S-Methyl-L-Ergothioneine to L-Ergothioneine Ratio in Urine Is a Marker of Cystine Lithiasis in a Cystinuria Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice Care
2.2. Knocking Out Slc22a4 in the Type B Cystinuric Mouse Model (Slc7a9−/−)
2.3. Cystine Calculi Detection by X-ray In Vivo Imaging
2.4. Sample Collection
2.5. L-Erg and S-Met-L-Erg Determination
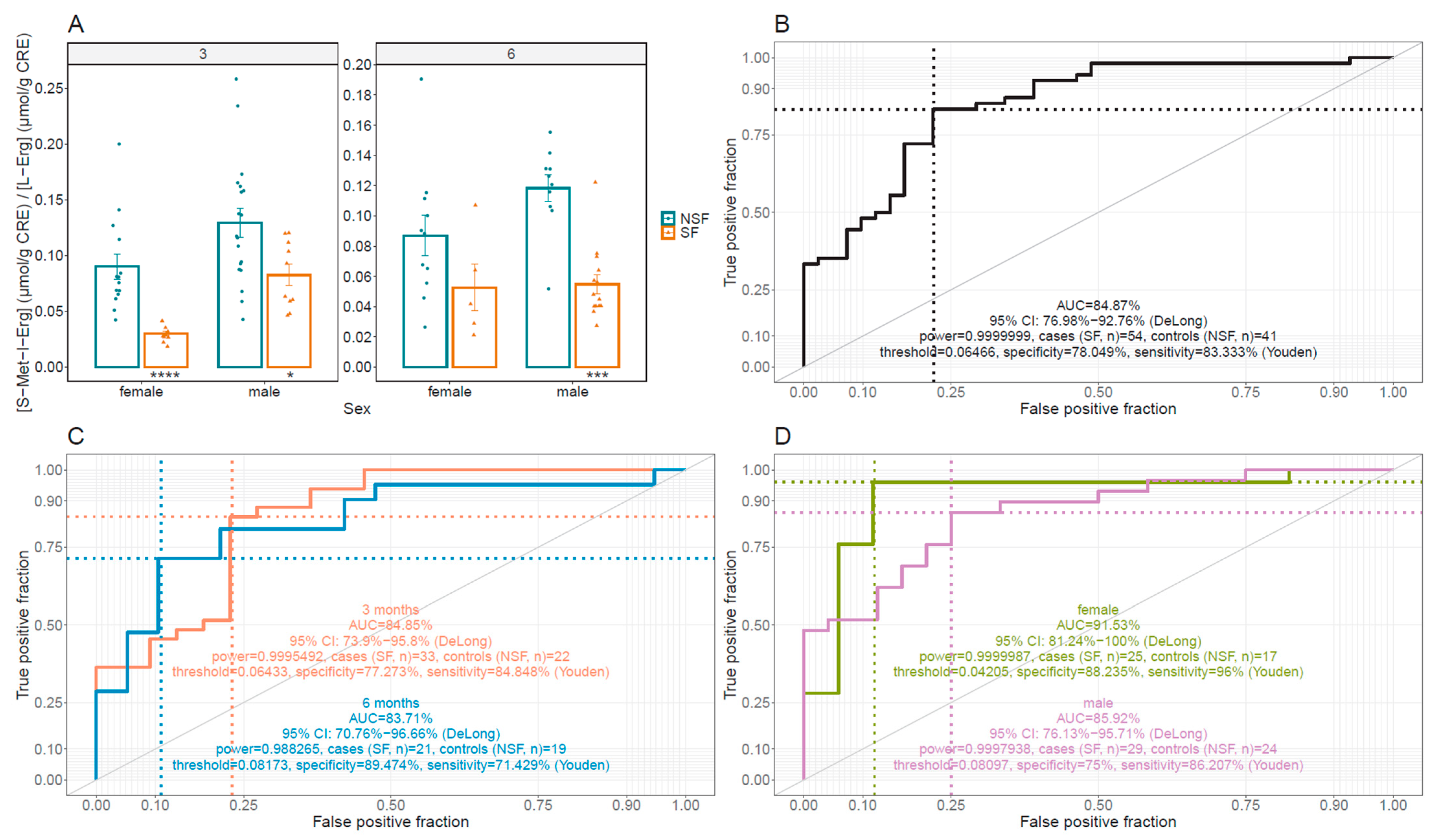
2.6. Statistical and Prediction Analysis
3. Results
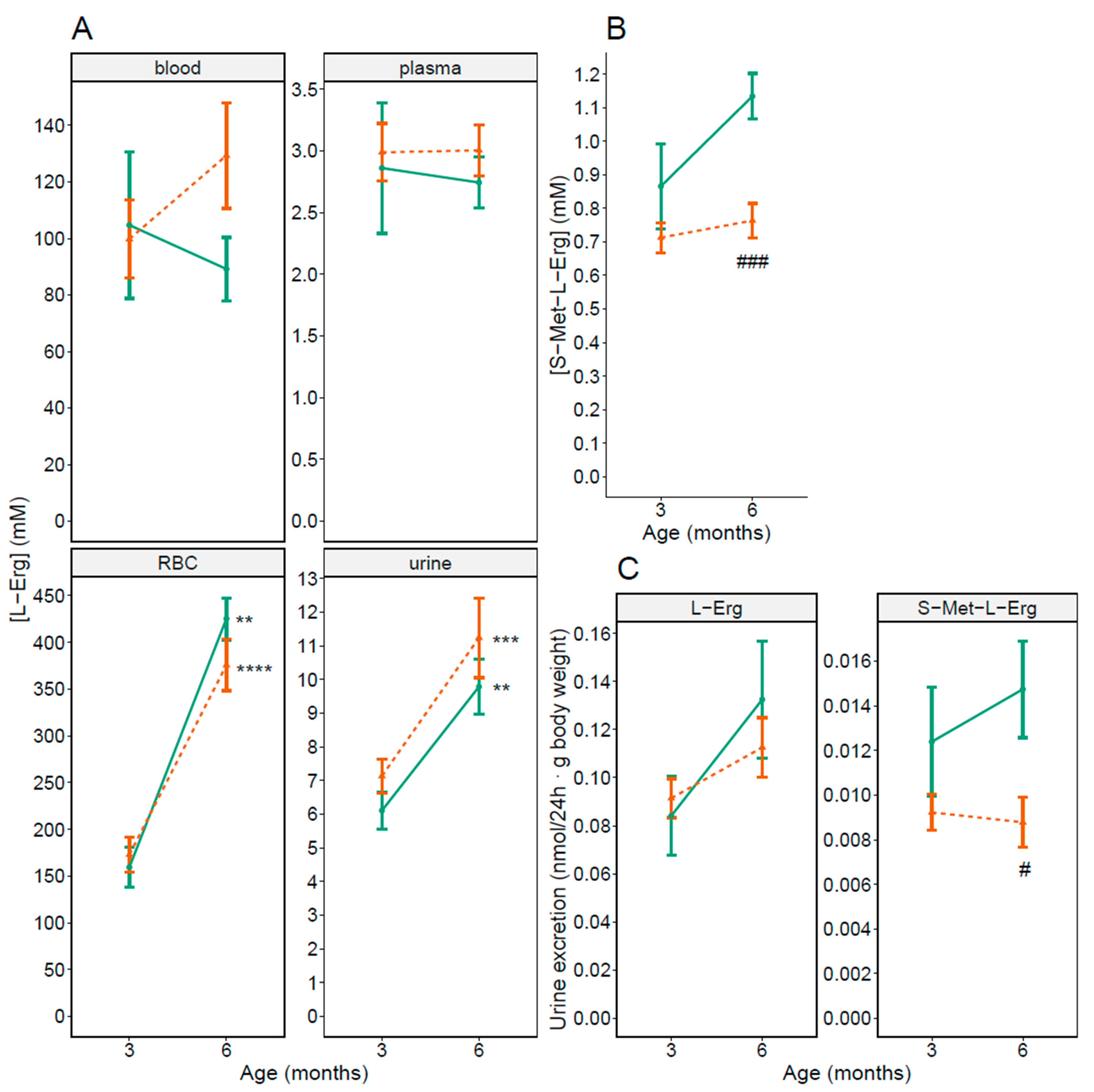
3.1. L-Erg and S-Met-L-Erg in Male Mice Blood and Urine
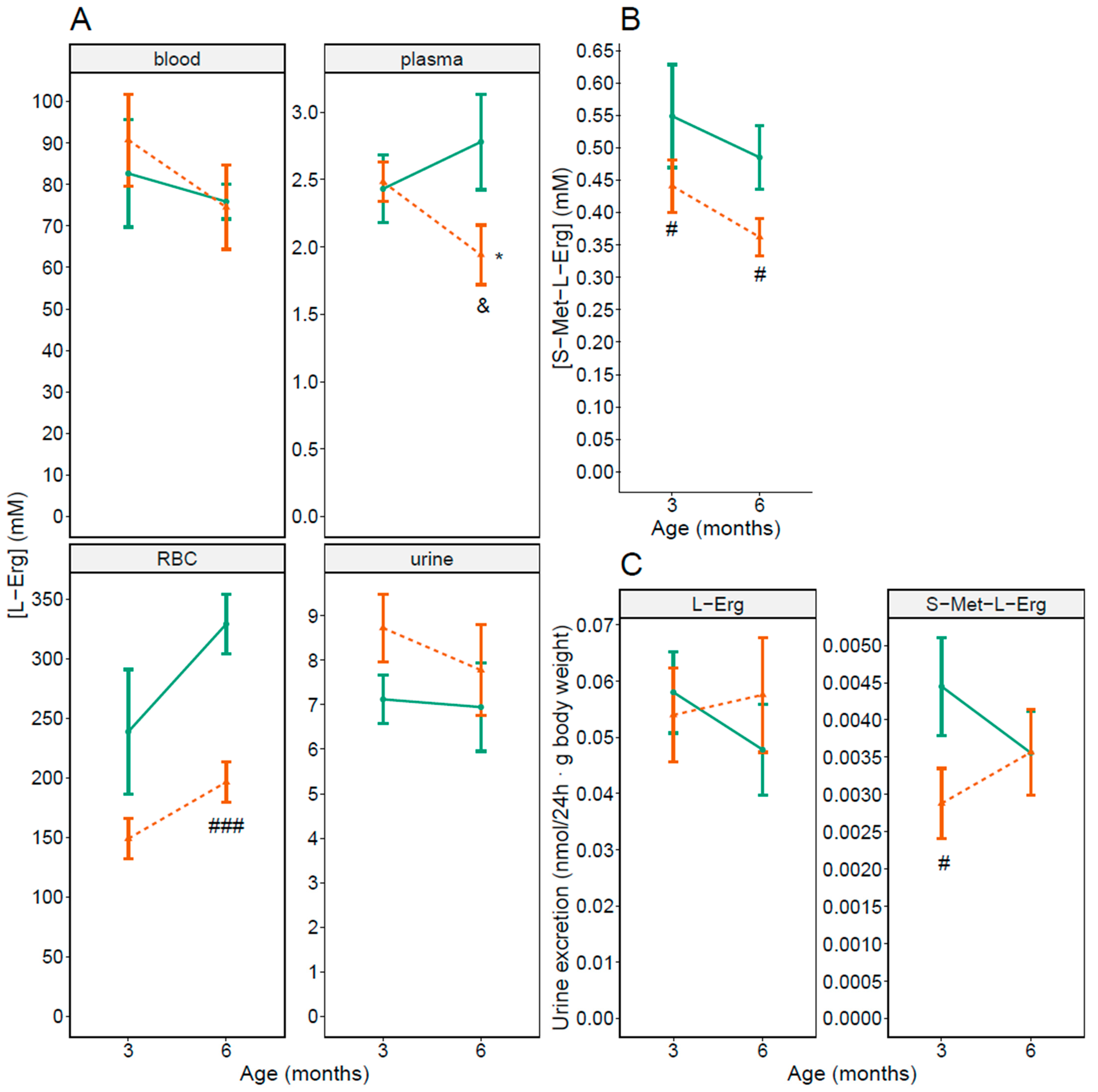
3.2. L-Erg and S-Met-L-Erg in Female Mice Blood and Urine
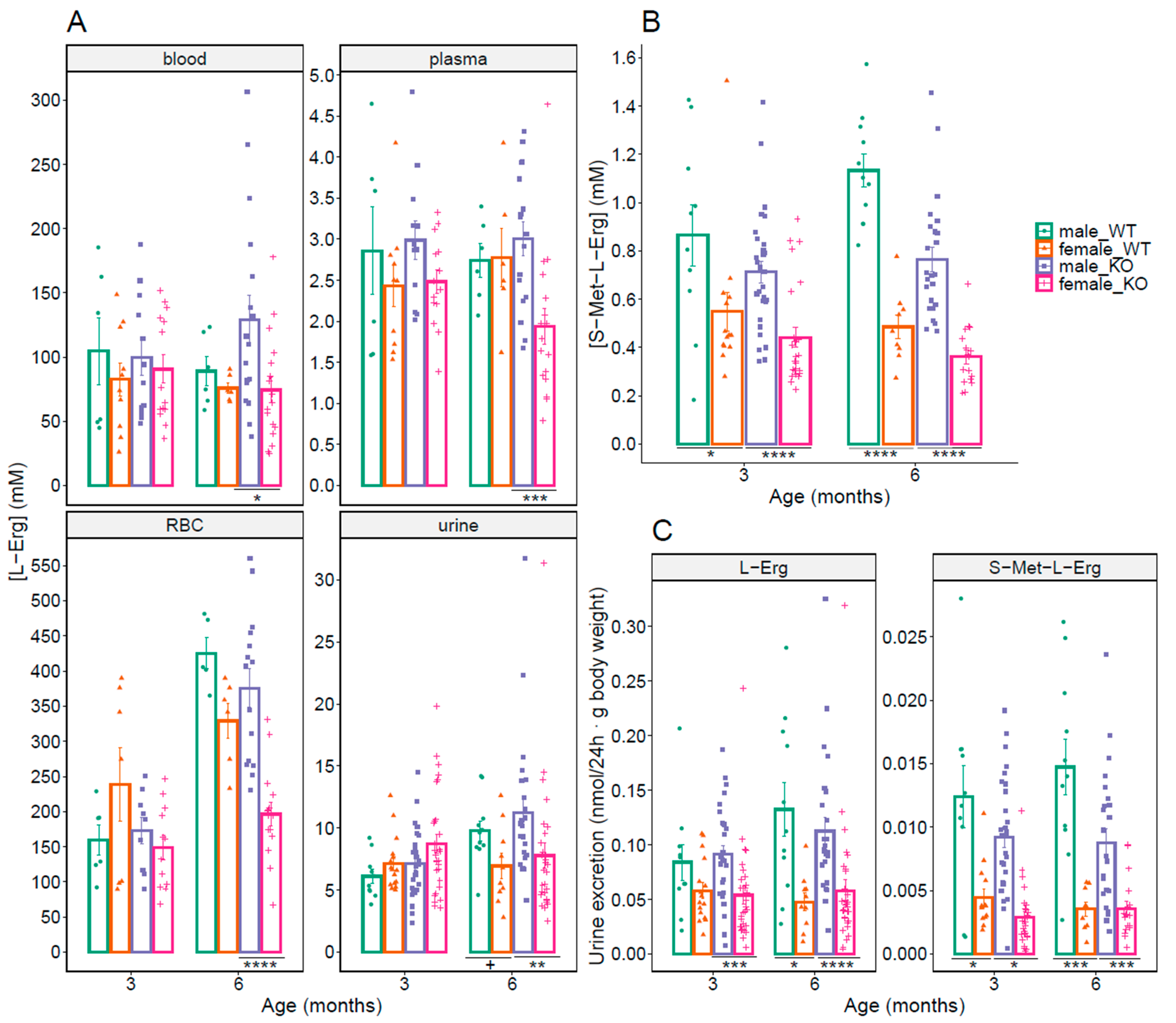
3.3. Sex-Related Differences in L-Erg and S-Met-L-Erg in Blood and Urine
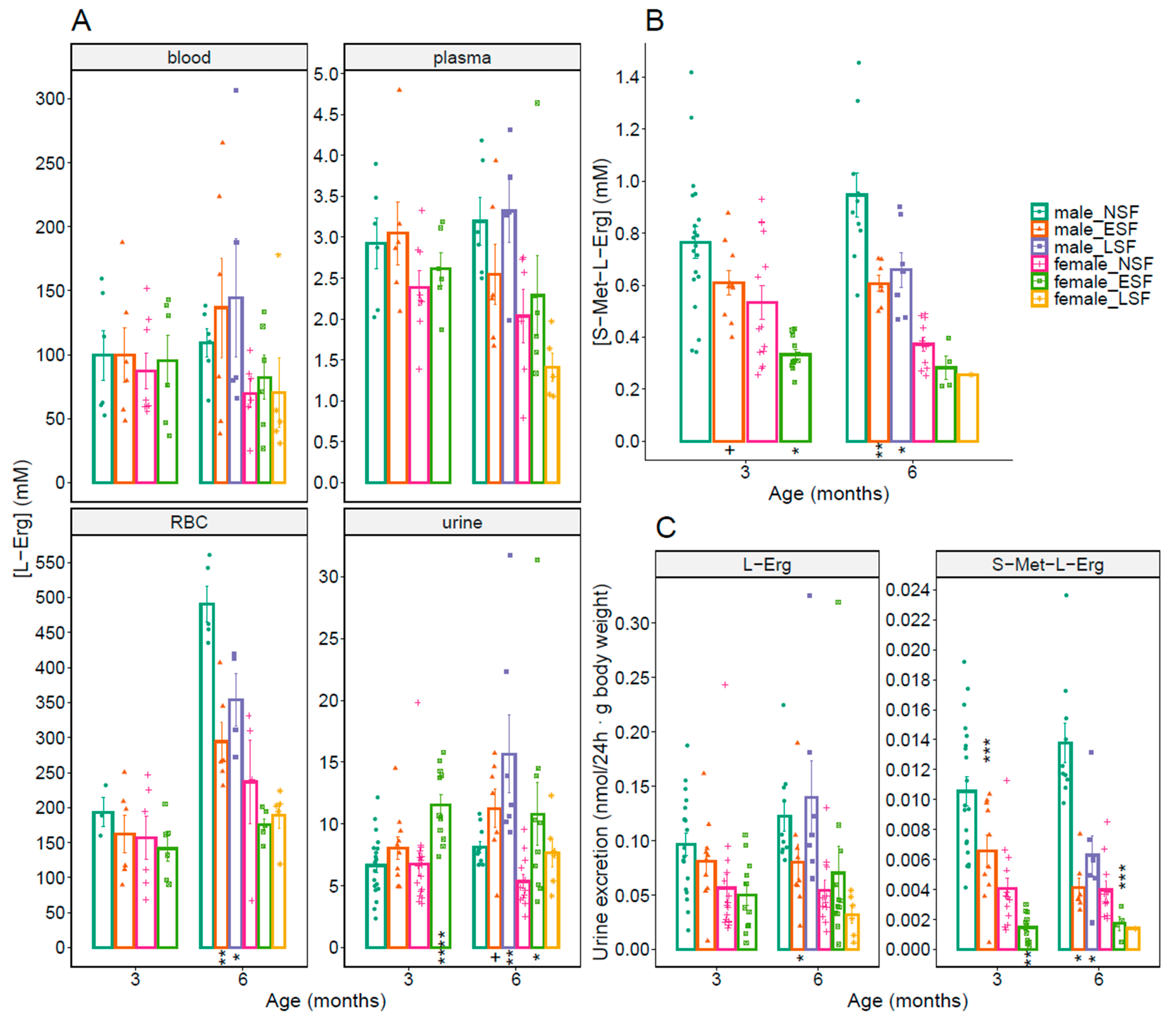
3.4. L-Erg and S-Met-L-Erg Urine Concentration in Lithiasic Mice
3.5. OCTN1 as a Cystine Lithiasis Modulator
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta 2012, 1822, 784–793. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, K.M.; Masuda, S.; Fujitani, Y.; Fukuda, F.; Tani, A. Production of ergothioneine by Methylobacterium species. Front. Microbiol. 2015, 6, 1185. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.; Bauer, T.; Surek, B.; Schömig, E.; Gründemann, D. Cyanobacteria produce high levels of ergothioneine. Food Chem. 2011, 129, 1766–1769. [Google Scholar] [CrossRef]
- Melville, D.B.; Horner, W.H.; Lubschez, R. Tissue ergothioneine. J. Biol. Chem. 1954, 206, 221–228. [Google Scholar] [CrossRef]
