Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation
Abstract
1. Introduction
2. Reactive Oxygen Species and Sperm Functions
3. Antioxidants in Oxidative Stress-Induced Male Infertility
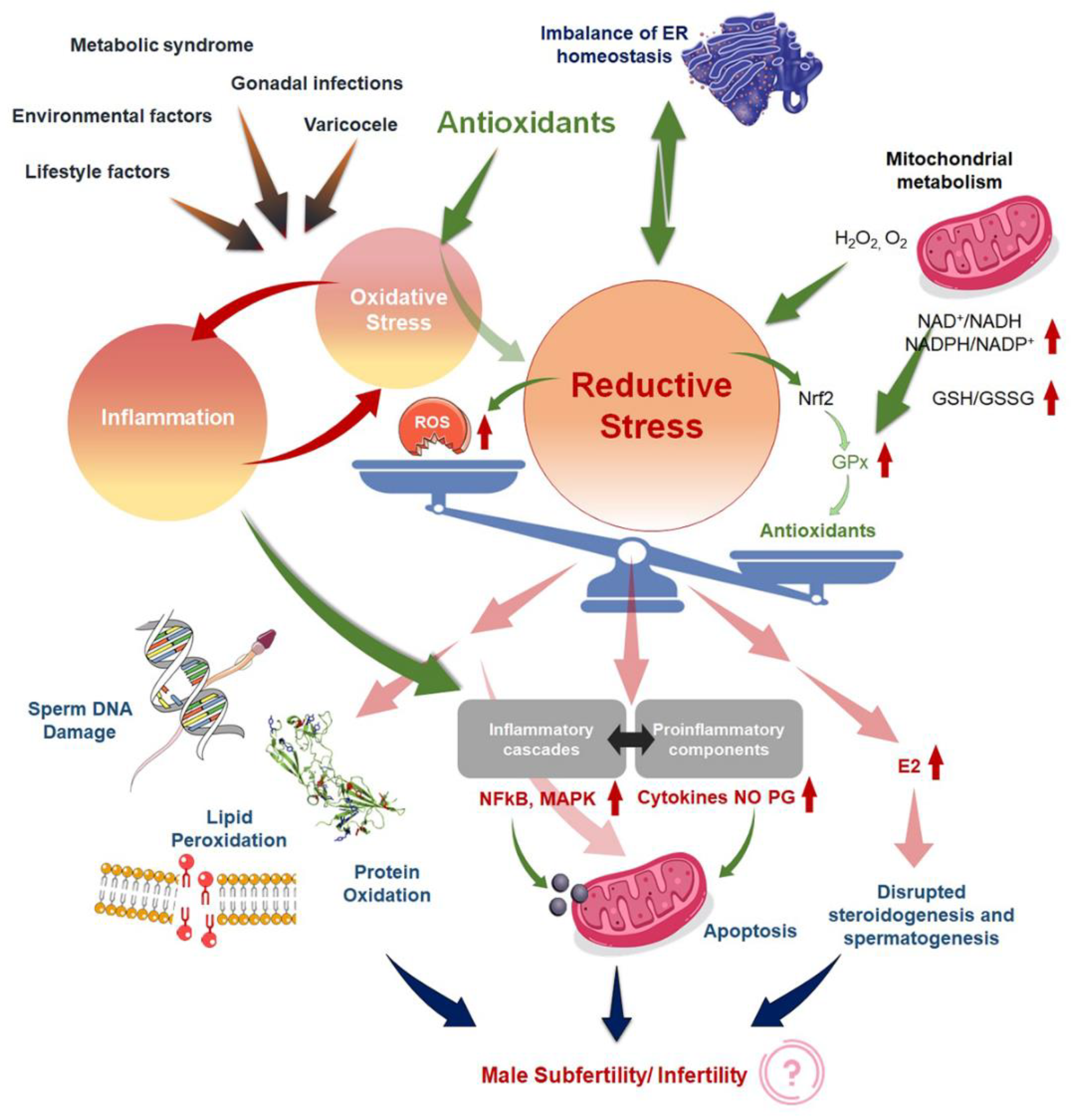
4. Induction of Reductive Stress
5. Antioxidants-Induced Reductive Stress
6. Oxidative Stress and Inflammation: Interdependence and Overlaps
7. ‘Antioxidant Paradox’ in the Light of the Interdependent Nature of Oxidative Stress and Inflammation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Aktan, G.; Doğru-Abbasoğlu, S.; Küçükgergin, C.; Kadıoğlu, A.; Özdemirler-Erata, G.; Koçak-Toker, N. Mystery of idiopathic male infertility: Is oxidative stress an actual risk? Fertil. Steril. 2013, 99, 1211–1215. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Sengupta, P.; Dutta, S. Coenzyme q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J. Men’s Health 2021, 39, 346–351. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Sengupta, P. Impact of coenzyme q10 and selenium on seminal fluid parameters and antioxidant status in men with idiopathic infertility. Biol. Trace Elem. Res. 2021, 199, 1246–1252. [Google Scholar] [CrossRef]
- Agarwal, A.; Sengupta, P. Oxidative stress and its association with male infertility. In Male Infertility; Springer: Berlin/Heidelberg, Germany, 2020; pp. 57–68. [Google Scholar]
- Sengupta, P.; Dutta, S.; Krajewska-Kulak, E. The disappearing sperms: Analysis of reports published between 1980 and 2015. Am. J. Men’s Health 2017, 11, 1279–1304. [Google Scholar] [CrossRef]
- Pant, P.R. Factors affecting male infertility. J. Inst. Med. 2009, 31, 10–12. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular alterations in spermatozoa of a family case living in the land of fires. A first look at possible transgenerational effects of pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 world health organization laboratory methods for the examination of human semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, P.K.; Janoo, G.; Bhurtu, A.; Teeluck, A.R.; Soogund, M.Z.S.; Pursun, M.; Huang, F. Tobacco smoking and semen quality in infertile males: A systematic review and meta-analysis. BMC Public Health 2019, 19, 36. [Google Scholar] [CrossRef]
- Kovac, J.R.; Khanna, A.; Lipshultz, L.I. The effects of cigarette smoking on male fertility. Postgrad. Med. 2015, 127, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Ramgir, S.S.; Abilash, V. Impact of smoking and alcohol consumption on oxidative status in male infertility and sperm quality. Indian J. Pharm. Sci. 2019, 81, 933–945. [Google Scholar] [CrossRef]
- Muthusami, K.; Chinnaswamy, P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil. Steril. 2005, 84, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D. Lifestyle causes of male infertility. Arab J. Urol. 2018, 16, 10–20. [Google Scholar] [CrossRef]
- Fronczak, C.M.; Kim, E.D.; Barqawi, A.B. The insults of illicit drug use on male fertility. J. Androl. 2012, 33, 515–528. [Google Scholar] [CrossRef]
- Sansone, A.; Di Dato, C.; de Angelis, C.; Menafra, D.; Pozza, C.; Pivonello, R.; Isidori, A.; Gianfrilli, D. Smoke, alcohol and drug addiction and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Baskaran, S.; Dutta, S.; Sengupta, P.; Khorshid, H.R.K.; Esteves, S.; Gilany, K.; Hedayati, M. Reactive oxygen species-induced alterations in h19-igf2 methylation patterns, seminal plasma metabolites, and semen quality. J. Assist. Reprod. Genet. 2019, 36, 241–253. [Google Scholar] [CrossRef]
- Dutta, S.; Biswas, A.; Sengupta, P. Obesity, endocrine disruption and male infertility. Asian Pac. J. Reprod. 2019, 8, 195–202. [Google Scholar] [CrossRef]
