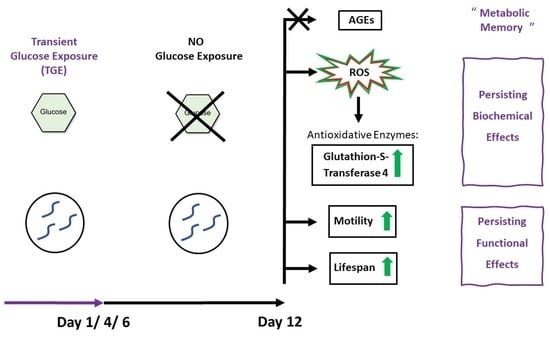
Protective Effects of Transient Glucose Exposure in Adult C. elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Maintenance
2.2. Activation of the Antioxidative Response
2.3. Formation of Advanced Glycation Endproducts (AGEs)
2.4. Motility
2.5. Life Span
2.6. Statistical Methods
3. Results
3.1. Activation of the Antioxidative Response
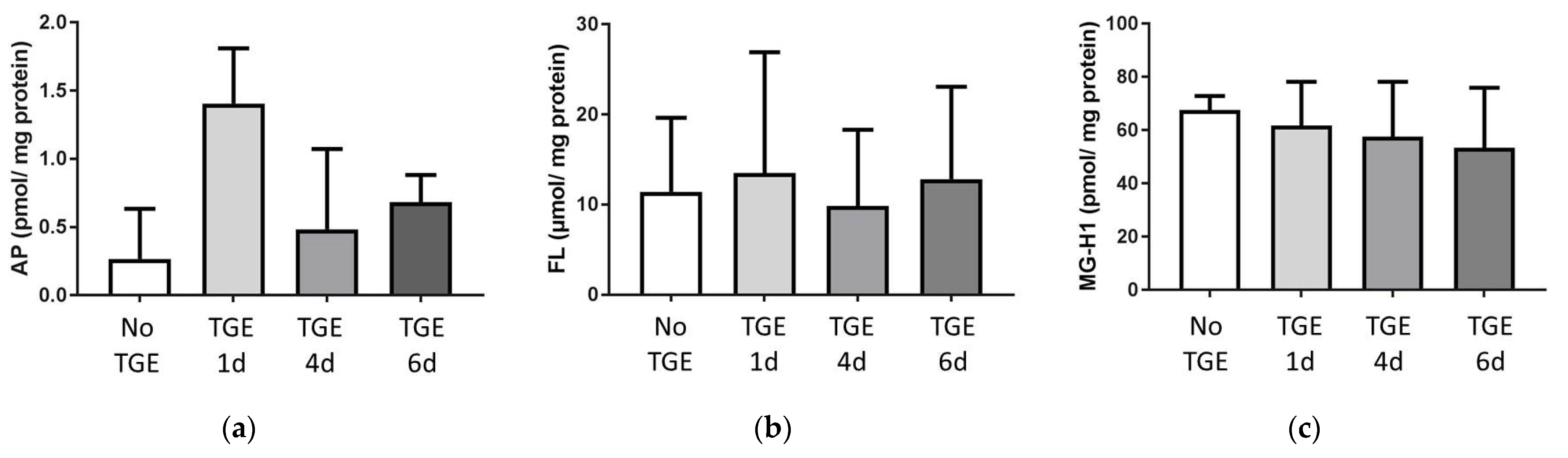
3.2. Formation of Advanced Glycation Endproducts (AGEs)
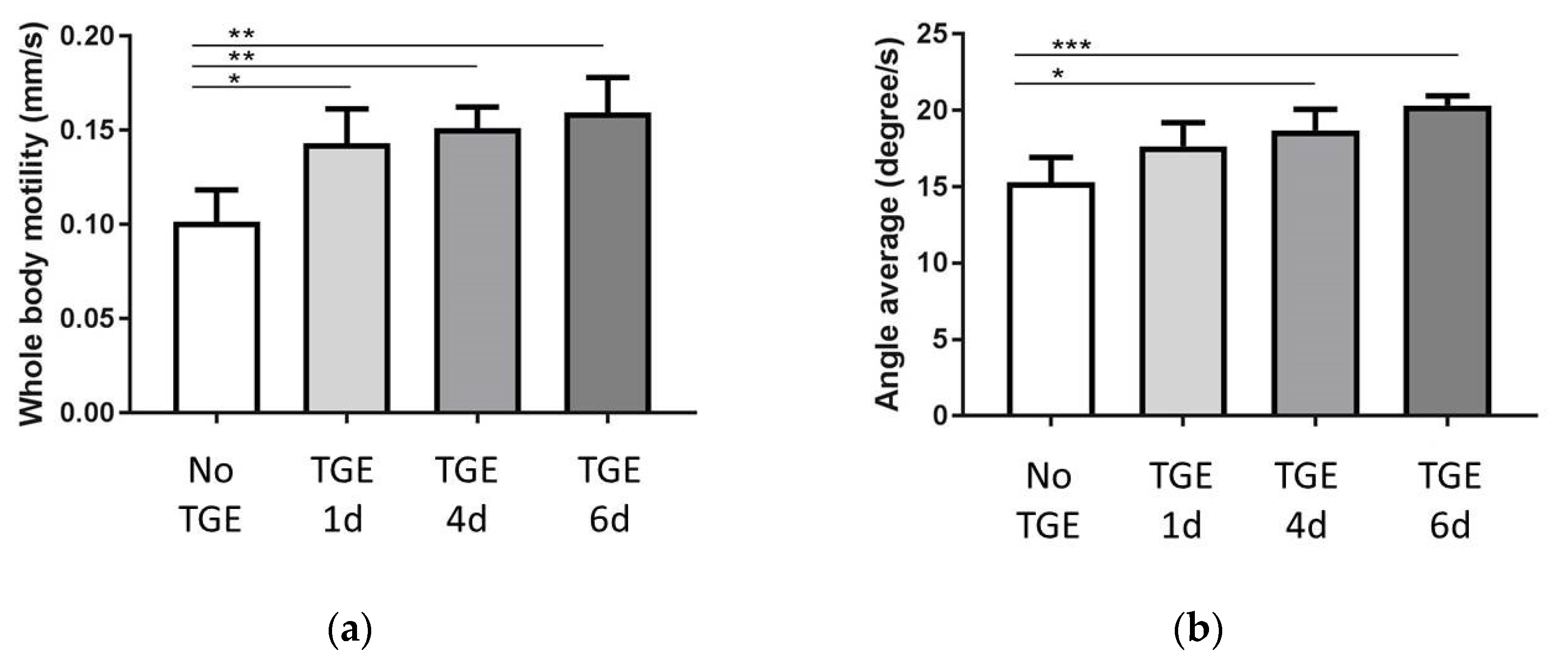
3.3. Motility
3.4. Life Span
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Lachin, J.M.; Genuth, S.; Nathan, D.M.; Zinman, B.; Rutledge, B.N. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—Revisited. Diabetes 2008, 57, 995–1001. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- ADVANCE Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef]
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Strokov, I.A.; Manukhina, E.B.; Bakhtina, L.Y.; Malyshev, I.Y.; Zoloev, G.K.; Kazikhanova, S.I.; Ametov, A.S. The Function of Endogenous Protective Systems in Patients with Insulin-Dependent Diabetes Mellitus and Polyneuropathy: Effect of Antioxidant Therapy. Bull. Exp. Biol. Med. 2000, 130, 986–990. [Google Scholar] [CrossRef]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P., Jr. A zebrafish model of diabetes mellitus and metabolic memory. J. Vis. Exp. JoVE 2013, 72, e50232. [Google Scholar] [CrossRef]
- Watts, J.L.; Ristow, M. Lipid and Carbohydrate Metabolism in Caenorhabditis elegans. Genetics 2017, 207, 413–446. [Google Scholar]
- Schlotterer, A.; Kukudov, G.; Bozorgmehr, F.; Hutter, H.; Du, X.; Oikonomou, D.; Ibrahim, Y.; Pfisterer, F.; Rabbani, N.; Thornalley, P.; et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes 2009, 58, 2450–2456. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sell, D.R.; Genuth, S. Glycation products as markers and predictors of the progression of diabetic complications. Ann. N. Y. Acad. Sci. 2005, 1043, 567–581. [Google Scholar] [CrossRef]