- Salt, H.B. The ergothioneine content of the blood in health and disease. Biochem. J. 1931, 25, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Leone, E.; Mann, T. Ergothioneine in the seminal vesicle secretion. Nature 1951, 168, 205–206. [Google Scholar] [CrossRef]
- Tokuhiro, S.; Yamada, R.; Chang, X.; Suzuki, A.; Kochi, Y.; Sawada, T.; Suzuki, M.; Nagasaki, M.; Ohtsuki, M.; Ono, M.; et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat. Genet. 2003, 35, 341–348. [Google Scholar] [CrossRef]
- Taubert, D.; Jung, N.; Goeser, T.; Schomig, E. Increased ergothioneine tissue concentrations in carriers of the Crohn’s disease risk-associated 503F variant of the organic cation transporter OCTN1. Gut 2009, 58, 312–314. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.; Ye, P.; Yew, T.S.Z.; Lim, K.H.S.; Halliwell, B. Liver ergothioneine accumulation in a guinea pig model of non-alcoholic fatty liver disease. A possible mechanism of defence? Free Radic. Res. 2016, 50, 14–25. [Google Scholar] [CrossRef]
- Ye, P.; Cheah, I.K.; Halliwell, B. High fat diets and pathology in the guinea pig. Atherosclerosis or liver damage? Biochim. Biophys. Acta-Mol. Basis Dis. 2013, 1832, 355–364. [Google Scholar] [CrossRef]
- Grigat, S.; Harlfinger, S.; Pal, S.; Striebinger, R.; Golz, S.; Geerts, A.; Lazar, A.; Schömig, E.; Gründemann, D. Probing the substrate specificity of the ergothioneine transporter with methimazole, hercynine, and organic cations. Biochem. Pharmacol. 2007, 74, 309–316. [Google Scholar] [CrossRef]