- Leisegang, K.; Dutta, S. Do lifestyle practices impede male fertility? Andrologia 2021, 53, e13595. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Karkada, I.; Chinni, S. Endocrinopathies and male infertility. Life 2022, 12, 10. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D. Ros and antioxidants: Achieving the balance between when to use the synthetic antioxidants. Oxidat. Med. Cell. Long. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Tilmisany, A.M.; Al-Sawaf, H. Mechanisms of male infertility: Role of antioxidants. Curr. Drug Metab. 2005, 6, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sekhon, L.H. The role of antioxidant therapy in the treatment of male infertility. Hum. Fertil. 2010, 13, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10, 15–21. [Google Scholar] [CrossRef]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Izuka, E.; Menuba, I.; Sengupta, P.; Dutta, S.; Nwagha, U. Antioxidants, anti-inflammatory drugs and antibiotics in the treatment of reproductive tract infections and their association with male infertility. Chem. Biol. Lett. 2020, 7, 156–165. [Google Scholar]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Baskaran, S.; Sengupta, P.; Dutta, S.; Mokarram, P.; Saliminejad, K.; Sadeghi, M.R. Oxidative stress-induced alterations in seminal plasma antioxidants: Is there any association with keap1 gene methylation in human spermatozoa? Andrologia 2019, 51, e13159. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S. Hormones in male reproduction and fertility. Asian Pac. J. Reprod. 2019, 8, 187–188. [Google Scholar] [CrossRef]
- Zhou, W.; De Iuliis, G.N.; Dun, M.D.; Nixon, B. Characteristics of the epididymal luminal environment responsible for sperm maturation and storage. Front. Endocrinol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological role of ros in sperm function. In Male Infertility; Springer: Berlin/Heidelberg, Germany, 2020; pp. 337–345. [Google Scholar]
- Agarwal, A.; Prabakaran, S.A. Oxidative stress and antioxidants in male infertility: A difficult balance. Int. J. Reprod. Med. 2005, 3, 1–8. [Google Scholar]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583. [Google Scholar] [CrossRef]
- O’Flaherty, C. The enzymatic antioxidant system of human spermatozoa. Adv. Androl. 2014, 2014, 626374. [Google Scholar] [CrossRef]
- Yan, L.; Liu, J.; Wu, S.; Zhang, S.; Ji, G.; Gu, A. Seminal superoxide dismutase activity and its relationship with semen quality and sod gene polymorphism. J. Assist. Reprod. Genet. 2014, 31, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Ann. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, L.; Kurashova, N.; Bairova, T.; Dolgikh, M.; Ershova, O.; Dashiev, B.; Korytov, L.; Koroleva, N. Role of glutathione-s-transferase family genes in male infertility. Bull. Exp. Biol. Med. 2017, 163, 643–645. [Google Scholar] [CrossRef]
- Agarwal, A.; Leisegang, K.; Sengupta, P. Oxidative stress in pathologies of male reproductive disorders. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–27. [Google Scholar]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. BioMed. 2016, 14, 231. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the origin of sperm DNA fragmentation: Role of apoptosis, immaturity and oxidative stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Ceretti, E.; Donato, F.; Bergamo, P.; Zani, C.; Viola, G.C.V.; Notari, T.; Pappalardo, S.; Zani, D.; Ubaldi, S.; et al. Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in italy: The fast randomized controlled trial. Eur. Urol. Focus, 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Rad. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef]
- Barratt, C.L.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; van der Poel, S.; St John, B.; Sigman, M. The diagnosis of male infertility: An analysis of the evidence to support the development of global who guidance—challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. J. Am. Med. Assoc. 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet 2004, 364, 1219–1228. [Google Scholar] [CrossRef]