- Schlotterer, A.; Kolibabka, M.; Lin, J.; Acunman, K.; Dietrich, N.; Sticht, C.; Fleming, T.; Nawroth, P.; Hammes, H.P. Methylglyoxal induces retinopathy-type lesions in the absence of hyperglycemia: Studies in a rat model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 4141–4153. [Google Scholar] [CrossRef]
- Shipanova, I.N.; Glomb, M.A.; Nagaraj, R.H. Protein modification by methylglyoxal: Chemical nature and synthetic mechanism of a major fluorescent adduct. Arch. Biochem. Biophys. 1997, 344, 29–36. [Google Scholar] [CrossRef]
- UKPDS-Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Misra, A.; Bloomgarden, Z. Metabolic memory: Evolving concepts. J. Diabetes 2018, 10, 186–187. [Google Scholar] [CrossRef]
- Laiteerapong, N.; Ham, S.A.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 2019, 42, 416–426. [Google Scholar]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Ceriello, A. The emerging challenge in diabetes: The “metabolic memory”. Vascul. Pharmacol. 2012, 57, 133–138. [Google Scholar] [CrossRef]
- Alcántar-Fernández, J.; Navarro, R.E.; Salazar-Martínez, A.M.; Pérez-Andrade, M.E.; Miranda-Ríos, J. Caenorhabditis elegans respond to high-glucose diets through a network of stress-responsive transcription factors. PLoS ONE 2018, 13, e0199888. [Google Scholar] [CrossRef]
- Lee, S.J.; Murphy, C.T.; Kenyon, C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009, 10, 379–391. [Google Scholar] [CrossRef]
- Franco-Juárez, B.; Mejía-Martínez, F.; Moreno-Arriola, E.; Hernández-Vázquez, A.; Gómez-Manzo, S.; Marcial-Quino, J.; Arreguín-Espinosa, R.; Velázquez-Arellano, A.; Ortega-Cuellar, D. A high glucose diet induces autophagy in a HLH-30/TFEB-dependent manner and impairs the normal lifespan of C. elegans. Aging 2018, 10, 2657–2667. [Google Scholar] [CrossRef]