- Tucker, R.A.J.; Cheah, I.K.; Halliwell, B. Specificity of the ergothioneine transporter natively expressed in HeLa cells. Biochem. Biophys. Res. Commun. 2019, 513, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kubo, Y.; Iwata, D.; Kato, S.; Sudo, T.; Sugiura, T.; Kagaya, T.; Wakayama, T.; Hirayama, A.; Sugimoto, M.; et al. Gene Knockout and Metabolome Analysis of Carnitine/Organic Cation Transporter OCTN1. Pharm. Res. 2010, 27, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Palacín, M. The role of amino acid transporters in inherited and acquired diseases. Biochem. J. 2011, 436, 193–211. [Google Scholar] [CrossRef]
- Wong, K.A.; Pardy, C.; Pillay, S.; Athanasiou, T.; Rottenberg, G.; Bultitude, M.; Chandra, A.; Thomas, K. Can the Presence of Crystalluria Predict Stone Formation in Patients with Cystinuria? J. Endourol. 2016, 30, 609–614. [Google Scholar] [CrossRef]
- Friedlander, J.I.; Antonelli, J.A.; Canvasser, N.E.; Morgan, M.S.C.; Mollengarden, D.; Best, S.; Pearle, M.S. Do Urinary Cystine Parameters Predict Clinical Stone Activity? J. Urol. 2018, 199, 495–499. [Google Scholar] [CrossRef]
- Prot-Bertoye, C.; Lebbah, S.; Daudon, M.; Tostivint, I.; Bataille, P.; Bridoux, F.; Brignon, P.; Choquenet, C.; Cochat, P.; Combe, C.; et al. CKD and its risk factors among patients with cystinuria. Clin. J. Am. Soc. Nephrol. 2015, 10, 842–851. [Google Scholar] [CrossRef]
- Assimos, D.G.; Leslie, S.W.; Ng, C.; Streem, S.B.; Hart, L.J. The impact of cystinuria on renal function. J. Urol. 2002. [Google Scholar] [CrossRef]
- Purohit, R.S.; Stoller, M.L. Stone clustering of patients with cystine urinary stone formation. Urology 2004, 63, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Sahota, A.; Tischfield, J.A.; Goldfarb, D.S.; Ward, M.D.; Hu, L. Cystinuria: Genetic aspects, mouse