- Stanner, S.; Hughes, J.; Kelly, C.; Buttriss, J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Pub. Health Nutr. 2004, 7, 407–422. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochr. Datab. Syst. Rev. 2012, 2012, CD007176. [Google Scholar] [CrossRef]
- Giussani, D.A.; Niu, Y.; Herrera, E.A.; Richter, H.G.; Camm, E.J.; Thakor, A.S.; Kane, A.D.; Hansell, J.A.; Brain, K.L.; Skeffington, K.L.; et al. Heart disease link to fetal hypoxia and oxidative stress. Adv. Fetal Neonat. Physiol. 2014, 814, 77–87. [Google Scholar]
- Busetto, G.; Agarwal, A.; Virmani, A.; Antonini, G.; Ragonesi, G.; Del Giudice, F.; Micic, S.; Gentile, V.; De Berardinis, E. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: A double-blind placebo-controlled study. Andrologia 2018, 50, e12927. [Google Scholar] [CrossRef]
- Sengupta, P.; Agarwal, A.; Pogrebetskaya, M.; Roychoudhury, S.; Durairajanayagam, D.; Henkel, R. Role of withania somnifera (ashwagandha) in the management of male infertility. Reprod. Biomed. Online 2018, 36, 311–326. [Google Scholar] [CrossRef]
- Torres-Arce, E.; Vizmanos, B.; Babio, N.; Márquez-Sandoval, F.; Salas-Huetos, A. Dietary antioxidants in the treatment of male infertility: Counteracting oxidative stress. Biology 2021, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis. Ecotoxicol. Env. Saf. 2021, 226, 112878. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Altered expression of daam1 and prep induced by cadmium toxicity is counteracted by melatonin in the rat testis. Genes 2021, 12, 1016. [Google Scholar] [CrossRef] [PubMed]
- Tikkiwal, M.; Ajmera, R.L.; Mathur, N.K. Effect of zinc administration on seminal zinc and fertility of oligospermic males. Indian J. Physiol. Pharmacol. 1987, 31, 30–34. [Google Scholar]
- Kessopoulou, E.; Powers, H.J.; Sharma, K.K.; Pearson, M.J.; Russell, J.M.; Cooke, I.D.; Barratt, C.L. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin e to treat reactive oxygen species associated male infertility. Fertil. Steril. 1995, 64, 825–831. [Google Scholar] [CrossRef]
- Omu, A.E.; Dashti, H.; Al-Othman, S. Treatment of asthenozoospermia with zinc sulphate: Andrological, immunological and obstetric outcome. Eur. J. Obs. Gynecol. Reprod. Biol. 1998, 79, 179–184. [Google Scholar] [CrossRef]
- Rolf, C.; Cooper, T.; Yeung, C.; Nieschlag, E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin c and vitamin e: A randomized, placebo-controlled, double-blind study. Hum. Reprod. 1999, 14, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef]
- Balercia, G.; Regoli, F.; Armeni, T.; Koverech, A.; Mantero, F.; Boscaro, M. Placebo-controlled double-blind randomized trial on the use of l-carnitine, l-acetylcarnitine, or combined l-carnitine and l-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil. Steril. 2005, 84, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.; Glass, S.; Campagnone, J.; Pryor, J.L. Carnitine for the treatment of idiopathic asthenospermia: A randomized, double-blind, placebo-controlled trial. Fertil. Steril. 2006, 85, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.J.; Hazout, A.; Panteix, G.; Robert, F.; Rollet, J.; Cohen-Bacrie, P.; Chapuis, F.; Clément, P.; Benkhalifa, M. Antioxidants to reduce sperm DNA fragmentation: An unexpected adverse effect. Reprod. Biomed. Online 2007, 14, 418–421. [Google Scholar] [CrossRef]
- Tremellen, K.; Miari, G.; Froiland, D.; Thompson, J. A randomised control trial examining the effect of an antioxidant (menevit) on pregnancy outcome during ivf-icsi treatment. Austr. N. Z. J. Obs. Gynaecol. 2007, 47, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Alkan, Z.; Wong, K. Selenium supplementation does not affect testicular selenium status or semen quality in north american men. J. Androl. 2009, 30, 525–533. [Google Scholar] [CrossRef]
