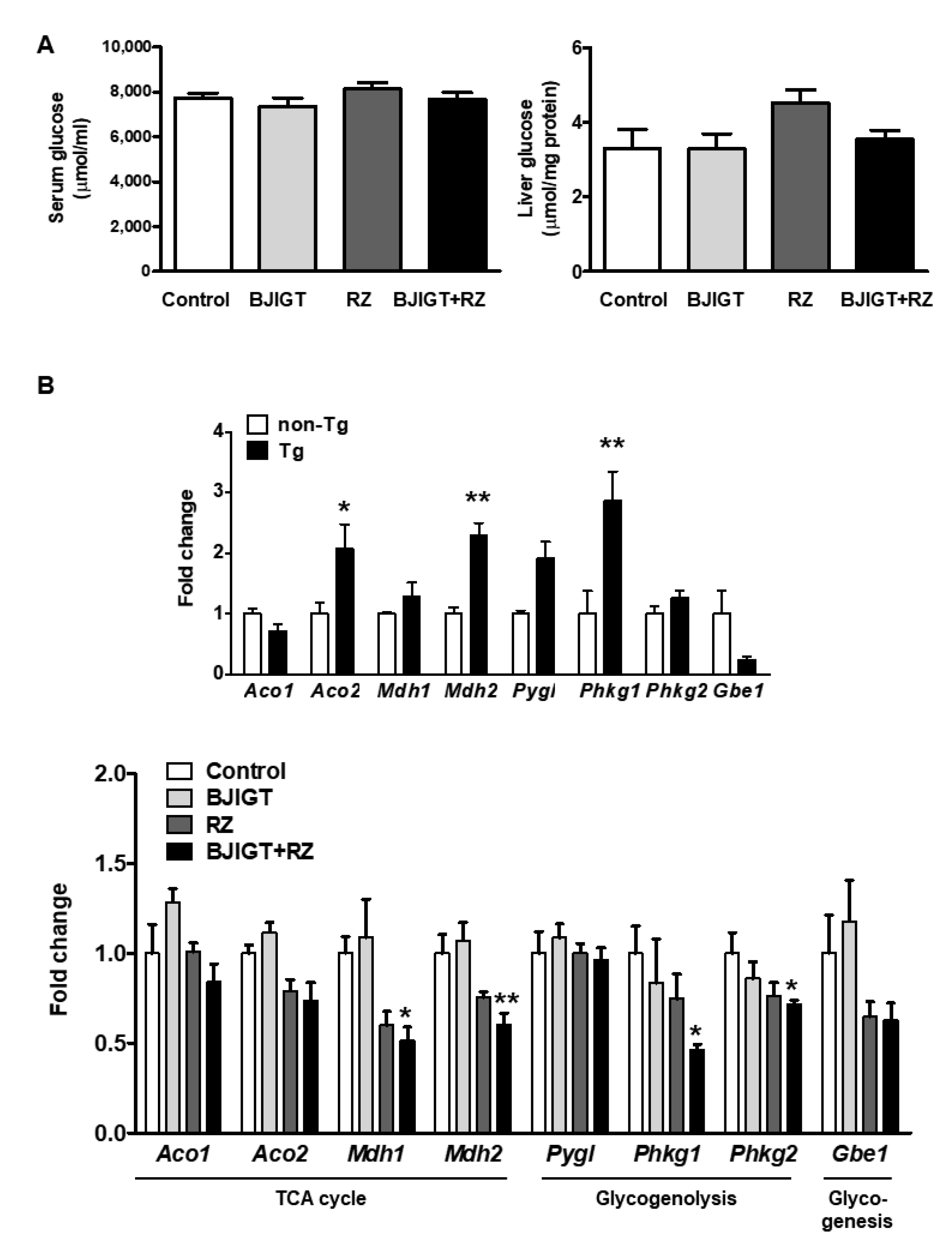
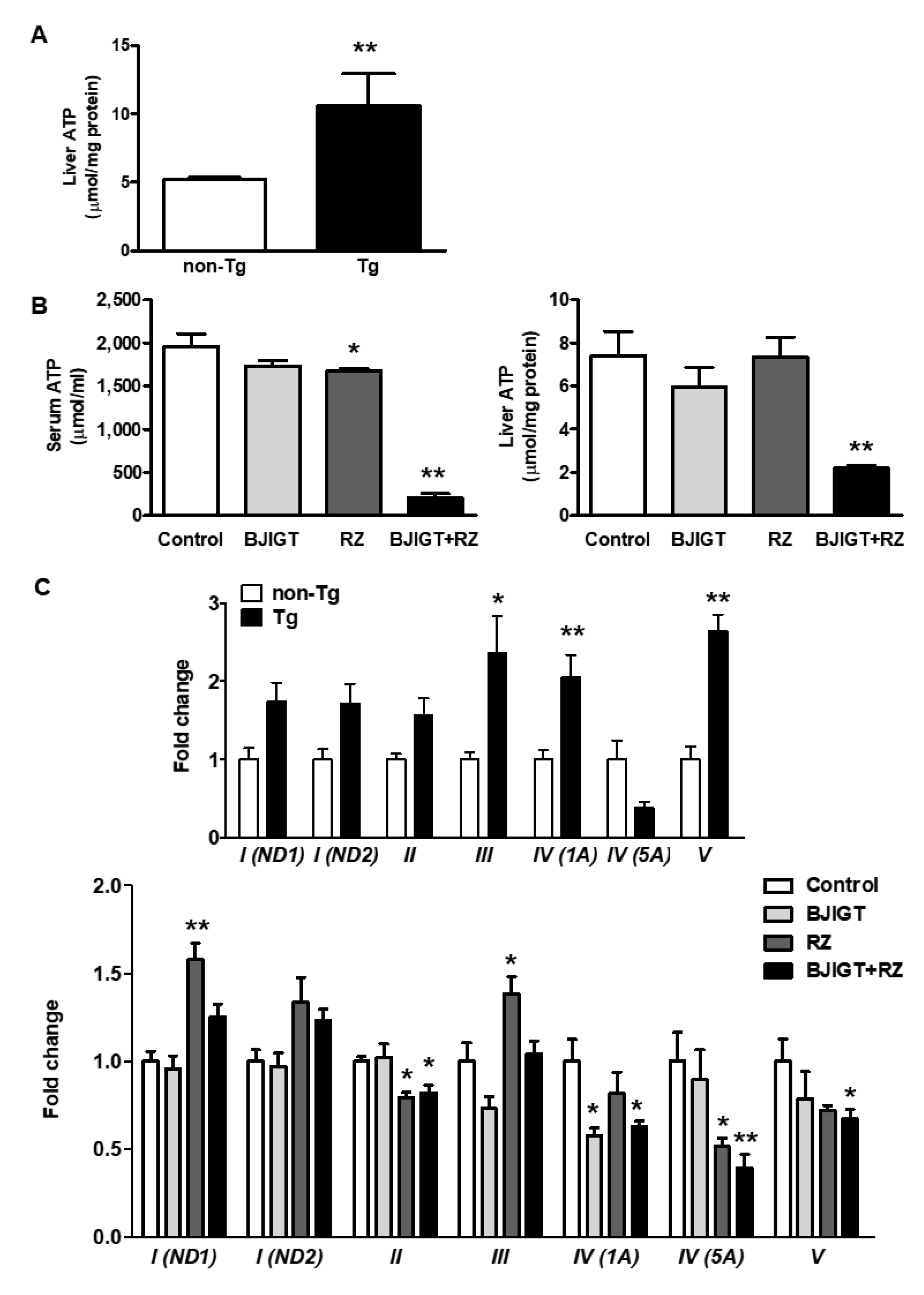
3.5. Metabolic Dysfunction in the Liver of hSOD1G93A Mice Was Improved by Combined Administration of BJIGT and RZ
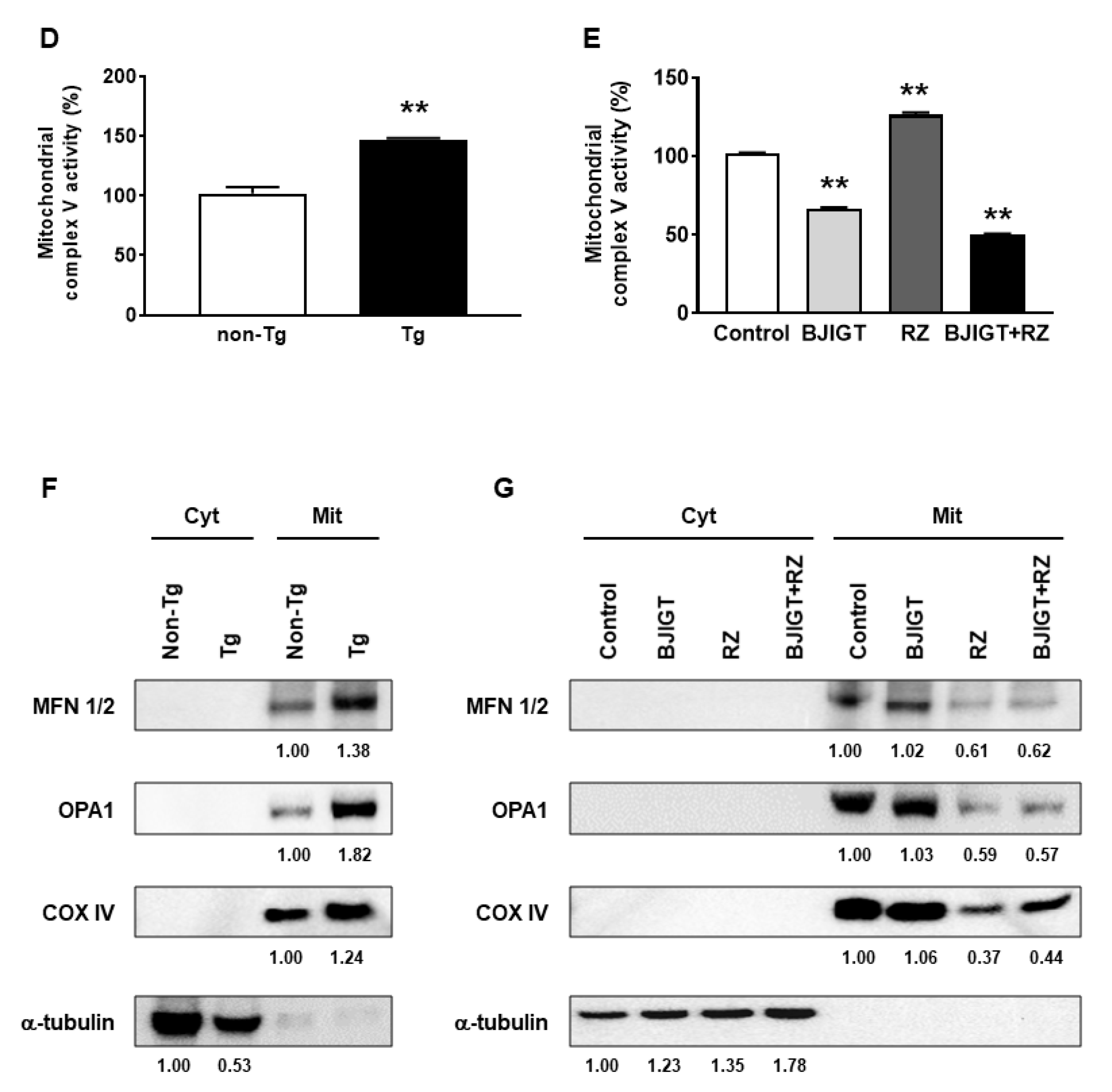
3.6. Dysfunction of the OXPHOS System and Abnormal Mitochondrial Fusion in the Liver of hSOD1G93A Mice Was Improved by Combined Administration of BJIGT and RZ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossi, S.; Cozzolino, M.; Carri, M.T. Old versus New Mechanisms in the Pathogenesis of ALS. Brain Pathol. 2016, 26, 276–286. [Google Scholar] [CrossRef]
- McDonald, T.S.; Kumar, V.; Fung, J.N.; Woodruff, T.M.; Lee, J.D. Glucose clearance and uptake is increased in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis through an insulin-independent mechanism. FASEB J. 2021, 35, e21707. [Google Scholar] [CrossRef] [PubMed]
- Tefera, T.W.; Borges, K. Metabolic Dysfunctions in Amyotrophic Lateral Sclerosis Pathogenesis and Potential Metabolic Treatments. Front. Neurosci. 2016, 10, 611. [Google Scholar] [CrossRef]
- Bouteloup, C.; Desport, J.C.; Clavelou, P.; Guy, N.; Derumeaux-Burel, H.; Ferrier, A.; Couratier, P. Hypermetabolism in ALS patients: An early and persistent phenomenon. J. Neurol. 2009, 256, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Mattiazzi, M.; D’Aurelio, M.; Gajewski, C.D.; Martushova, K.; Kiaei, M.; Beal, M.F.; Manfredi, G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 2002, 277, 29626–29633. [Google Scholar] [CrossRef] [Green Version]
- Jaarsma, D.; Haasdijk, E.D.; Grashorn, J.A.; Hawkins, R.; van Duijn, W.; Verspaget, H.W.; London, J.; Holstege, J.C. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol. Dis. 2000, 7, 623–643. [Google Scholar]