models, and a new approach to therapy. Urolithiasis 2019, 47, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Furuichi, K.; Toyama, T.; Kitajima, S.; Hara, A.; Iwata, Y.; Sakai, N.; Shimizu, M.; Kaneko, S.; Isozumi, N.; et al. Impairment of the carnitine/organic cation transporter 1-ergothioneine axis is mediated by intestinal transporter dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Feliubadaló, L.; Arbonés, M.L.; Mañas, S.; Chillarón, J.; Visa, J.; Rodés, M.; Rousaud, F.; Zorzano, A.; Palacín, M.; Nunes, V. Slc7a9-deficient mice develop cystinuria non-l and cystine urolithiasis. Hum. Mol. Genet. 2003, 12, 2097–2108. [Google Scholar] [CrossRef]
- Font-Llitjós, M.; Feliubadaló, L.; Espino, M.; Clèries, R.; Mañas, S.; Frey, I.M.; Puertas, S.; Colell, G.; Palomo, S.; Aranda, J.; et al. Slc7a9 knockout mouse is a good cystinuria model for antilithiasic pharmacological studies. Am. J. Physiol. Physiol. 2007, 293, F732–F740. [Google Scholar] [CrossRef][Green Version]
- Sotgia, S.; Pisanu, E.; Pintus, G.; Erre, G.L.; Pinna, G.A.; Deiana, L.; Carru, C.; Zinellu, A. Plasma L-ergothioneine measurement by high-performance liquid chromatography and capillary electrophoresis after a pre-column derivatization with 5-iodoacetamidofluorescein (5-IAF) and fluorescence detection. PLoS ONE 2013, 8, e70374. [Google Scholar] [CrossRef]
- Sotgia, S.; Zinellu, A.; Pintus, G.; Pinna, G.A.; Deiana, L.; Carru, C. Quantification of L-ergothioneine in whole blood by hydrophilic interaction ultra-performance liquid chromatography and UV-detection. J. Sep. Sci. 2013, 36, 1002–1006. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Zinellu, E.; Chessa, R.; Deiana, L.; Carru, C. Assay for the simultaneous determination of guanidinoacetic acid, creatinine and creatine in plasma and urine by capillary electrophoresis UV-detection. J. Sep. Sci. 2006, 29, 704–708. [Google Scholar] [CrossRef]
- Xu, J.; Yadan, J.C. Synthesis of L-(+)-Ergothioneine. J. Org. Chem. 1995, 60, 6296–6301. [Google Scholar] [CrossRef]
- Kumosani, T.A. L-ergothioneine level in red blood cells of healthy human males in the Western province of Saudi Arabia. Exp. Mol. Med. 2001, 33, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.B.; Mackenzie, C.G. The effect of age, sex, and androgen on blood ergothioneine. J. Biol. Chem. 1957, 225, 651–657. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine: A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.M.Y.; Yew, T.S.Z.Z.; Lim, K.H.C.S.; Halliwell, B. Administration of Pure Ergothioneine to Healthy Human Subjects: Uptake, Metabolism, and Effects on Biomarkers of Oxidative Damage and Inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef]
- Wang, L.-Z.; Thuya, W.-L.; Toh, D.S.-L.; Lie, M.G.-L.; Lau, J.-Y.A.; Kong, L.-R.; Wan, S.-C.; Chua, K.-N.; Lee, E.J.-D.; Goh, B.-C. Quantification of L-ergothioneine in human plasma and erythrocytes by liquid chromatography-tandem mass spectrometry. J. Mass Spectrom. 2013, 48, 406–412. [Google Scholar] [CrossRef]
- Mitsuyama, H.; May, J.M. Uptake and antioxidant effects of ergothioneine in human erythrocytes. Clin. Sci. 1999, 97, 407–411. [Google Scholar] [CrossRef]
- Tang, R.M.Y.; Cheah, I.K.-M.; Yew, T.S.K.; Halliwell, B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 2018, 8, 1601. [Google Scholar] [CrossRef]