- Tunc, O.; Thompson, J.; Tremellen, K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod. Biomed. Online 2009, 18, 761–768. [Google Scholar] [CrossRef]
- Gual-Frau, J.; Abad, C.; Amengual, M.J.; Hannaoui, N.; Checa, M.A.; Ribas-Maynou, J.; Lozano, I.; Nikolaou, A.; Benet, J.; García-Peiró, A. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade i varicocele patients. Hum. Fertil. 2015, 18, 225–229. [Google Scholar] [CrossRef]
- Stenqvist, A.; Oleszczuk, K.; Leijonhufvud, I.; Giwercman, A. Impact of antioxidant treatment on DNA fragmentation index: A double-blind placebo-controlled randomized trial. Andrology 2018, 6, 811–816. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Safarinejad, S.; Shafiei, N.; Safarinejad, S. Effects of the reduced form of coenzyme q10 (ubiquinol) on semen parameters in men with idiopathic infertility: A double-blind, placebo controlled, randomized study. J. Urol. 2012, 188, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Micic, S.; Lalic, N.; Djordjevic, D.; Bojanic, N.; Bogavac-Stanojevic, N.; Busetto, G.M.; Virmani, A.; Agarwal, A. Double-blind, randomised, placebo-controlled trial on the effect of l-carnitine and l-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia 2019, 51, e13267. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: A randomized clinical trial. J. Am. Med. Assoc. 2020, 323, 35–48. [Google Scholar] [CrossRef]
- Greabu, M.; Battino, M.; Mohora, M.; Olinescu, R.; Totan, A.; Didilescu, A. Oxygen, a paradoxical element. Rom. J. Intern. Med. 2008, 46, 125–135. [Google Scholar]
- Bioveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. Biochem. J. 1973, 134, 707. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants–quo vadis? Trends Pharm. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- John Aitken, R.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.-F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Alahmar, A.T.; D’souza, U.J.A. Reproductive tract infection, inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 75–84. [Google Scholar]
- O’Flaherty, C.N.; de Lamirande, E.; Gagnon, C. Reactive oxygen species and protein kinases modulate the level of phospho-mek-like proteins during human sperm capacitation. Biol. Reprod. 2005, 73, 94–105. [Google Scholar] [CrossRef]
- Garratt, M.; Bathgate, R.; de Graaf, S.P.; Brooks, R.C. Copper-zinc superoxide dismutase deficiency impairs sperm motility and in vivo fertility. Reproduction 2013, 146, 297–304. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, C.; Dermitzaki, E.; Avgoustinaki, P.; Malliaraki, N.; Mytaras, V.; Margioris, A.N. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (hpg) axis. Hormones 2015, 14, 549–562. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Sengupta, P.; Dutta, S.; Karkada, I.R. Obesity, systemic inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 92–98. [Google Scholar]
- Irez, T.; Bicer, S.; Sahin, E.; Dutta, S.; Sengupta, P. Cytokines and adipokines in the regulation of spermatogenesis and semen quality. Chem. Biol. Lett. 2020, 7, 131–139. [Google Scholar]
- Mathur, P.P.; Huang, L.; Kashou, A.; Vaithinathan, S.; Agarwal, A. Environmental toxicants and testicular apoptosis. Open Reprod. Sci. J. 2011, 3, 114–124. [Google Scholar]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef]
- Wendel, A. Measurement of in vivo lipid peroxidation and toxicological significance. Free Rad. Biol. Med. 1987, 3, 355–358. [Google Scholar] [CrossRef]
- Castagne, V.; Lefevre, K.; Natero, R.; Becker, D.; Clarke, P. An optimal redox status for the survival of axotomized ganglion cells in the developing retina. Neuroscience 1999, 93, 313–320. [Google Scholar] [CrossRef]
