Sulfane Sulfur Regulates LasR-Mediated Quorum Sensing and Virulence in Pseudomonas aeruginosa PAO1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Reagents
2.2. Gene Knockout and Complementation
2.3. Detection of H2S and Sulfane Sulfur
2.4. Rhamnolipids Production Measurement
2.5. Pyocyanin Quantitation Assay
2.6. Lettuce Leaf Model of Infection
2.7. Transcriptomic Analysis of PAO1 and PaΔH2S
2.8. Reporter Plasmids Construction and Fluorescence Assays
2.9. Protein Expression and Purification
2.10. Electrophoretic Mobility Shift Assay (EMSA)
2.11. In Vitro Transcription–Translation Analysis
2.12. LC-MS/MS Analysis of LasR
2.13. Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
3. Results
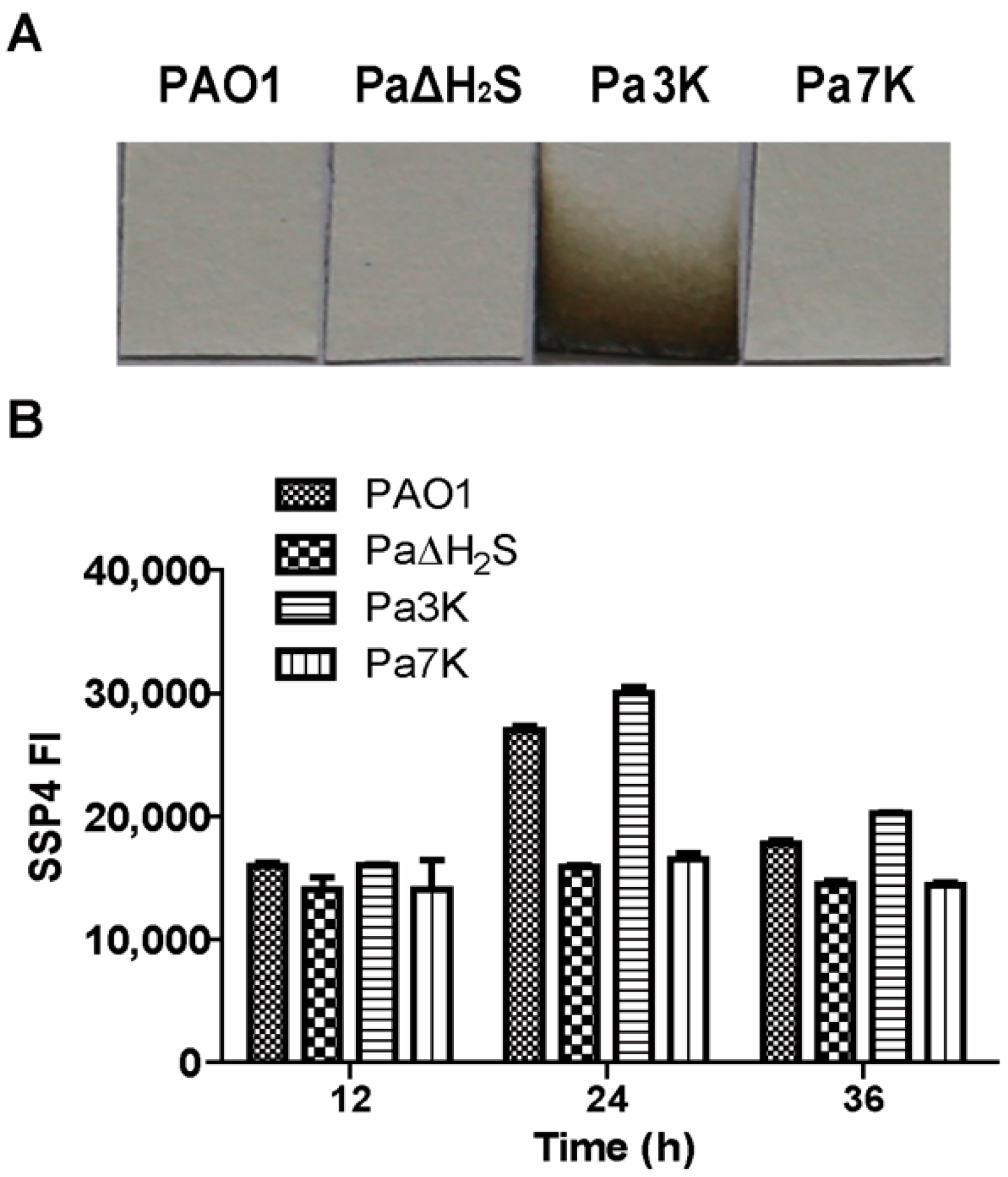
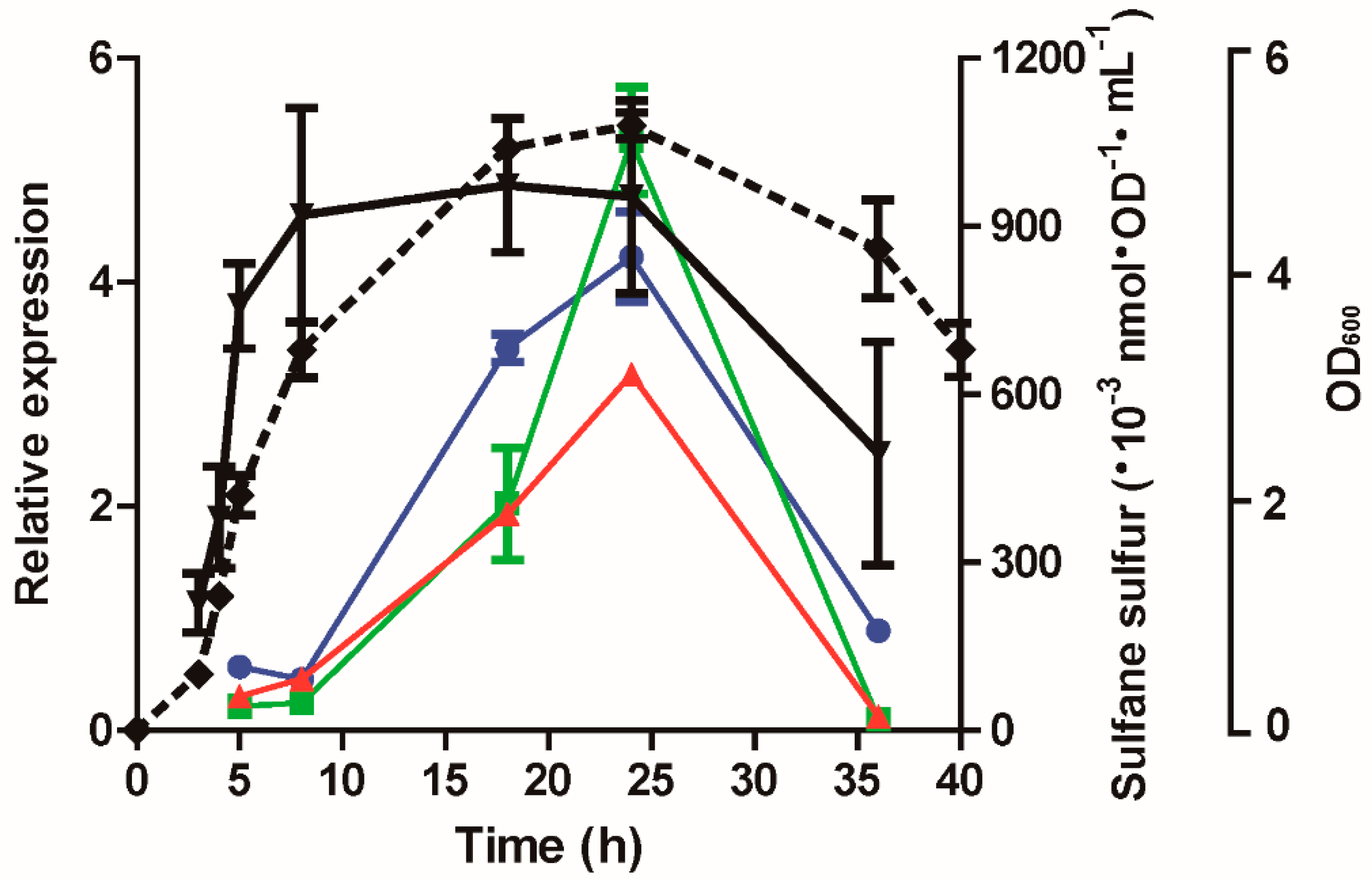
3.1. H2S and Sulfane Sulfur Production by P. aeruginosa PAO1 and Its Mutants
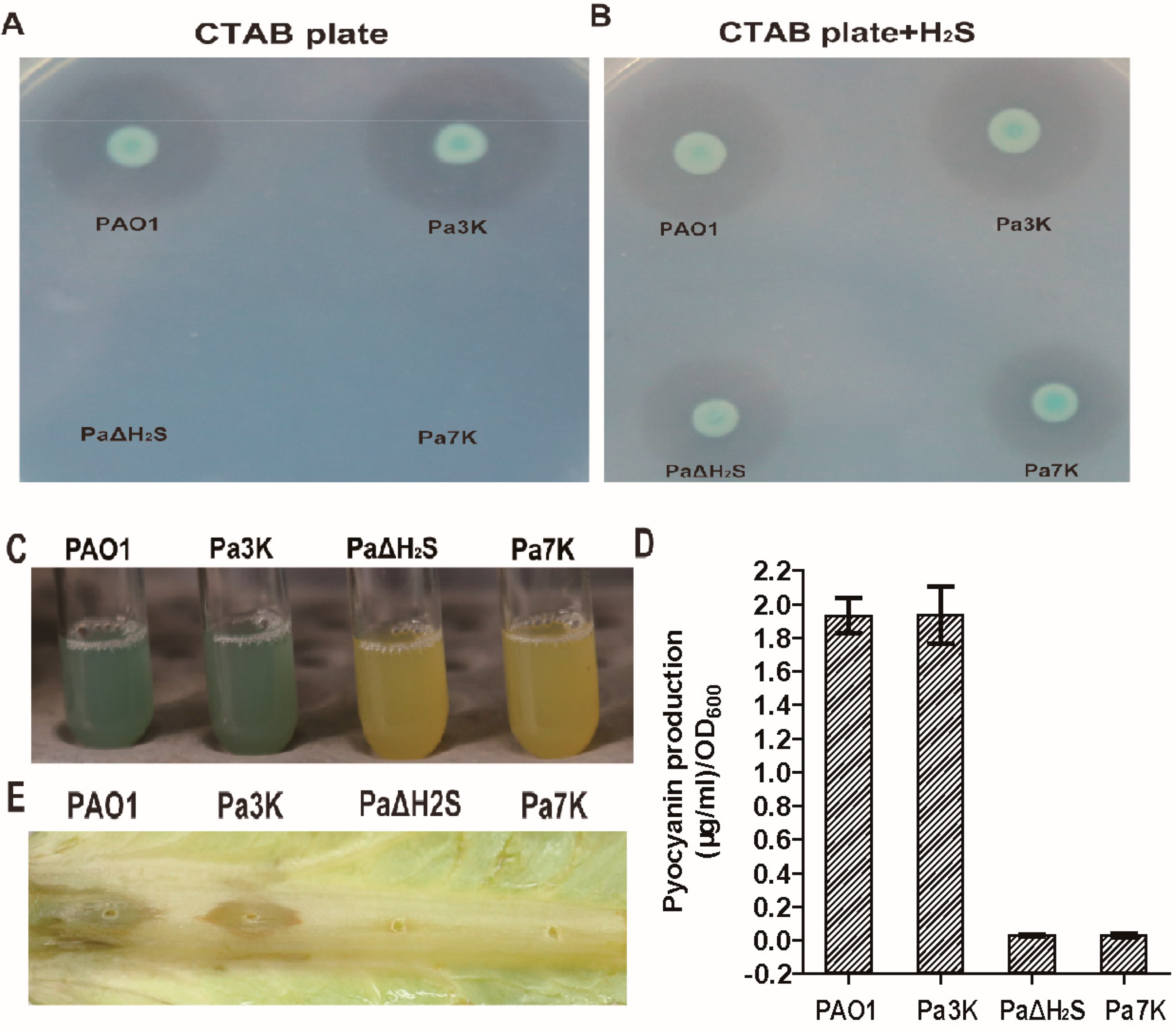
3.2. Virulence Factors and Pathogenicity of PAO1 and Its Mutant Strains
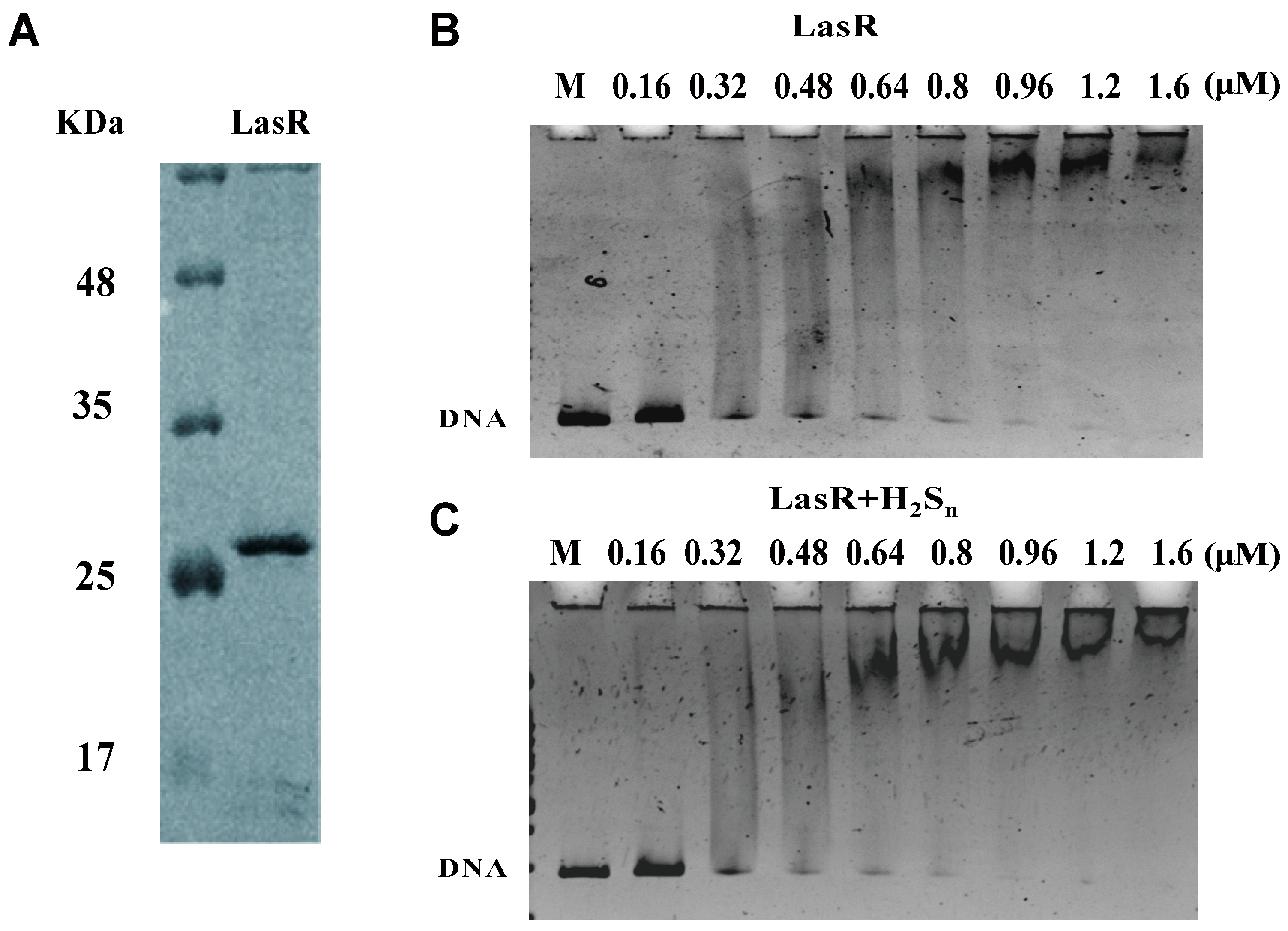
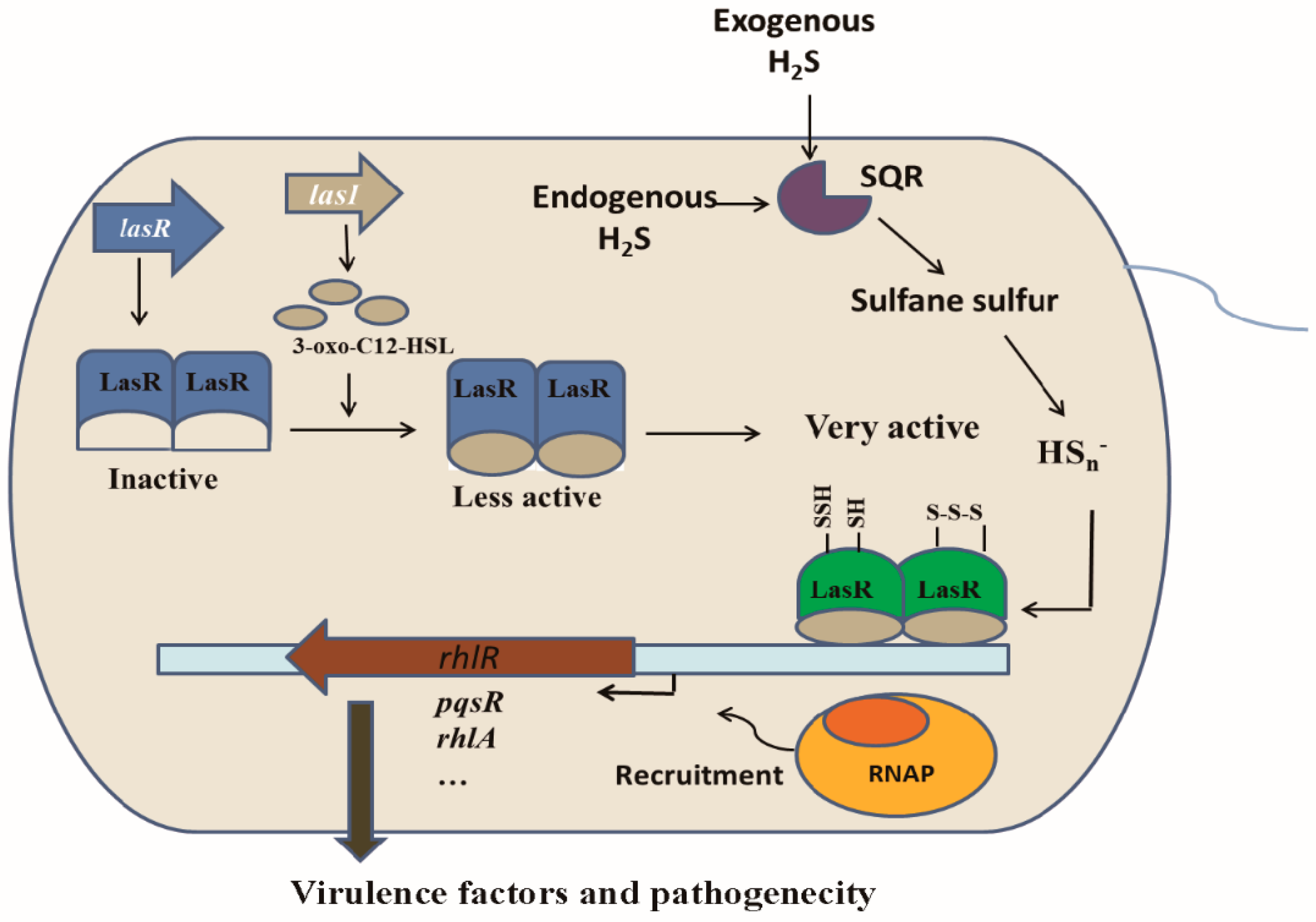
3.3. Linking H2S/Sulfane Sulfur to LasR
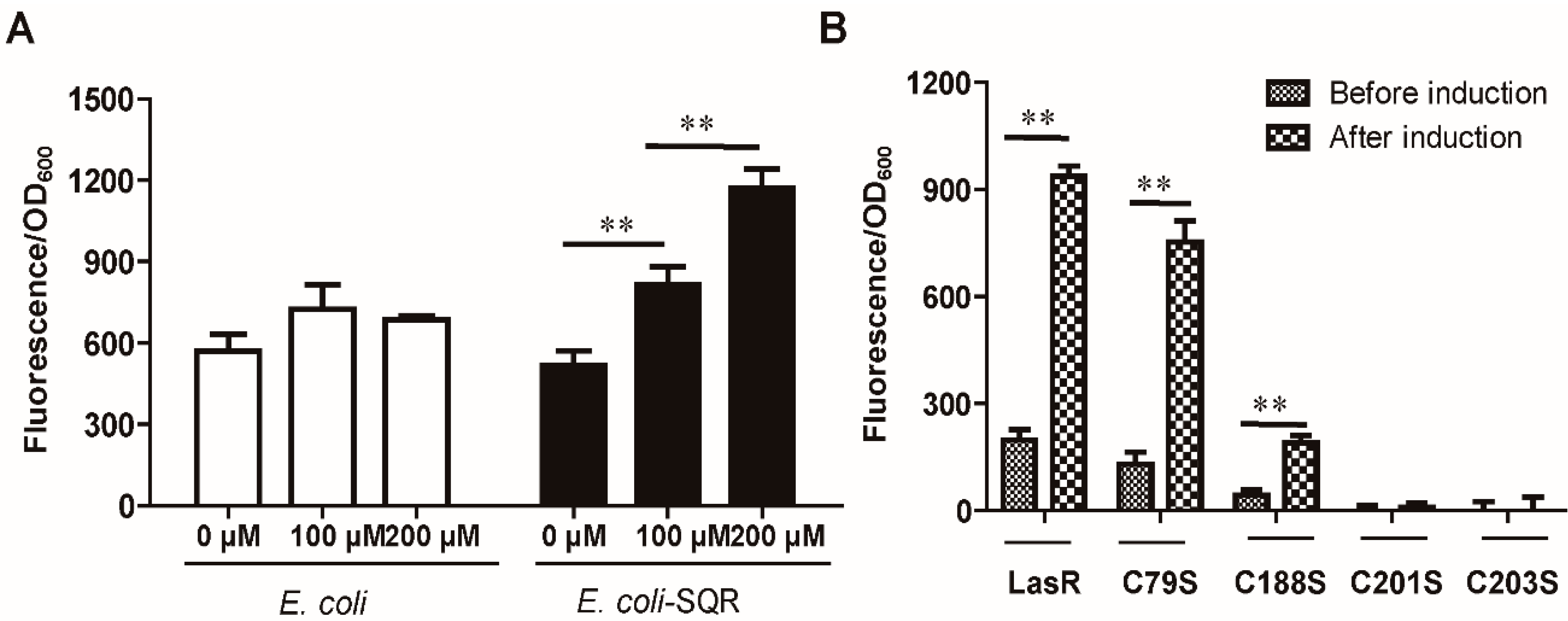
3.4. LasR Senses H2S through Sulfane Sulfur
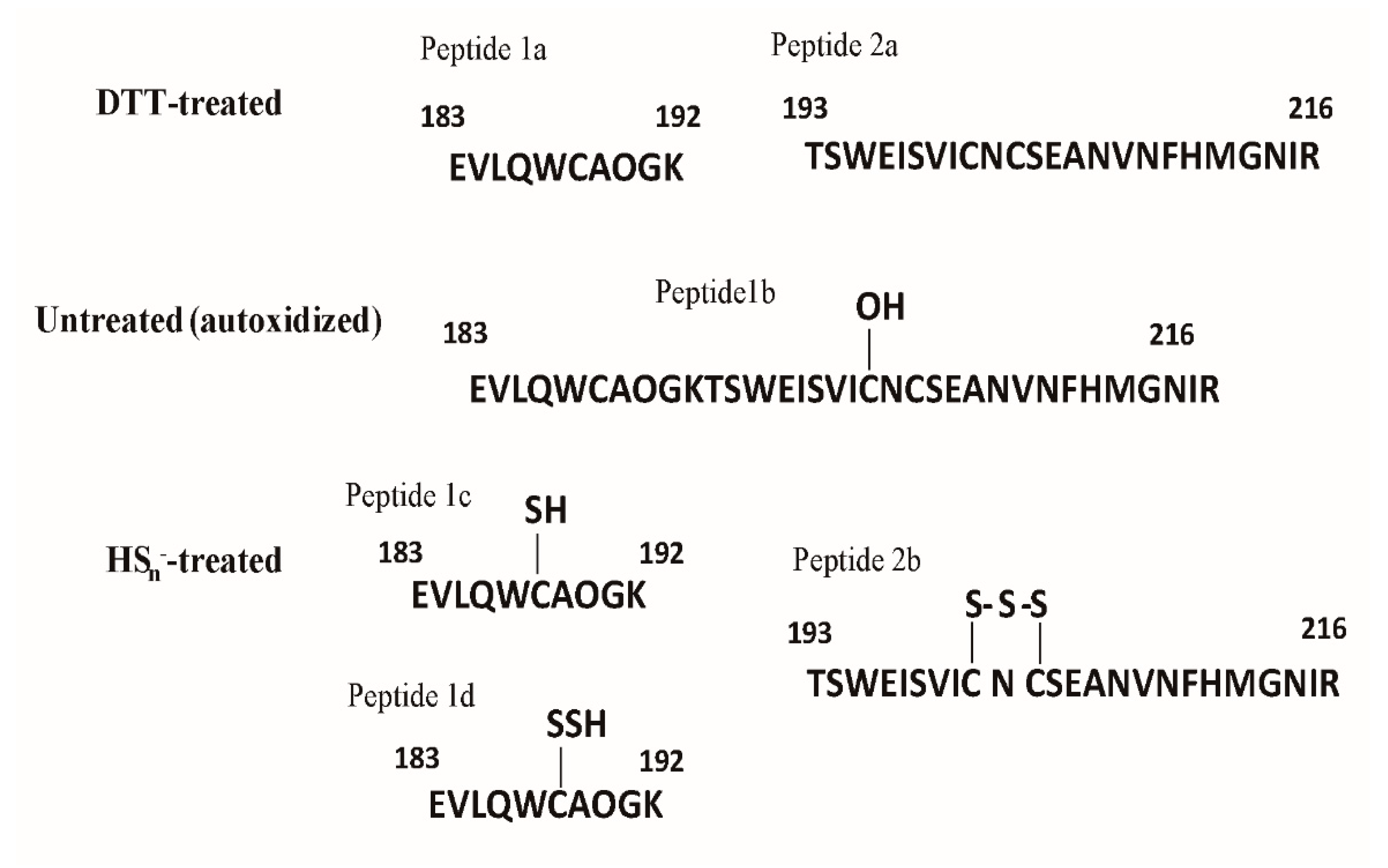
3.5. Characterization of LasR Modification
3.6. HSn− Modifies Cys188, Cys201, and Cys203 of LasR
3.7. The Expression of lasB, rhlR, and lasI Was Affected by Cellular Sulfane Sulfur
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimura, H. Hydrogen sulfide and polysulfides as signaling molecules. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 131–159. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.H.; Wong, P.T.-H.; Bian, J. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Panopoulos, P.; Coletta, C.; Papapetropoulos, A.; Szabo, C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem. Pharmacol. 