- Woodard, L.E.; Welch, R.C.; Veach, R.A.; Beckermann, T.M.; Sha, F.; Weinman, E.J.; Ikizler, T.A.; Tischfield, J.A.; Sahota, A.; Wilson, M.H. Metabolic consequences of cystinuria. BMC Nephrol. 2019, 20, 227. [Google Scholar] [CrossRef]
- Flurkey, K.; Mcurrer, J.; Harrison, D.E. Mouse Models in Aging Research. Mouse Biomed. Res. 2007, 637–672. [Google Scholar] [CrossRef]
- Sotgia, S.; Zinellu, A.; Mangoni, A.A.; Pintus, G.; Attia, J.; Carru, C.; McEvoy, M. Clinical and biochemical correlates of serum L-ergothioneine concentrations in community-dwelling middle-aged and older adults. PLoS ONE 2014, 9, e84918. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Banga, R.S.; Kapitsinou, P.; Ramaiah, M.; Lawrence, J.; Kambhampati, G.; Gruenwald, A.; Bottinger, E.; Glicklich, D.; Tellis, V.; et al. Human and murine kidneys show gender- and species-specific gene expression differences in response to injury. PLoS ONE 2009, 4, e4802. [Google Scholar] [CrossRef] [PubMed]
- Kwekel, J.C.; Desai, V.G.; Moland, C.L.; Vijay, V.; Fuscoe, J.C. Sex differences in kidney gene expression during the life cycle of F344 rats. Biol. Sex Differ. 2013, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, M.E.; Krambeck, A.E.; Bergstralh, E.J.; Milliner, D.S.; Lieske, J.C.; Rule, A.D. Temporal Trends in Incidence of Kidney Stones Among Children: A 25-Year Population Based Study. J. Urol. 2012, 188, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Routh, J.C.; Graham, D.A.; Nelson, C.P. Epidemiological Trends in Pediatric Urolithiasis at United States Freestanding Pediatric Hospitals. J. Urol. 2010, 184, 1100–1105. [Google Scholar] [CrossRef]