- Scaricamazza, S.; Salvatori, I.; Giacovazzo, G.; Loeffler, J.P.; Rene, F.; Rosina, M.; Quessada, C.; Proietti, D.; Heil, C.; Rossi, S.; et al. Skeletal-Muscle Metabolic Reprogramming in ALS-SOD1(G93A) Mice Predates Disease Onset and Is A Promising Therapeutic Target. iScience 2020, 23, 101087. [Google Scholar] [CrossRef]
- Allen, S.P.; Duffy, L.M.; Shaw, P.J.; Grierson, A.J. Altered age-related changes in bioenergetic properties and mitochondrial morphology in fibroblasts from sporadic amyotrophic lateral sclerosis patients. Neurobiol. Aging 2015, 36, 2893–2903. [Google Scholar] [CrossRef]
- Sasaki, S.; Warita, H.; Murakami, T.; Abe, K.; Iwata, M. Ultrastructural study of mitochondria in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 2004, 107, 461–474. [Google Scholar] [CrossRef]
- Finkelstein, A.; Kunis, G.; Seksenyan, A.; Ronen, A.; Berkutzki, T.; Azoulay, D.; Koronyo-Hamaoui, M.; Schwartz, M. Abnormal changes in NKT cells, the IGF-1 axis, and liver pathology in an animal model of ALS. PLoS ONE 2011, 6, e22374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Hirayama, K.; Terao, K. Hepatic ultrastructural changes and liver dysfunction in amyotrophic lateral sclerosis. Arch. Neurol. 1987, 44, 103–106. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, E.J. Relationship between Liver Pathology and Disease Progression in a Murine Model of Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2018, 18, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.B. Deciphering the metabolic fates of herbal constituents and the interactions of herbs with human metabolic system. Chin. J. Nat. Med. 2019, 17, 801–802. [Google Scholar] [CrossRef]
- Stickel, F.; Schuppan, D. Herbal medicine in the treatment of liver diseases. Dig. Liver Dis. 2007, 39, 293–304. [Google Scholar] [CrossRef]