- Symeonidis, E.N.; Evgeni, E.; Palapelas, V.; Koumasi, D.; Pyrgidis, N.; Sokolakis, I.; Hatzichristodoulou, G.; Tsiampali, C.; Mykoniatis, I.; Zachariou, A.; et al. Redox balance in male infertility: Excellence through moderation-“μέτρον ἄριστον”. Antioxidants 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A.; Henkel, R.; Finelli, R.; Robert, K.A.; Iovine, C.; Baskaran, S. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Rad. Biol. Med. 2020, 152, 375–385. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Limphong, P.; Pieper, J.; Liu, Q.; Rodesch, C.K.; Christians, E.; Benjamin, I.J. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012, 26, 1442–1451. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Korge, P.; Calmettes, G.; Weiss, J.N. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta 2015, 1847, 514–525. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M. Vitamin e and the risk of prostate cancer: The selenium and vitamin e cancer prevention trial (select). J. Am. Med. Assoc. 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Brewer, A.C.; Mustafi, S.B.; Murray, T.V.; Rajasekaran, N.S.; Benjamin, I.J. Reductive stress linked to small hsps, g6pd, and nrf2 pathways in heart disease. Antioxid. Redox Signal. 2013, 18, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Mentor, S.; Fisher, D. Aggressive antioxidant reductive stress impairs brain endothelial cell angiogenesis and blood brain barrier function. Curr. Neurovas. Res. 2017, 14, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A. Antioxidants: An overview on the natural and synthetic types. Eur. Chem. Bull. 2017, 6, 365–375. [Google Scholar] [CrossRef]
- Miller, E.R., III; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin e supplementation may increase all-cause mortality. Ann. Int. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Eng. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Fraga, C.G.; Motchnik, P.A.; Shigenaga, M.K.; Helbock, H.J.; Jacob, R.A.; Ames, B.N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Nat. Acad. Sci. USA 1991, 88, 11003–11006. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Gajewski, E.; Dizdaroglu, M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J. 1991, 273, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Amengual, M.; Gosálvez, J.; Coward, K.; Hannaoui, N.; Benet, J.; García-Peiró, A.; Prats, J. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia 2013, 45, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. BioMed. 2016, 14, 729. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Sengupta, P.; Dutta, S.; Calogero, A.E. Coenzyme q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin. Exp. Reprod. Med. 2021, 48, 150–155. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97–104. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochr. Datab. Syst. Rev. 2014, 12, CD007411. [Google Scholar] [CrossRef]
- Garg, H.; Kumar, R. An update on the role of medical treatment including antioxidant therapy in varicocele. Asian J. Androl. 2016, 18, 222. [Google Scholar]
- Alahmar, A.T. The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2018, 45, 57. [Google Scholar] [CrossRef]
