Fisetin Protects HaCaT Human Keratinocytes from Fine Particulate Matter (PM2.5)-Induced Oxidative Stress and Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Preparation of PM2.5 Stock Solutions
2.3. Cell Culture and Cell Viability
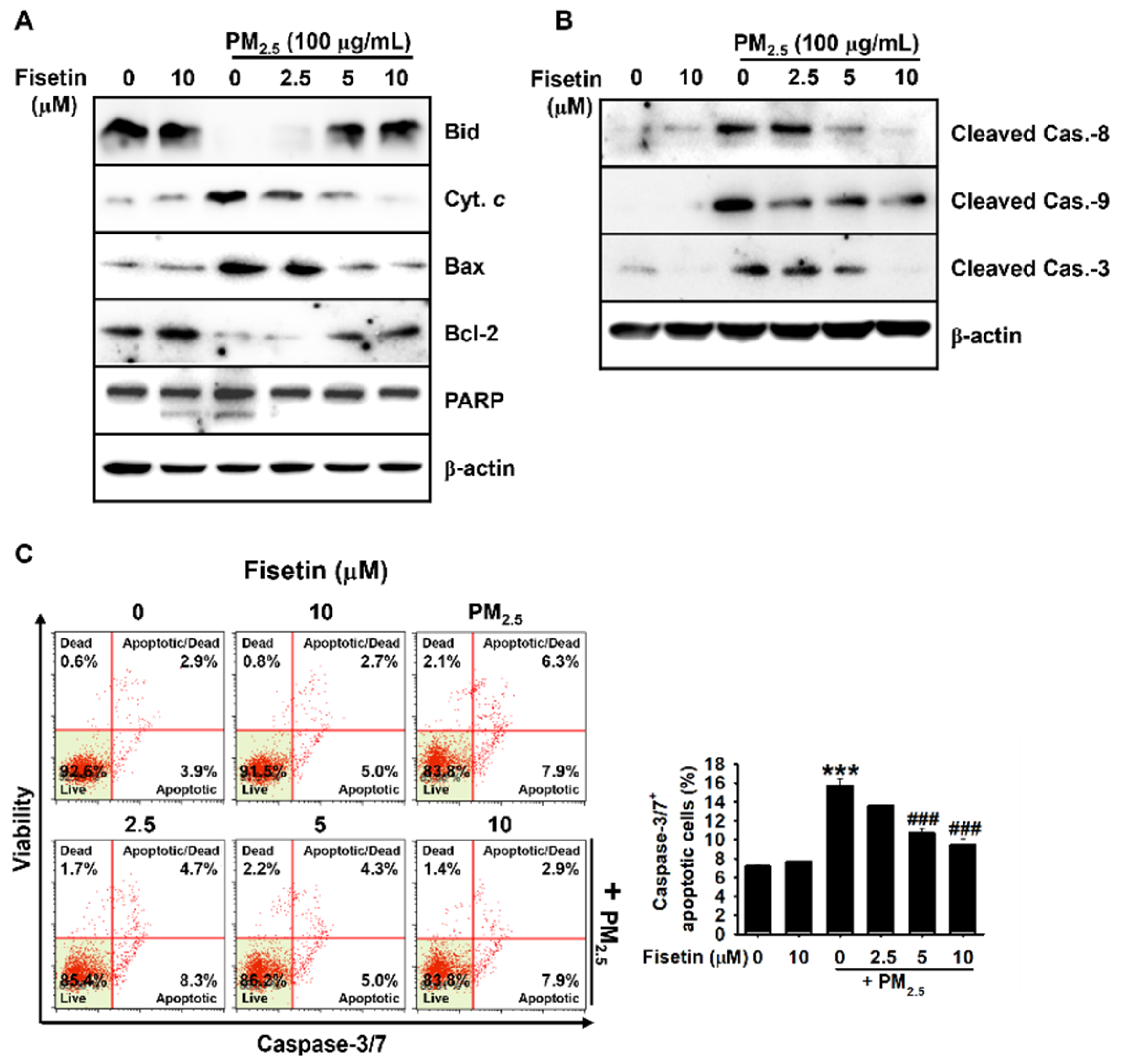
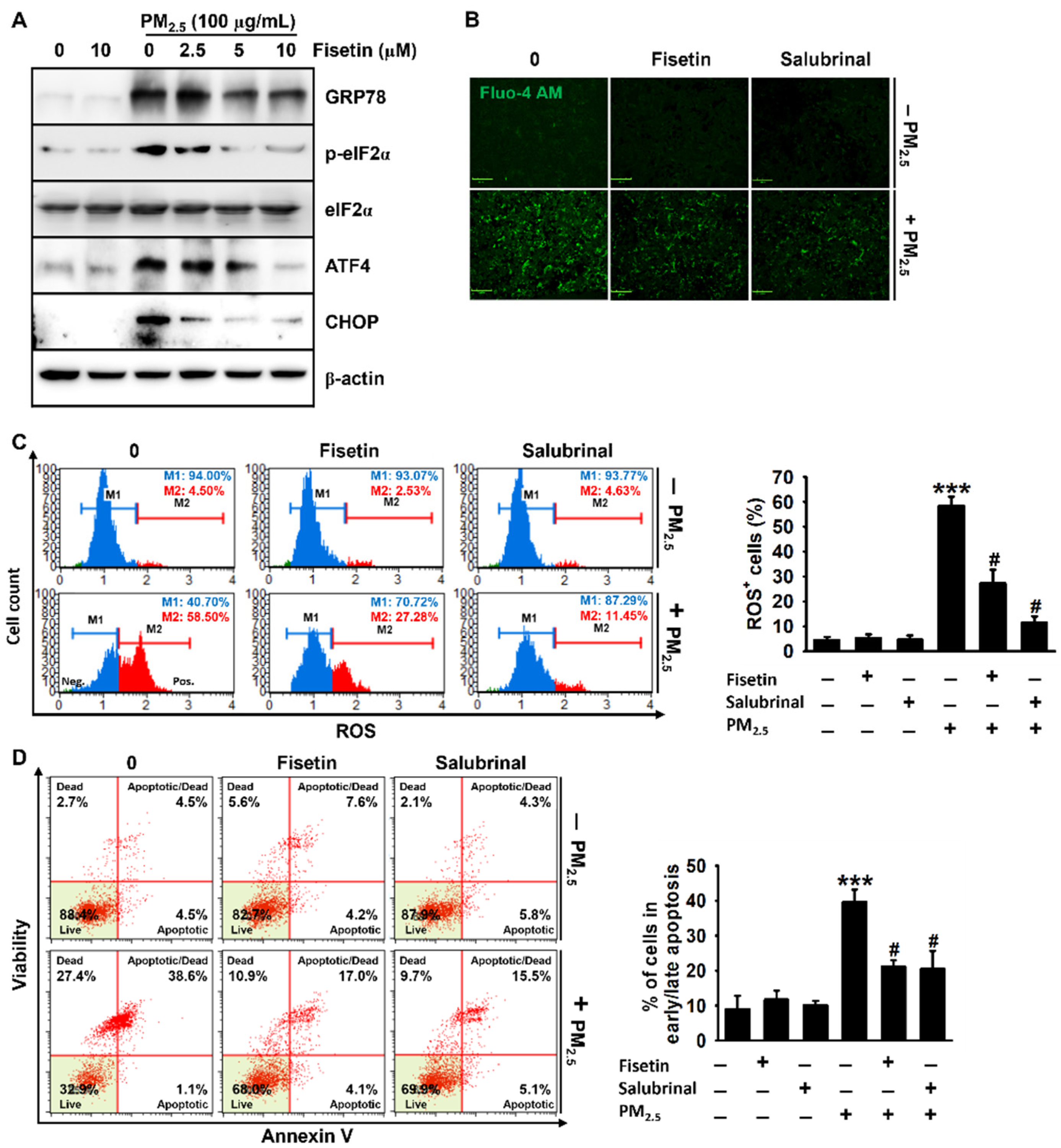
2.4. Annexin V Staining
2.5. Protein Extraction and Western Blotting
2.6. Caspase-3/7 Activity
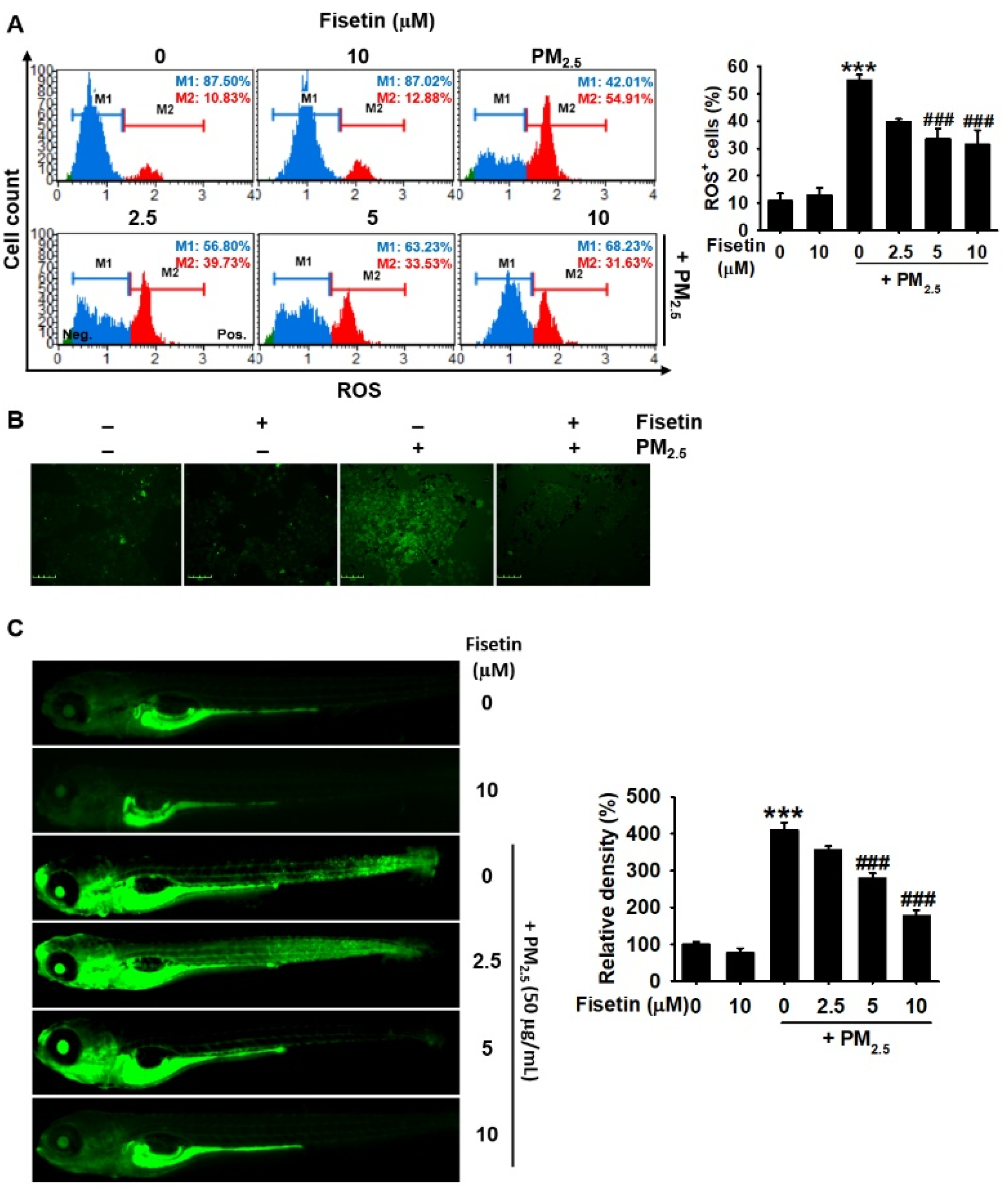
2.7. Intracellular Production of ROS
2.8. Cytosolic Ca2+ Levels
2.9. ROS Staining in Zebrafish Larvae
2.10. Statistical Analyses
3. Results
3.1. Fisetin Protects HaCaT Keratinocytes from PM2.5-Induced Apoptosis
3.2. Fisetin Inhibits PM2.5-Induced Apoptosis by Modulating the Expression of Apoptosis-Related Proteins
3.3. Fisetin Inhibits PM2.5-Induced Production of ROS
3.4. Fisetin Inhibits PM2.5-Induced Apoptosis by Alleviating ER Stress
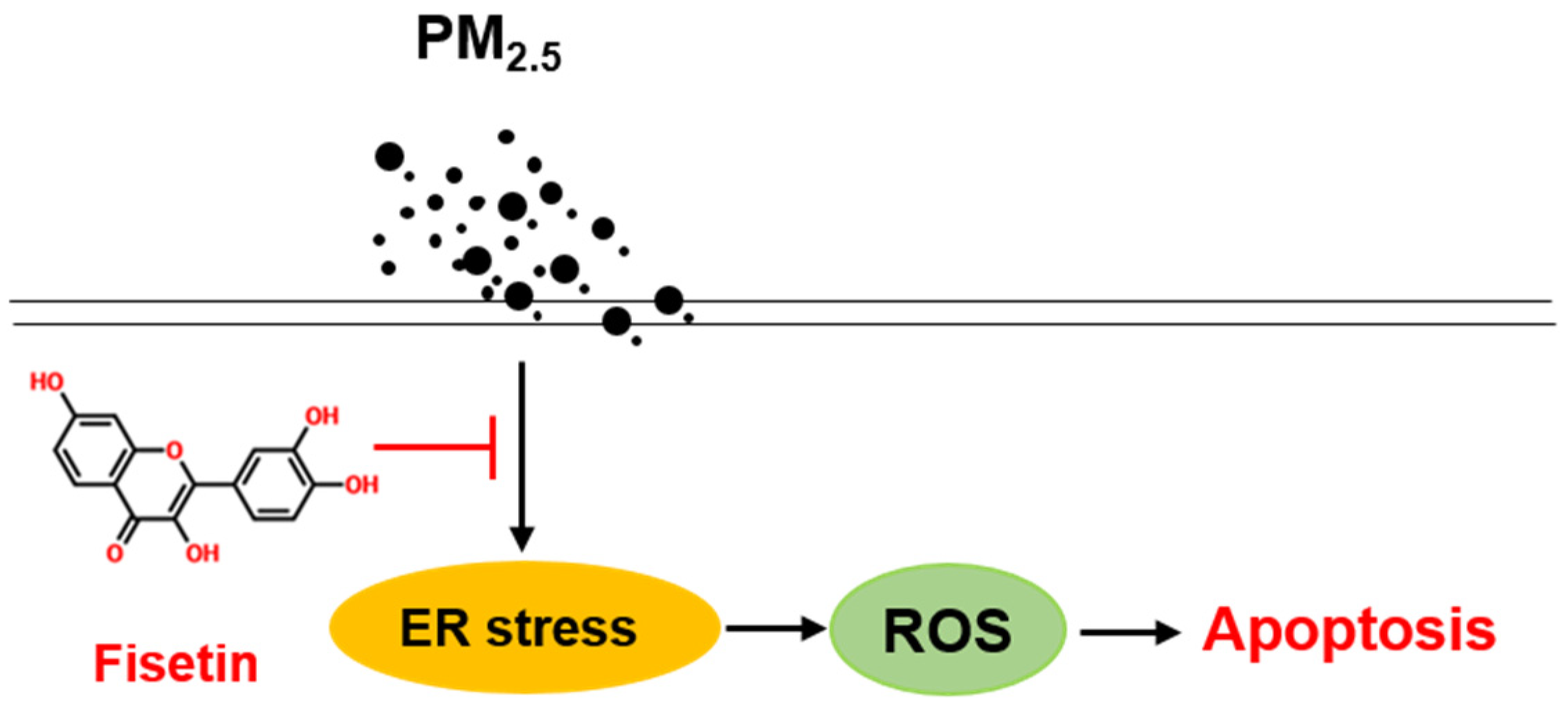
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, C.-C.; Hsieh, W.-Y.; Tsai, C.-H.; Chen, C.-Y.; Chang, H.-F.; Lin, C.-S. In vitro and in vivo experimental studies of PM2.5 on disease progression. Int. J. Environ. Res. Public Health 2018, 15, 1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Ji, Y.; Sun, H.; Hui, F.; Hu, J.; Wu, Y.; Fang, J.; Lin, H.; Wang, J.; Duan, H.; et