- Casado, M.; Sierra, C.; Batllori, M.; Artuch, R.; Ormazabal, A. A targeted metabolomic procedure for amino acid analysis in different biological specimens by ultra-high-performance liquid chromatography–tandem mass spectrometry. Metabolomics 2018, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Shohani, A.; Khorami, H.; Mahdavi, K.N.; Izad Panahi, M.H.; Alizadeh, F.; Azizi, M. The effect of selenium supplementation on cystine crystal volume in patients with cystinuria. BioMedicine 2018, 8, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Zee, T.; Bose, N.; Zee, J.; Beck, J.N.; Yang, S.; Parihar, J.; Yang, M.; Damodar, S.; Hall, D.; O’Leary, M.N.; et al. α-Lipoic acid treatment prevents cystine urolithiasis in a mouse model of cystinuria. Nat. Med. 2017, 23, 288–290. [Google Scholar] [CrossRef] [PubMed]
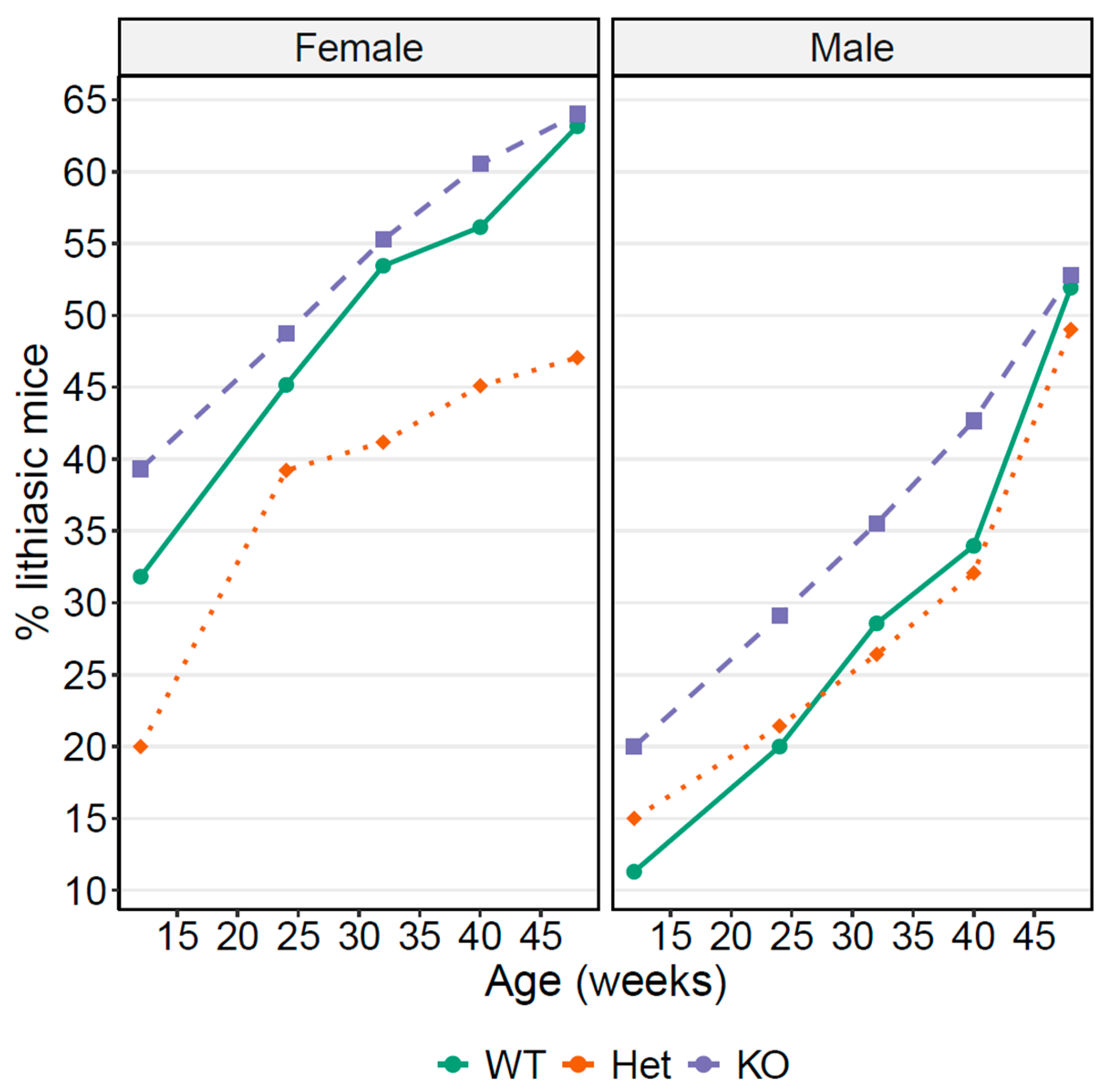
| Sex | Slc22a4 Genotype | Week 12 | Week 24 | Week 32 | Week 40 | Week 48 |
|---|---|---|---|---|---|---|
| Female | WT (+/+) | 66 | 62 | 58 | 57 | 57 |
| Female | Het (+/−) | 55 | 51 | 51 | 51 | 51 |
| Female | KO (−/−) | 89 | 78 | 76 | 76 | 75 |
| Male | WT (+/+) | 89 | 78 | 76 | 76 | 75 |
| Male | Het (+/−) | 60 | 60 | 56 | 53 | 52 |
| Male | KO (−/−) | 90 | 79 | 76 | 75 | 72 |
| Compound | Slc7a9 Genotype | Slc22a4 Genotype | N | Mean | se | p-Value * |
|---|---|---|---|---|---|---|
| L-Erg | WT (+/+) | WT (+/+) | 33 | 1.960 | 0.188 | |
| L-Erg | KO (−/−) | WT (+/+) | 58 | 1.789 | 0.087 | 0.1964 |
| L-Erg | KO (−/−) | Het (+/−) | 5 | 0.691 | 0.088 | 0.0382 |