- Silver, E.W.; Eskenazi, B.; Evenson, D.P.; Block, G.; Young, S.; Wyrobek, A.J. Effect of antioxidant intake on sperm chromatin stability in healthy nonsmoking men. J. Androl. 2005, 26, 550–556. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C.C.; Borchert, A.; Heydeck, D.; Kuhn, H. Redox control in mammalian embryo development. Antiox. Redox Signal. 2010, 13, 833–875. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.J.; Kind, K.L.; Thompson, J.G. Redox regulation of early embryo development. Reproduction 2002, 123, 479–486. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Milzani, A.; Rossi, R. Is ascorbate able to reduce disulfide bridges? A cautionary note. Nitric Oxide 2008, 19, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.; Entezami, F.; Lichtblau, I.; Belloc, S.; Cohen, M.; Dale, B. Oxidative stress and fertility: Incorrect assumptions and ineffective solutions? Zygote 2014, 22, 80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bleau, G.; Lemarbre, J.; Faucher, G.; Roberts, K.D.; Chapdelaine, A. Semen selenium and human fertility. Fertil. Steril. 1984, 42, 890–894. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidat. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr. Risk factors for lung cancer and for intervention effects in caret, the beta-carotene and retinol efficacy trial. J. Nat. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. The effect of ascorbate and α-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 1999, 14, 505–512. [Google Scholar] [CrossRef]
- Verma, A.; Kanwar, K. Human sperm motility and lipid peroxidation in different ascorbic acid concentrations: An in vitro analysis. Andrologia 1998, 30, 325–329. [Google Scholar] [CrossRef]
- Moilanen, J.; Hovatta, O.; Lindroth, L. Vitamin e levels in seminal plasma can be elevated by oral administration of vitamin e in infertile men. Int. J. Androl. 1993, 16, 165–166. [Google Scholar] [CrossRef]
- DePalma, A.F.; Rothman, R.H.; Lewinnek, G.E.; Canale, S.T. Anterior interbody fusion for severe cervical disc degeneration. Surg. Gynecol. Obs. 1972, 134, 755–758. [Google Scholar]
- Purvis, K.; Christiansen, E. Infection in the male reproductive tract. Impact, diagnosis and treatment in relation to male infertility. Int. J. Androl. 1993, 16, 1–13. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Mahmoud, A.M.; Depuydt, C.E.; Zalata, A.A.; Christophe, A.B. Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: The andrologist’s viewpoint. Hum. Reprod. Update 1999, 5, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Chhikara, B.S. Reproductive inflammatory mediators and male infertility. Chem. Biol. Lett. 2020, 7, 73–74. [Google Scholar]
- Hales, D.B.; Diemer, T.; Hales, K.H. Role of cytokines in testicular function. Endocrine 1999, 10, 201–217. [Google Scholar] [CrossRef]
- Söder, O.; Sultana, T.; Jonsson, C.; Wahlgren, A.; Petersen, C.; Holst, M. The interleukin-1 system in the testis. Andrologia 2000, 32, 52–55. [Google Scholar] [PubMed]
- Diemer, T.; Hales, D.B.; Weidner, W. Immune-endocrine interactions and leydig cell function: The role of cytokines. Andrologia 2003, 35, 55–63. [Google Scholar] [CrossRef]
- Maegawa, M.; Kamada, M.; Irahara, M.; Yamamoto, S.; Yoshikawa, S.; Kasai, Y.; Ohmoto, Y.; Gima, H.; Thaler, C.J.; Aono, T. A repertoire of cytokines in human seminal plasma. J. Reprod. Immunol. 2002, 54, 33–42. [Google Scholar] [CrossRef]
- Theam, O.C.; Dutta, S.; Sengupta, P. Role of leucocytes in reproductive tract infections and male infertility. Chem. Biol. Lett. 2020, 7, 124–130. [Google Scholar]
- Cudicini, C.; Lejeune, H.; Gomez, E.; Bosmans, E.; Ballet, F.; Saez, J.; Jégou, B. Human leydig cells and sertoli cells are producers of interleukins-1 and -6. J. Clin. Endocrinol. Metab. 1997, 82, 1426–1433. [Google Scholar] [CrossRef]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allerg. Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef]
- Keck, C.; Gerber-Schäfer, C.; Clad, A.; Wilhelm, C.; Breckwoldt, M. Seminal tract infections: Impact on male fertility and treatment options. Hum. Reprod. Update 1998, 4, 891–903. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Hassan, M.F.; Biswas, A. Role of toll-like receptors in the reproductive tract inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 113–123. [Google Scholar]