2013, 86, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Sawa, T.; Kitajima, N.; Ono, K.; Inoue, H.; Ihara, H.; Motohashi, H.; Yamamoto, M.; Suematsu, M.; Kurose, H.; et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012, 8, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.C.; Missner, A.; Kugler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, D. Hydrogen sulfide: Clandestine microbial messenger? Trends Microbiol. 2006, 14, 456–462. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Lü, C.; Xia, Y.; Xin, Y.; Liu, H.; Xun, L.; Liu, H. FisR activates sigma(54)-dependent transcription of sulfide-oxidizing genes inCupriavidus pinatubonensisJMP134. Mol. Microbiol. 2017, 105, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Shen, J.; Fang, M.; Zhang, Y.; Hori, K.; Trinidad, J.C.; Bauer, C.E.; Giedroc, D.P.; Masuda, S. Sulfide-responsive transcriptional repressor SqrR functions as a master regulator of sulfide-dependent photosynthesis. Proc. Natl. Acad. Sci. USA 2017, 114, 2355–2360. [Google Scholar] [CrossRef] [Green Version]
- Luebke, J.L.; Shen, J.; Bruce, K.E.; Kehl-Fie, T.; Peng, H.; Skaar, E.P.; Giedroc, D.P. The CsoR-like sulfurtransferase repressor (CstR) is a persulfide sensor in Staphylococcus aureus. Mol. Microbiol. 2014, 94, 1343–1360. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Cao, Q.; Pang, X.; Xia, Y.; Xun, L.; Liu, H. Sulfane sulfur-activated actinorhodin production and sporulation is maintained by a natural gene circuit in Streptomyces coelicolor. Microb. Biotechnol. 2020, 13, 1917–1932. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, H.; Cui, F.; Liu, H.; Xun, L. Recombinant Escherichia coli with sulfide:quinone oxidoreductase and persulfide dioxygenase rapidly oxidises sulfide to sulfite and thiosulfate via a new pathway. Environ. Microbiol. 2016, 18, 5123–5136. [Google Scholar] [CrossRef]