al. Nanoscale characterization of PM2.5 airborne pollutants reveals high adhesiveness and aggregation capability of soot particles. Sci. Rep. 2015, 5, 11232. [Google Scholar] [CrossRef]
- Marshall, J. PM 2.5. Proc. Natl. Acad. Sci. USA 2013, 110, 8756. [Google Scholar] [CrossRef] [Green Version]
- Sram, R.J.; Veleminsky, M., Jr.; Veleminsky, M., Sr.; Stejskalová, J. The impact of air pollution to central nervous system in children and adults. Neuro Endocrinol. Lett. 2017, 38, 389–396. [Google Scholar]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar]
- Wei, T.; Tang, M. Biological effects of airborne fine particulate matter (PM2.5) exposure on pulmonary immune system. Environ. Toxicol. Pharmacol. 2018, 60, 195–201. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Madduma Hewage, S.R.; Ryu, Y.S.; Oh, M.C.; Kwon, T.K.; Chae, S.; Hyun, J.W. Fisetin induces apoptosis and endoplasmic reticulum stress in human non-small cell lung cancer through inhibition of the MAPK signaling pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 9615–9624. [Google Scholar] [CrossRef]
- Xu, Z.; Ding, W.; Deng, X. PM2.5, Fine Particulate Matter: A Novel Player in the Epithelial-Mesenchymal Transition? Front. Physiol. 2019, 10, 1404. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.-M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrukh, M.R.; Nissar, U.A.; Afnan, Q.; Rafiq, R.A.; Sharma, L.; Amin, S.; Kaiser, P.; Sharma, P.R.; Tasduq, S.A. Oxidative stress mediated Ca2+ release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J. Dermatol. Sci. 2014, 75, 24–35. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Pihán, P.; Carreras-Sureda, A.; Hetz, C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017, 24, 1478–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, J.C.; Arnoult, D.; Youle, R.J. Control of mitochondrial permeability by Bcl-2 family members. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2004, 1644, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.-Y.; Kim, J.-H.; Cho, J.Y. Fisetin suppresses macrophage-mediated inflammatory responses by blockade of Src and Syk. Biomol. Ther. 2015, 23, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol 2020, 18, e3000411. [Google Scholar]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Teng, Y.; Gao, M.; Wang, J.; Kong, Q.; Hua, H.; Luo, T.; Jiang, Y. Inhibition of eIF2α dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis. 2014, 5, e1060. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Zhang, X.-y.; Han, R.; Zhang, T.-t.; Chen, C.; Qin, Z.-h.; Sheng, R. The endoplasmic reticulum stress inhibitor salubrinal inhibits the activation of autophagy and neuroprotection induced by brain ischemic preconditioning. Acta Pharm. Sin. 2013, 34, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, J.; Friedman, A.J. Exogenous factors in skin barrier repair. J. Drugs Derm. 2016, 15, 1289–1294. [Google Scholar]
- Liao, Z.; Nie, J.; Sun, P. The impact of particulate matter (PM2.5) on skin barrier revealed by transcriptome analysis: Focusing on cholesterol metabolism. Toxicol. Rep. 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kang, Z.; Jiang, S.; Zhao, J.; Yan, S.; Xu, F.; Xu, J. Effects of ambient fine particles PM2.5 on human HaCaT cells. Int. J. Environ. Res. Public Health 2017, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.Y.; Lyu, J.L.; Liu, Y.J.; Chien, T.Y.; Hsu, H.C.; Wen, K.C.; Chiang, H.M. Fisetin regulates Nrf2 expression and the inflammation-related signaling pathway to prevent UVB-induced skin damage in hairless mice. Int. J. Mol. Sci. 2017, 18, 2118. [Google Scholar] [CrossRef] [Green Version]