- Potts, J.M.; Sharma, R.; Pasqualotto, F.; Nelson, D.; Hall, G.; Agarwal, A. Association of ureaplasma urealyticum with abnormal reactive oxygen species levels and absence of leukocytospermia. J. Urol. 2000, 163, 1775–1778. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Selvam, M.K.P.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C. Sperm DNA fragmentation: A new guideline for clinicians. World J. Men’s Health 2020, 38, 412. [Google Scholar] [CrossRef]
- Yu, B.; Huang, Z. Variations in antioxidant genes and male infertility. BioMed Res. Int. 2015, 2015, 513196. [Google Scholar] [CrossRef]
- Carrell, D.T.; Aston, K.I. The search for snps, cnvs, and epigenetic variants associated with the complex disease of male infertility. Syst. Biol. Reprod. Med. 2011, 57, 17–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kemal Duru, N.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Ko, E.Y. Oxidative stress in the pathophysiology of male infertility. Andrologia 2021, 53, e13581. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cao, X.; Pang, D.; Li, C.; Luo, Q.; Zou, Y.; Feng, B.; Li, L.; Cheng, A.; Chen, Z. Is male infertility associated with increased oxidative stress in seminal plasma? A-meta analysis. Oncotarget 2018, 9, 24494. [Google Scholar] [CrossRef]
- Naz, R.K.; Evans, L. Presence and modulation of interleukin-12 in seminal plasma of fertile and infertile men. J. Androl. 1998, 19, 302–307. [Google Scholar]
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabryś, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machoń-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative stress is associated with reduced sperm motility in normal semen. Am. J. Men’s Health 2020, 14, 1557988320939731. [Google Scholar] [CrossRef] [PubMed]
- Gruschwitz, M.S.; Brezinschek, R.; Brezinschek, H.P. Cytokine levels in the seminal plasma of infertile males. J. Androl. 1996, 17, 158–163. [Google Scholar] [PubMed]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Brit. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell. Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Collis, C.S.; Yang, M.; Kelly, M.; Diplock, A.T.; Halliwell, B.; Rice-Evans, C. The effects of iron and vitamin c co-supplementation on oxidative damage to DNA in healthy volunteers. Biochem. Biophys. Res. Comm. 1998, 246, 293–298. [Google Scholar] [CrossRef]
- Beatty, E.; England, T.; Geissler, C.; Aruoma, O.; Halliwell, B. Effects of antioxidant vitamin supplementation on markers of DNA damage and plasma antioxidants. Proc. Nutr. Soc. 1999, 58, 44. [Google Scholar]
- Podmore, I.D.; Griffiths, H.R.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Vitamin c exhibits pro-oxidant properties. Nature 1998, 392, 559. [Google Scholar] [CrossRef]
- Son, Y.O.; Pratheeshkumar, P.; Roy, R.V.; Hitron, J.A.; Wang, L.; Divya, S.P.; Xu, M.; Luo, J.; Chen, G.; Zhang, Z.; et al. Antioncogenic and oncogenic properties of nrf2 in arsenic-induced carcinogenesis. J. Biol. Chem. 2015, 290, 27090–27100. [Google Scholar] [CrossRef]
- Djuric, Z.; Kashif, M.; Fleming, T.; Muhammad, S.; Piel, D.; von Bauer, R.; Bea, F.; Herzig, S.; Zeier, M.; Pizzi, M.; et al. Targeting activation of specific nf-κb subunits prevents stress-dependent atherothrombotic gene expression. Mol. Med. 2012, 18, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant Regime | Study Population | Sperm Parameters | Study |
|---|---|---|---|
| Zinc sulphate (220 mg) for 4 months | oligospermic males (n = 14) | 21.4% (3/14) of patients achieved pregnancy | [62] |
| 600 mg of vitamin E daily for 3 months | Infertility with high ROS | No change in SC, Smot and Smorph | [63] |
| Zinc sulphate (500 mg) for 3 months | Asthenozoospermia (n = 100) | Improved pregnancy (22.5%) vs. placebo (4.3%) | [64] |