- Schlotterer, A.; Pfisterer, F.; Kukudov, G.; Heckmann, B.; Henriquez, D.; Morath, C.; Krämer, B.K.; Hammes, H.-P.; Schwenger, V.; Morcos, M. Neuronal damage and shortening of lifespan in C. elegans by peritoneal dialysis fluid: Protection by glyoxalase-1. Biomed. Rep. 2018, 8, 540–546. [Google Scholar] [CrossRef]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef]
- Hansen, M.; Chandra, A.; Mitic, L.L.; Onken, B.; Driscoll, M.; Kenyon, C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008, 4, e24. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef]
- Tauffenberger, A.; Vaccaro, A.; Parker, J.A. Fragile lifespan expansion by dietary mitohormesis in C. elegans. Aging 2016, 8, 50–61. [Google Scholar] [CrossRef]
- Bazopoulou, D.; Knoefler, D.; Zheng, Y.; Ulrich, K.; Oleson, B.J.; Xie, L.; Kim, M.; Kaufmann, A.; Lee, Y.-T.; Dou, Y.; et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature 2019, 576, 301–305. [Google Scholar] [CrossRef]
- Leiers, B.; Kampkotter, A.; Grevelding, C.G.; Link, C.D.; Johnson, T.E.; Henkle-Duhrsen, K. A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic. Biol. Med. 2003, 34, 1405–1415. [Google Scholar] [CrossRef]
- Detienne, G.; Van de Walle, P.; De Haes, W.; Schoofs, L.; Temmerman, L. SKN-1-independent transcriptional activation of glutathione S-transferase 4 (GST-4) by EGF signaling. Worm 2016, 5, e1230585. [Google Scholar] [CrossRef][Green Version]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef]
- Kohl, K.; Fleming, T.; Acunman, K.; Hammes, H.P.; Morcos, M.; Schlotterer, A. Plate-based Large-scale Cultivation of Caenorhabditis elegans: Sample Preparation for the Study of Metabolic Alterations in Diabetes. J. Vis. Exp. JoVE 2018, 138, 58117. [Google Scholar] [CrossRef] [PubMed]