| L-Erg | KO (−/−) | KO (−/−) | 3 | 0.176 | 0.028 | 0.0138 |
| S-Met-L-Erg | WT (+/+) | WT (+/+) | 24 | 0.285 | 0.042 | |
| S-Met-L-Erg | KO (−/−) | WT (+/+) | 56 | 0.152 | 0.013 | 0.0022 |
| S-Met-L-Erg | KO (−/−) | Het (+/−) | 5 | bql | ||
| S-Met-L-Erg | KO (−/−) | KO (−/−) | 3 | bql |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López de Heredia, M.; Muñoz, L.; Carru, C.; Sotgia, S.; Zinellu, A.; Serra, C.; Llebaria, A.; Kato, Y.; Nunes, V. S-Methyl-L-Ergothioneine to L-Ergothioneine Ratio in Urine Is a Marker of Cystine Lithiasis in a Cystinuria Mouse Model. Antioxidants 2021, 10, 1424. https://doi.org/10.3390/antiox10091424
López de Heredia M, Muñoz L, Carru C, Sotgia S, Zinellu A, Serra C, Llebaria A, Kato Y, Nunes V. S-Methyl-L-Ergothioneine to L-Ergothioneine Ratio in Urine Is a Marker of Cystine Lithiasis in a Cystinuria Mouse Model. Antioxidants. 2021; 10(9):1424. https://doi.org/10.3390/antiox10091424
Chicago/Turabian StyleLópez de Heredia, Miguel, Lourdes Muñoz, Ciriaco Carru, Salvatore Sotgia, Angelo Zinellu, Carmen Serra, Amadeu Llebaria, Yukio Kato, and Virginia Nunes. 2021. "S-Methyl-L-Ergothioneine to L-Ergothioneine Ratio in Urine Is a Marker of Cystine Lithiasis in a Cystinuria Mouse Model" Antioxidants 10, no. 9: 1424. https://doi.org/10.3390/antiox10091424
APA StyleLópez de Heredia, M., Muñoz, L., Carru, C., Sotgia, S., Zinellu, A., Serra, C., Llebaria, A., Kato, Y., & Nunes, V. (2021). S-Methyl-L-Ergothioneine to L-Ergothioneine Ratio in Urine Is a Marker of Cystine Lithiasis in a Cystinuria Mouse Model. Antioxidants, 10(9), 1424. https://doi.org/10.3390/antiox10091424