- Cherney, M.M.; Zhang, Y.; Solomonson, M.; Weiner, J.H.; James, M.N. Crystal Structure of Sulfide: Quinone Oxidoreductase from Acidithiobacillus ferrooxidans: Insights into Sulfidotrophic Respiration and Detoxification. J. Mol. Biol. 2010, 398, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, K.; Li, H.; Wang, Q.; Yang, Y.; Li, K.; Xia, Y.; Xun, L. Synthetic Gene Circuits Enable Escherichia coli to Use Endogenous H2S as a Signaling Molecule for Quorum Sensing. ACS Synth. Biol. 2019, 8, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Yan, Z.; Fan, K.; Li, H.; Zhao, R.; Xia, Y.; Xun, L.; Liu, H. OxyR senses sulfane sulfur and activates the genes for its removal in Escherichia coli. Redox Biol. 2019, 26, 101293. [Google Scholar] [CrossRef] [PubMed]
- Xuan, G.; Lü, C.; Xu, H.; Chen, Z.; Li, K.; Liu, H.; Liu, H.; Xia, Y.; Xun, L. Sulfane Sulfur is an intrinsic signal activating MexR-regulated antibiotic resistance in Pseudomonas aeruginosa. Mol. Microbiol. 2020, 114, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lü, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, M.; Wang, T.; Shao, M.; Chen, Z.; Liu, H.; Xia, Y.; Xun, L. Sensitive Method for Reliable Quantification of Sulfane Sulfur in Biological Samples. Anal. Chem. 2019, 91, 11981–11986. [Google Scholar] [CrossRef]
- Li, K.; Xin, Y.; Xuan, G.; Zhao, R.; Liu, H.; Xia, Y.; Xun, L. Escherichia coli Uses Separate Enzymes to Produce H2S and Reactive Sulfane Sulfur From L-cysteine. Front. Microbiol. 2019, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A Universal Defense Against Antibiotics in Bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and Kinetic Analysis of H2S Production by Human Mercaptopyruvate Sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [Green Version]
- Akaike, T.; Ida, T.; Wei, F.-Y.; Nishida, M.; Kumagai, Y.; Alam, M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, X.; Li, H.; Liu, H.; Xia, Y.; Xun, L. The Complete Pathway for Thiosulfate Utilization in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2018, 84, e01241-18. [Google Scholar] [CrossRef] [Green Version]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [Green Version]