- Shore, D.E.; Carr, C.E.; Ruvkun, G. Induction of cytoprotective pathways is central to the extension of lifespan conferred by multiple longevity pathways. PLoS Genetics. 2012, 8, e1002792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Mitochondrial hormesis and diabetic complications. Diabetes 2015, 64, 663–672. [Google Scholar] [CrossRef] [PubMed]

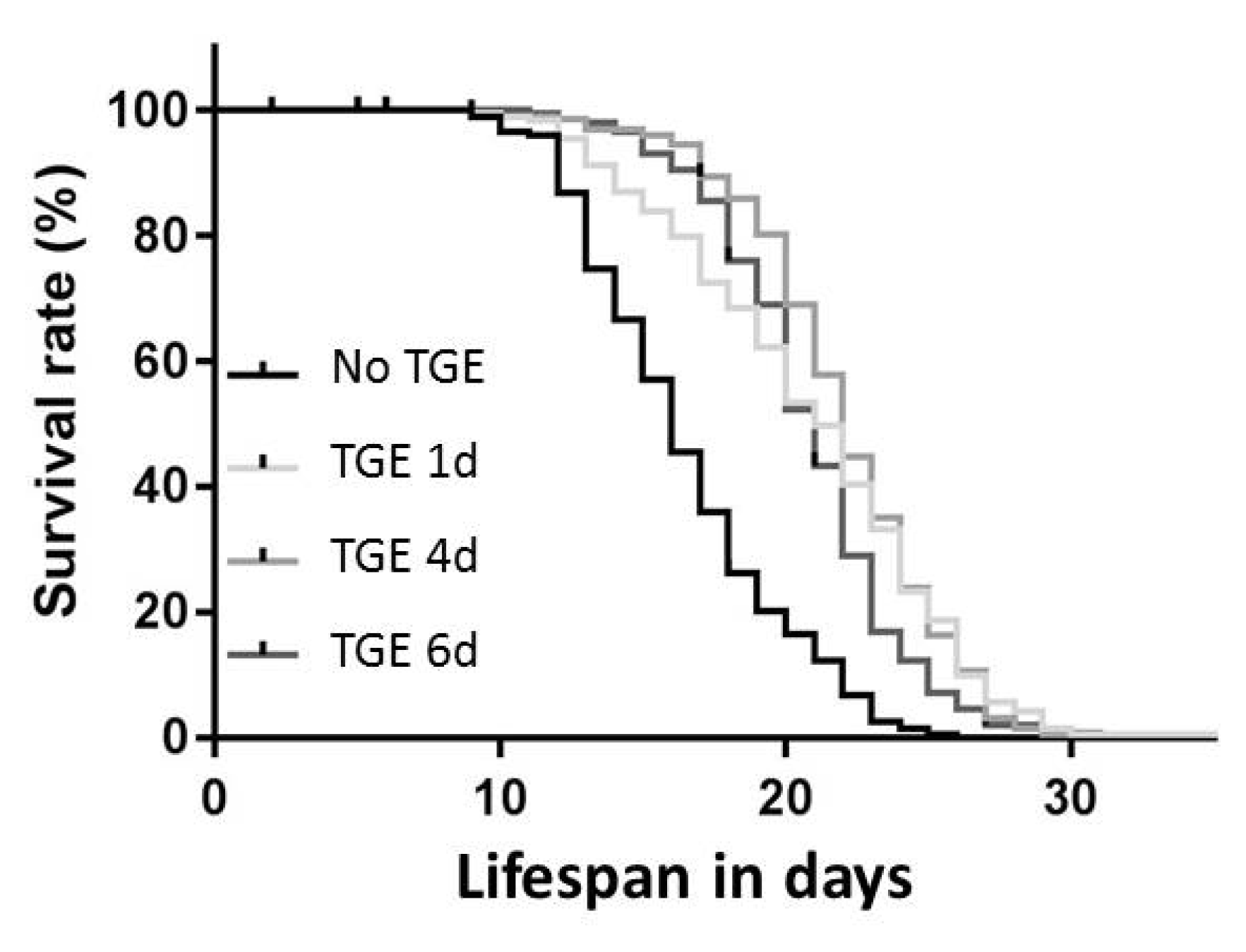
| Glucose Exposure (mM) | Duration (Days) | Life Span (Days) Mean ± SD | Change in % as Compared to Controls | p-Value | n |
|---|---|---|---|---|---|
| 0 | 0 | 16 ± 1.3 | n. a. | n. a. | 197 |
| 400 | 1 | 21 ± 2.4 | 27 | <0.01 | 194 |
| 400 | 4 | 22 ± 1.2 | 34 | <0.001 | 197 |
| 400 | 6 | 21 ± 1.4 | 26 | <0.01 | 197 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo, K.; Samigullin, A.; Humpert, P.M.; Fleming, T.; Özer, K.; Schlotterer, A.; Hammes, H.-P.; Morcos, M. Protective Effects of Transient Glucose Exposure in Adult C. elegans. Antioxidants 2022, 11, 160. https://doi.org/10.3390/antiox11010160
Murillo K, Samigullin A, Humpert PM, Fleming T, Özer K, Schlotterer A, Hammes H-P, Morcos M. Protective Effects of Transient Glucose Exposure in Adult C. elegans. Antioxidants. 2022; 11(1):160. https://doi.org/10.3390/antiox11010160
Chicago/Turabian StyleMurillo, Katharina, Azat Samigullin, Per M. Humpert, Thomas Fleming, Kübra Özer, Andrea Schlotterer, Hans-Peter Hammes, and Michael Morcos. 2022. "Protective Effects of Transient Glucose Exposure in Adult C. elegans" Antioxidants 11, no. 1: 160. https://doi.org/10.3390/antiox11010160
APA StyleMurillo, K., Samigullin, A., Humpert, P. M., Fleming, T., Özer, K., Schlotterer, A., Hammes, H.-P., & Morcos, M. (2022). Protective Effects of Transient Glucose Exposure in Adult C. elegans. Antioxidants, 11(1), 160. https://doi.org/10.3390/antiox11010160