| 1 g vitamin C and 800 mg vitamin E daily for 56 days | Asthenozoospermia | No change in SC, Smot and Smorph | [65] |
| 1 g of vitamins C and E daily for 2 months | Idiopathic infertility (38 infertile men with previous IVF/ICSI) | No change in SC, Smot and Smorph | [66] |
| L-carnitine 3 g daily; L-acetyl carnitine 3 g daily; L-carnitine (2 g daily) and L-acetyl carnitine combination (1 g daily) | Idiopathic asthenozoospermia (Placebo group = 15, L-carnitine group = 15; L-acetylcarnitine group = 15; L-carnitine and L-acetylcarnitine combined group = 15) | Increase in sperm motility and normal sperm morphology | [67] |
| 1 g Carnitine and 500 mg L-acetyl carnitine daily for 24 weeks | Asthenozoospermia | No improvement in Smot | [68] |
| 400 mg each vitamins C and E, 18 mg β-carotene, 500 μmol zinc, 1 μmol selenium daily for 90 days | 38 infertile men with At least two failed IVF or ICSI | Increased chromatin decondensation | [69] |
| Menevit (lycopene, vitamins E, C, zinc, selenium, folate, garlic oil) daily for three months | 60 infertile men | No significant difference in DFI | [70] |
| 300 mg selenium daily for 48 weeks | Normozoospermia | No improvement in Smot and Smorph | [71] |
| Menevit (lycopene, vitamins E, C, zinc, selenium, folate, garlic oil) daily for three months | 50 infertile men with elevated OS | No change in SC, Smot and Smorph | [72] |
| 500 mg L-carnitine, 60 mg vitamin C, 20 mg coenzyme Q10, 10 mg vitamin E, 200 µg vitamin B, 91 µg vitamin B12, 10 mg zinc, 50 µg selenium once daily for 3 months | Prospective observational study with 20 men with grade 1 varicocele and primary or secondary infertility | No change in SC, Smot and Smorph | [73] |
| 30 mg vitamin C, 5 mg vitamin E, 0.5 µg vitamin B12, 750 mg L-carnitine, 10 mg coenzyme Q10, 100 µg folic acid, 5 mg zinc, 25 µg selenium twice daily for 6 months | 7 infertile men with sperm DFI >25%Treatment group (37 patients) Placebo group (40 patients) | No change in SC, DFI | [74] |
| Coenzyme Q10 = 200 mg daily | Idiopathic infertility (Placebo group = 114, Treatment group = 114) | Increase in sperm concentration, motility, and normal sperm morphology | [75] |
| L-carnitine = 1 g, L-acetylcarnitine = 0.5 g, fumarate = 0.725 g, fructose = 1 g, citric acid = 50 mg, zinc = 10 mg, coenzyme Q10 = 20 mg, selenium = 50 µg, Vit C = 90 mg, folic acid = 200 µg, Vit B12 = 1.5 µg daily for 3 months | Idiopathic oligoasthenozoospermia (Placebo group = 50, Treatment group = 50) | Increase in semen volume, progressive motility, and vitality in placebo group. Decrease in sperm DNA fragmentation index in treatment group. | [76] |
| 5 mg folic acid, 30 mg zinc once daily for 6 months | 1773 men planning to undergo infertility treatment with spouse | No improvement in Smot and Smorph | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Sengupta, P.; Roychoudhury, S.; Chakravarthi, S.; Wang, C.W.; Slama, P. Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation. Antioxidants 2022, 11, 167. https://doi.org/10.3390/antiox11010167
Dutta S, Sengupta P, Roychoudhury S, Chakravarthi S, Wang CW, Slama P. Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation. Antioxidants. 2022; 11(1):167. https://doi.org/10.3390/antiox11010167
Chicago/Turabian StyleDutta, Sulagna, Pallav Sengupta, Shubhadeep Roychoudhury, Srikumar Chakravarthi, Chee Woon Wang, and Petr Slama. 2022. "Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation" Antioxidants 11, no. 1: 167. https://doi.org/10.3390/antiox11010167
APA StyleDutta, S., Sengupta, P., Roychoudhury, S., Chakravarthi, S., Wang, C. W., & Slama, P. (2022). Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation. Antioxidants, 11(1), 167. https://doi.org/10.3390/antiox11010167