- Cai, M.; Lee, S.H.; Yang, E.J. Bojungikgi-tang Improves Muscle and Spinal Cord Function in an Amyotrophic Lateral Sclerosis Model. Mol. Neurobiol. 2019, 56, 2394–2407. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, Y.J.; Sohn, E.; Yoon, J.; Kim, B.Y.; Jeong, S.J. Bojungikgi-Tang, a Traditional Herbal Formula, Exerts Neuroprotective Effects and Ameliorates Memory Impairments in Alzheimer’s Disease-Like Experimental Models. Nutrients 2018, 10, 1952. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.J.; Lee, H.J.; Kim, J.D.; Jung, H.J.; Bae, S.H.; Ryoo, H.M.; Kim, S.G. Changes of peripheral blood lymphocyte subtypes in patients with end stage cancer administered localized radiotherapy and bojungikki-tang. Evid. Based Complement. Altern. Med. 2014, 2014, 207613. [Google Scholar] [CrossRef]
- Sato, T.; Kita, K.; Sato, C.; Kaneda, A. Hochuekkito (Buzhongyiqitang), a herbal medicine, enhances cisplatininduced apoptosis in HeLa cells. Mol. Med. Rep. 2015, 12, 6215–6220. [Google Scholar] [CrossRef] [Green Version]
- Bensimon, G.; Lacomblez, L.; Meininger, V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994, 330, 585–591. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef] [Green Version]
- Parvez, M.K.; Rishi, V. Herb-Drug Interactions and Hepatotoxicity. Curr. Drug Metab. 2019, 20, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Klotz, U. Drug interactions with herbal medicines. Clin. Pharmacokinet. 2012, 51, 77–104. [Google Scholar] [CrossRef]
- van Kan, H.J.; Groeneveld, G.J.; Kalmijn, S.; Spieksma, M.; van den Berg, L.H.; Guchelaar, H.J. Association between CYP1A2 activity and riluzole clearance in patients with amyotrophic lateral sclerosis. Br. J. Clin. Pharmacol. 2005, 59, 310–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.E.; Ha, H.; Shin, H.K. Effects of Herbal Formulas Bojungikgi-tang and Palmijihwang-hwan on Inflammation in RAW 264.7 Cells and the Activities of Drug-Metabolizing Enzymes in Human Hepatic Microsomes. J. Med. Food 2018, 21, 1173–1187. [Google Scholar] [CrossRef]