- Winstanley, C.; Fothergill, J.L. The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosainfections. FEMS Microbiol. Lett. 2009, 290, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s Not Easy Being Green: A Narrative Review on the Microbiology, Virulence and Therapeutic Prospects of Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef]
- Donadu, M.; Usai, D.; Pinna, A.; Porcu, T.; Mazzarello, V.; Fiamma, M.; Marchetti, M.; Cannas, S.; Delogu, G.; Zanetti, S.; et al. In vitro activity of hybrid lavender essential oils against multidrug resistant strains of Pseudomonas aeruginosa. J. Infect. Dev. Ctries. 2018, 12, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Dong, Y.; Wu, D.; Bowler, M.; Zhang, L.; Song, H. QsIA disrupts LasR dimerization in antiactivation of bacterial quorum sensing. Proc. Natl. Acad. Sci. USA 2013, 110, 20765–20770. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Feng, L.; Rutherford, S.T.; Papenfort, K.; Bassler, B. Functional determinants of the quorum-sensing non-coding RNAs and their roles in target regulation. EMBO J. 2013, 32, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Suneby, E.G.; Herndon, L.R.; Schneider, T.L. Pseudomonas aeruginosa LasR·DNA Binding Is Directly Inhibited by Quorum Sensing Antagonists. ACS Infect. Dis. 2017, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Medina, G.; Juárez, K.; Díaz, R.; Soberón-Chávez, G. Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology 2003, 149, 3073–3081. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, K.B.; Kim, T.H.; Gupta, R.; Greenberg, E.P.; Schuster, M. Global position analysis of the Pseudomonas aeruginosa quorum-sensing transcription factor LasR. Mol. Microbiol. 2009, 73, 1072–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, M.; Urbanowski, M.L.; Greenberg, E.P. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. USA 2004, 101, 15833–15839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCready, A.; Paczkowski, J.E.; Henke, B.R.; Bassler, B.L. Structural determinants driving homoserine lactone ligand selection in the Pseudomonas aeruginosa LasR quorum-sensing receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Sappington, K.J.; Dandekar, A.A.; Oinuma, K.-I.; Greenberg, E.P. Reversible Signal Binding by the Pseudomonas aeruginosa Quorum-Sensing Signal Receptor LasR. mBio 2011, 2, e00011-11. [Google Scholar] [CrossRef] [Green Version]
- Whiteley, M.; Lee, K.M.; Greenberg, E.P. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 13904–13909. [Google Scholar] [CrossRef] [Green Version]
- Schuster, M.; Lostroh, P.; Ogi, T.; Greenberg, E.P. Identification, Timing, and Signal Specificity of Pseudomonas aeruginosa Quorum-Controlled Genes: A Transcriptome Analysis. J. Bacteriol. 2003, 185, 2066–2079. [Google Scholar] [CrossRef] [Green Version]
- Scholz, R.L.; Greenberg, E.P. Positive Autoregulation of an Acyl-Homoserine Lactone Quorum-Sensing Circuit Synchronizes the Population Response. mBio 2017, 8, e01079-17. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Oinuma, K.-I.; Smalley, N.E.; Schaefer, A.L.; Hamwy, O.; Greenberg, E.P.; Dandekar, A.A. The Pseudomonas aeruginosa Orphan Quorum Sensing Signal Receptor QscR Regulates Global Quorum Sensing Gene Expression by Activating a Single Linked Operon. mBio 2018, 9, e01274-18. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Asfahl, K.L.; Li, N.; Sun, F.; Xiao, J.; Shen, D.; Dandekar, A.A.; Wang, M. Conditional quorum-sensing induction of a cyanide-insensitive terminal oxidase stabilizes cooperating populations of Pseudomonas aeruginosa. Nat. Commun. 2019, 10, 4999. [Google Scholar] [CrossRef]