- Chamcheu, J.C.; Esnault, S.; Adhami, V.M.; Noll, A.L.; Banang-Mbeumi, S.; Roy, T.; Singh, S.S.; Huang, S.; Kousoulas, K.G.; Mukhtar, H. Fisetin, a 3,7,3′,4′-tetrahydroxyflavone inhibits the PI3K/Akt/mTOR and MAPK pathways and ameliorates psoriasis pathology in 2D and 3D organotypic human inflammatory skin models. Cells 2019, 8, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-C.; Zhou, Y.; Huang, S.-K. SHP-2 phosphatase controls aryl hydrocarbon receptor-mediated ER stress response in mast cells. Arch. Toxicol. 2017, 91, 1739–1748. [Google Scholar] [CrossRef]
- Sehgal, P.; Szalai, P.; Olesen, C.; Praetorius, H.A.; Nissen, P.; Christensen, S.B.; Engedal, N.; Moller, J.V. Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response. J. Biol. Chem. 2017, 292, 19656–19673. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Son, J.W.; Kim, J.; Kim, M.G.; Jeong, S.H.; Park, T.J.; Son, S.W.; Ryu, H.J. Particulate matter (PM)2.5 affects keratinocytes via endoplasmic reticulum (ER) stress-mediated suppression of apoptosis. Mol. Cell. Toxicol. 2020, 16, 129–137. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molagoda, I.M.N.; Kavinda, M.H.D.; Choi, Y.H.; Lee, H.; Kang, C.-H.; Lee, M.-H.; Lee, C.-M.; Kim, G.-Y. Fisetin Protects HaCaT Human Keratinocytes from Fine Particulate Matter (PM2.5)-Induced Oxidative Stress and Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Response. Antioxidants 2021, 10, 1492. https://doi.org/10.3390/antiox10091492