- McClain, C.J.; Price, S.; Barve, S.; Devalarja, R.; Shedlofsky, S. Acetaminophen hepatotoxicity: An update. Curr. Gastroenterol. Rep. 1999, 1, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Salucci, S.; Bartoletti Stella, A.; Battistelli, M.; Burattini, S.; Bavelloni, A.; Cocco, L.I.; Gobbi, P.; Faenza, I. How Inflammation Pathways Contribute to Cell Death in Neuro-Muscular Disorders. Biomolecules 2021, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [PubMed] [Green Version]
- Smith, D.L., Jr.; Pavela, G. Energy Balance as a Moderator of Neurologic Disease Risk and Progression. Neurotox. Res. 2020, 38, 242–248. [Google Scholar] [CrossRef]
- He, J.; Fu, J.; Zhao, W.; Ren, C.; Liu, P.; Chen, L.; Li, D.; Tang, L.; Zhou, L.; Zhang, Y.; et al. Hypermetabolism associated with worse prognosis of amyotrophic lateral sclerosis. J. Neurol. 2021. [Google Scholar] [CrossRef]
- Cattaneo, M.; Jesus, P.; Lizio, A.; Fayemendy, P.; Guanziroli, N.; Corradi, E.; Sansone, V.; Leocani, L.; Filippi, M.; Riva, N.; et al. The hypometabolic state: A good predictor of a better prognosis in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2021, 93, 41–47. [Google Scholar] [CrossRef]
- Ravera, S.; Torazza, C.; Bonifacino, T.; Provenzano, F.; Rebosio, C.; Milanese, M.; Usai, C.; Panfoli, I.; Bonanno, G. Altered glucose catabolism in the presynaptic and perisynaptic compartments of SOD1(G93A) mouse spinal cord and motor cortex indicates that mitochondria are the site of bioenergetic imbalance in ALS. J. Neurochem. 2019, 151, 336–350. [Google Scholar] [CrossRef]
- Vande Velde, C.; McDonald, K.K.; Boukhedimi, Y.; McAlonis-Downes, M.; Lobsiger, C.S.; Bel Hadj, S.; Zandona, A.; Julien, J.P.; Shah, S.B.; Cleveland, D.W. Misfolded SOD1 associated with motor neuron mitochondria alters mitochondrial shape and distribution prior to clinical onset. PLoS ONE 2011, 6, e22031. [Google Scholar] [CrossRef]