- Kafle, P.; Amoh, A.N.; Reaves, J.M.; Suneby, E.G.; Tutunjian, K.A.; Tyson, R.L.; Schneider, T.L. Molecular Insights into the Impact of Oxidative Stress on the Quorum-Sensing Regulator Protein LasR. J. Biol. Chem. 2016, 291, 11776–11786. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.K.; Vrla, G.D.; Fröhlich, K.; Gitai, Z. Surface association sensitizes Pseudomonas aeruginosa to quorum sensing. Nat. Commun. 2019, 10, 4118. [Google Scholar] [CrossRef]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamyshny, A. Improved cyanolysis protocol for detection of zero-valent sulfur in natural aquatic systems. Limnol. Oceanogr. Methods 2009, 7, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Lü, C.; Xia, Y.; Liu, D.; Zhao, R.; Gao, R.; Liu, H.; Xun, L. Cupriavidus necator H16 Uses Flavocytochrome c Sulfide Dehydrogenase to Oxidize Self-Produced and Added Sulfide. Appl. Environ. Microbiol. 2017, 83, e01610-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harighi, B. Genetic evidence for CheB- and CheR-dependent chemotaxis system in A. tumefaciens toward acetosyringone. Microbiol. Res. 2009, 164, 634–641. [Google Scholar] [CrossRef]
- Kolluru, G.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide Biol. Chem. 2013, 35, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Bibli, S.-I.; Luck, B.; Zukunft, S.; Wittig, J.; Chen, W.; Xian, M.; Papapetropoulos, A.; Hu, J.; Fleming, I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 2018, 18, 295–304. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Chen, F.; Xia, Y.; Lou, J.; Zhang, X.; Yang, N.; Sun, X.; Zhang, Q.; Zhuo, C.; et al. A Novel Signal Transduction Pathway that Modulates rhl Quorum Sensing and Bacterial Virulence in Pseudomonas aeruginosa. PLOS Pathog. 2014, 10, e1004340. [Google Scholar] [CrossRef] [Green Version]
- Filiatrault, M.J.; Picardo, K.F.; Ngai, H.; Passador, L.; Iglewski, B.H. Identification of Pseudomonas aeruginosa Genes Involved in Virulence and Anaerobic Growth. Infect. Immun. 2006, 74, 4237–4245. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.Z.; Chu, W.Q.; Qi, Q.S.; Xun, L.Y. New insights into the QuikChange (TM) process guide the use of Phusion DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 2015, 43, e12. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Peng, H.; Zhang, Y.; Trinidad, J.C.; Giedroc, D.P. Staphylococcus aureus sqr Encodes a Type II Sulfide: Quinone Oxidoreductase and Impacts Reactive Sulfur Speciation in Cells. Biochemistry 2016, 55, 6524–6534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular Insights into Quorum Sensing in the Human Pathogen Pseudomonas aeruginosa from the Structure of the Virulence Regulator LasR Bound to Its Autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef] [Green Version]
- Giedroc, D.P. A new player in bacterial sulfide-inducible transcriptional regulation. Mol. Microbiol. 2017, 105, 347–352. [Google Scholar] [CrossRef]
- Volkmer, B.; Heinemann, M. Condition-Dependent Cell Volume and Concentration of Escherichia coli to Facilitate Data Conversion for Systems Biology Modeling. PLoS ONE 2011, 6, e23126. [Google Scholar] [CrossRef] [Green Version]
- Dóka, É.; Pader, I.; Bíró, A.; Johansson, K.; Cheng, Q.; Ballagó, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passador, L.; Cook, J.M.; Gambello, M.J.; Rust, L.; Iglewski, B.H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 1993, 260, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Winzer, K.; Falconer, C.; Garber, N.C.; Diggle, S.P.; Camara, M.; Williams, P. The Pseudomonas aeruginosa Lectins PA-IL and PA-IIL Are Controlled by Quorum Sensing and by RpoS. J. Bacteriol. 2000, 182, 6401–6411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, M.; Greenberg, E.P. Early activation of quorum sensing in Pseudomonas aeruginosa reveals the architecture of a complex regulon. BMC Genom. 2007, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Weerapana, E.; Ulanovskaya, O.; Sun, F.; Liang, H.; Ji, Q.; Ye, Y.; Fu, Y.; Zhou, L.; Li, J.; et al. Proteome-wide Quantification and Characterization of Oxidation-Sensitive Cysteines in Pathogenic Bacteria. Cell Host Microbe 2013, 13, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wang, M.; Smalley, N.E.; Kostylev, M.; Schaefer, A.L.; Greenberg, E.P.; Dandekar, A.A.; Xu, F. Modulation of Pseudomonas aeruginosa Quorum Sensing by Glutathione. J. Bacteriol. 