Molagoda IMN, Kavinda MHD, Choi YH, Lee H, Kang C-H, Lee M-H, Lee C-M, Kim G-Y. Fisetin Protects HaCaT Human Keratinocytes from Fine Particulate Matter (PM2.5)-Induced Oxidative Stress and Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Response. Antioxidants. 2021; 10(9):1492. https://doi.org/10.3390/antiox10091492
Chicago/Turabian StyleMolagoda, Ilandarage Menu Neelaka, Mirissa Hewage Dumindu Kavinda, Yung Hyun Choi, Hyesook Lee, Chang-Hee Kang, Mi-Hwa Lee, Chang-Min Lee, and Gi-Young Kim. 2021. "Fisetin Protects HaCaT Human Keratinocytes from Fine Particulate Matter (PM2.5)-Induced Oxidative Stress and Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Response" Antioxidants 10, no. 9: 1492. https://doi.org/10.3390/antiox10091492
APA StyleMolagoda, I. M. N., Kavinda, M. H. D., Choi, Y. H., Lee, H., Kang, C.-H., Lee, M.-H., Lee, C.-M., & Kim, G.-Y. (2021). Fisetin Protects HaCaT Human Keratinocytes from Fine Particulate Matter (PM2.5)-Induced Oxidative Stress and Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Response. Antioxidants, 10(9), 1492. https://doi.org/10.3390/antiox10091492