- Ladd, A.C.; Keeney, P.M.; Govind, M.M.; Bennett, J.P., Jr. Mitochondrial oxidative phosphorylation transcriptome alterations in human amyotrophic lateral sclerosis spinal cord and blood. Neuromol. Med. 2014, 16, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Babbar, M.; Sheikh, M.S. Metabolic Stress and Disorders Related to Alterations in Mitochondrial Fission or Fusion. Mol. Cell. Pharmacol. 2013, 5, 109–133. [Google Scholar]
- Sharma, A.; Smith, H.J.; Yao, P.; Mair, W.B. Causal roles of mitochondrial dynamics in longevity and healthy aging. EMBO Rep. 2019, 20, e48395. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Chakrabarti, O. Mitochondrial hyperfusion: A friend or a foe. Biochem. Soc. Trans. 2020, 48, 631–644. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.R.; Yang, E.J. Combined Treatment with Herbal Medicine and Drug Ameliorates Inflammation and Metabolic Abnormalities in the Liver of an Amyotrophic Lateral Sclerosis Mouse Model. Antioxidants 2022, 11, 173. https://doi.org/10.3390/antiox11010173
Park HR, Yang EJ. Combined Treatment with Herbal Medicine and Drug Ameliorates Inflammation and Metabolic Abnormalities in the Liver of an Amyotrophic Lateral Sclerosis Mouse Model. Antioxidants. 2022; 11(1):173. https://doi.org/10.3390/antiox11010173
Chicago/Turabian StylePark, Hee Ra, and Eun Jin Yang. 2022. "Combined Treatment with Herbal Medicine and Drug Ameliorates Inflammation and Metabolic Abnormalities in the Liver of an Amyotrophic Lateral Sclerosis Mouse Model" Antioxidants 11, no. 1: 173. https://doi.org/10.3390/antiox11010173
APA StylePark, H. R., & Yang, E. J. (2022). Combined Treatment with Herbal Medicine and Drug Ameliorates Inflammation and Metabolic Abnormalities in the Liver of an Amyotrophic Lateral Sclerosis Mouse Model. Antioxidants, 11(1), 173. https://doi.org/10.3390/antiox11010173