2019, 201, e00685-00618. [Google Scholar] [CrossRef] [Green Version]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The Role of Diallyl Sulfides and Dipropyl Sulfides in the In Vitro Antimicrobial Activity of the Essential Oil of Garlic, Allium sativum L., and Leek, Allium porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.-M.; Yin, M.-C. In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J. Med. Microbiol. 2001, 50, 646–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-R.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Sun, T.-L.; Peng, H.; Huang, X.-M. Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 2018, 102, 7555–7564. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.-I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, G.; Lv, C.; Xu, H.; Li, K.; Liu, H.; Xia, Y.; Xun, L. Sulfane Sulfur Regulates LasR-Mediated Quorum Sensing and Virulence in Pseudomonas aeruginosa PAO1. Antioxidants 2021, 10, 1498. https://doi.org/10.3390/antiox10091498
Xuan G, Lv C, Xu H, Li K, Liu H, Xia Y, Xun L. Sulfane Sulfur Regulates LasR-Mediated Quorum Sensing and Virulence in Pseudomonas aeruginosa PAO1. Antioxidants. 2021; 10(9):1498. https://doi.org/10.3390/antiox10091498
Chicago/Turabian StyleXuan, Guanhua, Chuanjuan Lv, Huangwei Xu, Kai Li, Huaiwei Liu, Yongzhen Xia, and Luying Xun. 2021. "Sulfane Sulfur Regulates LasR-Mediated Quorum Sensing and Virulence in Pseudomonas aeruginosa PAO1" Antioxidants 10, no. 9: 1498. https://doi.org/10.3390/antiox10091498
APA StyleXuan, G., Lv, C., Xu, H., Li, K., Liu, H., Xia, Y., & Xun, L. (2021). Sulfane Sulfur Regulates LasR-Mediated Quorum Sensing and Virulence in Pseudomonas aeruginosa PAO1. Antioxidants, 10(9), 1498. https://doi.org/10.3390/antiox10091498





