Peroxiredoxin 6 Knockout Mice Demonstrate Anxiety Behavior and Attenuated Contextual Fear Memory after Receiving Acute Immobilization Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Tests
2.2.1. Acute Immobilization Stress (AIS)
2.2.2. Trace Fear Conditioning
2.2.3. Open Field Test
2.2.4. Elevated plus Maze Test
2.3. Cell Culture
2.4. Immunocytochemistry and Image Analysis
2.5. Western Blot (WB) Analysis
2.6. Intracellular ROS Accumulation Measurement
2.7. Statistical Analysis
3. Results
3.1. PRDX6 Expression Level Was Decreased in ARPE-19 Cells Treated with Stress Hormone Glucocorticoid
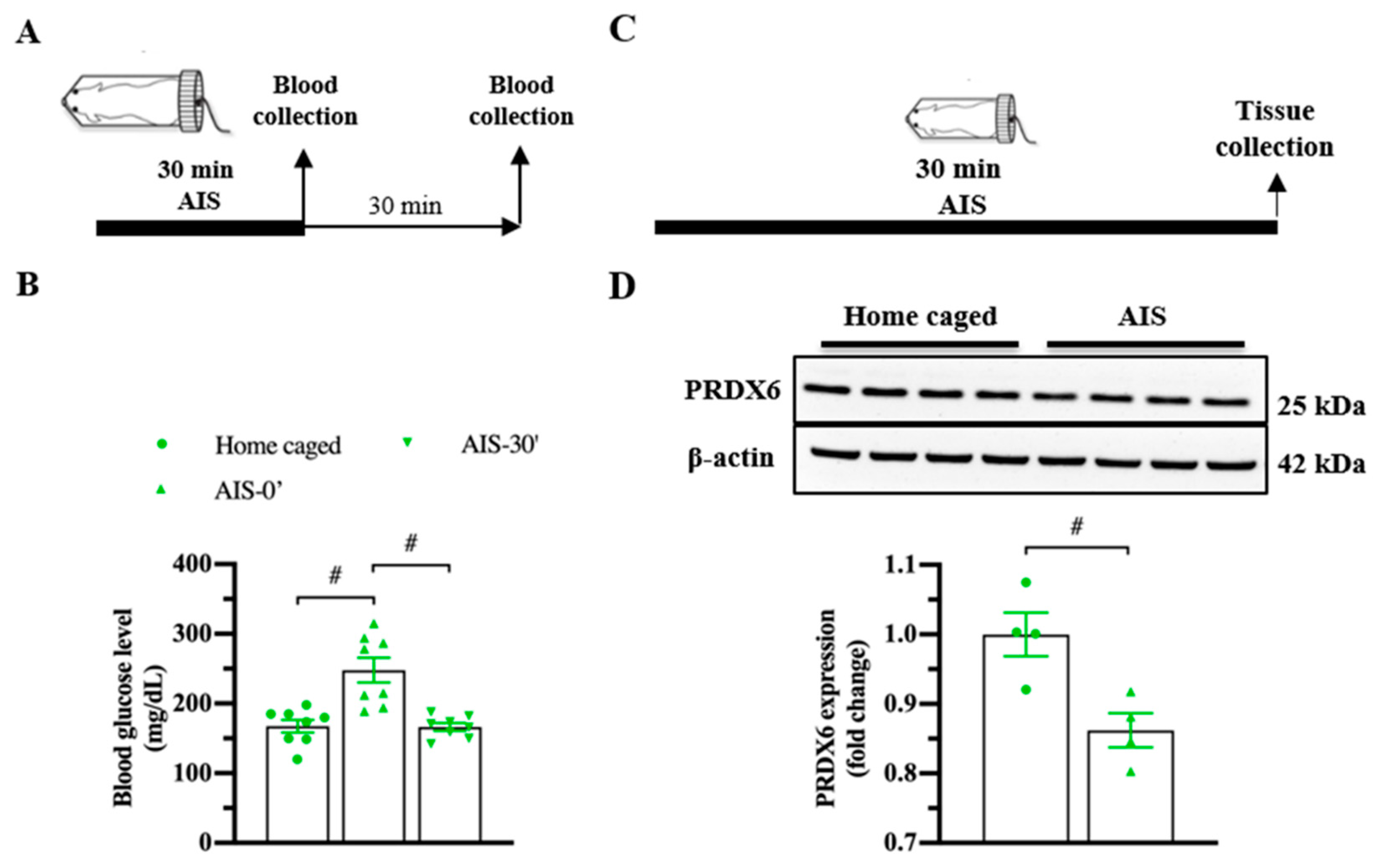
3.2. PRDX6 Expression Level Was Downregulated in Response to AIS and TFC
3.3. Decreased H2O2 Level in the Hippocampal CA1, Basolateral Amygdala and Medial Prefrontal Cortex in Response to AIS in Prdx6−/− Mice
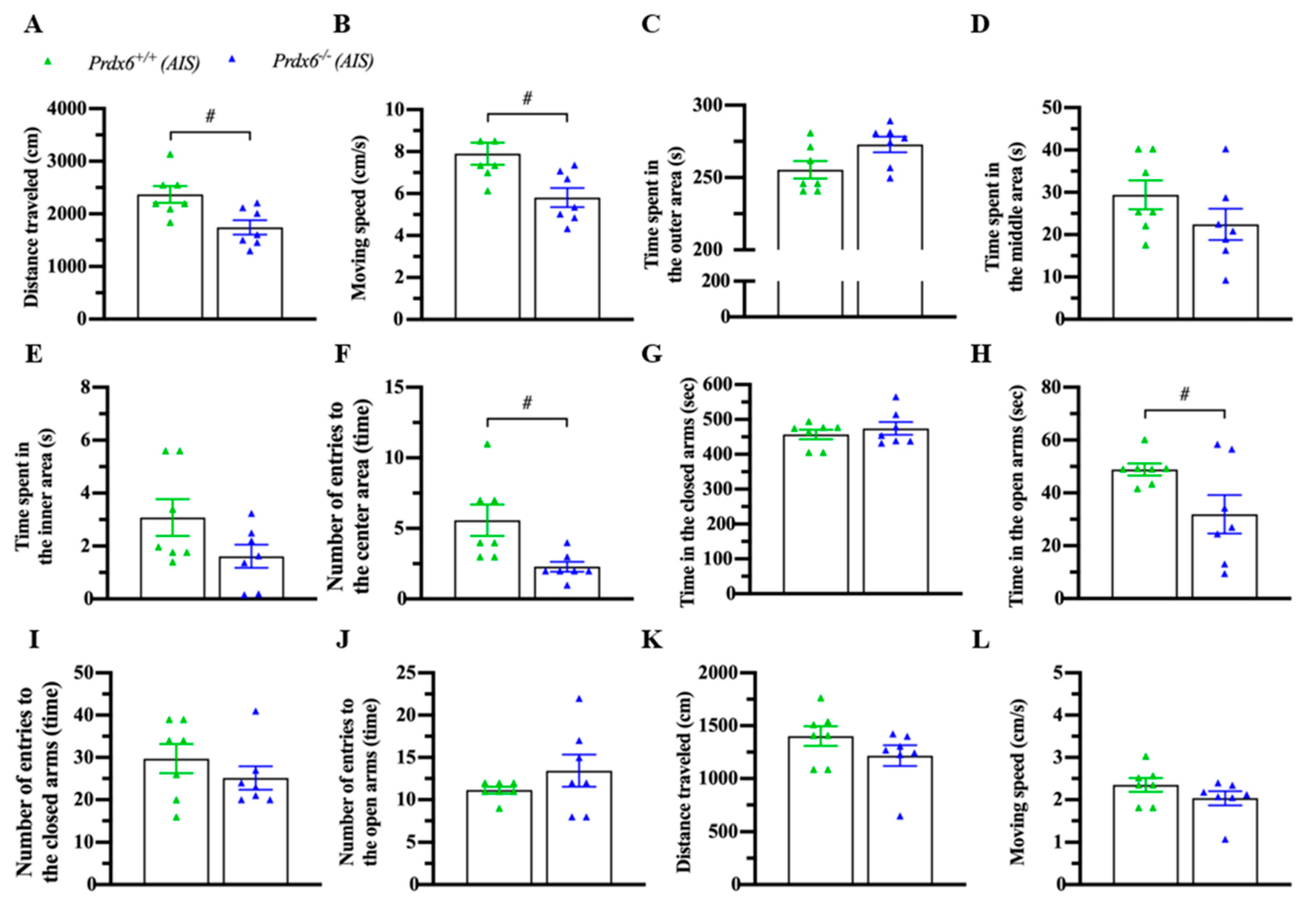
3.4. Prdx6−/− Mice Exhibited an Abnormal Locomotion and Anxiety Response after AIS
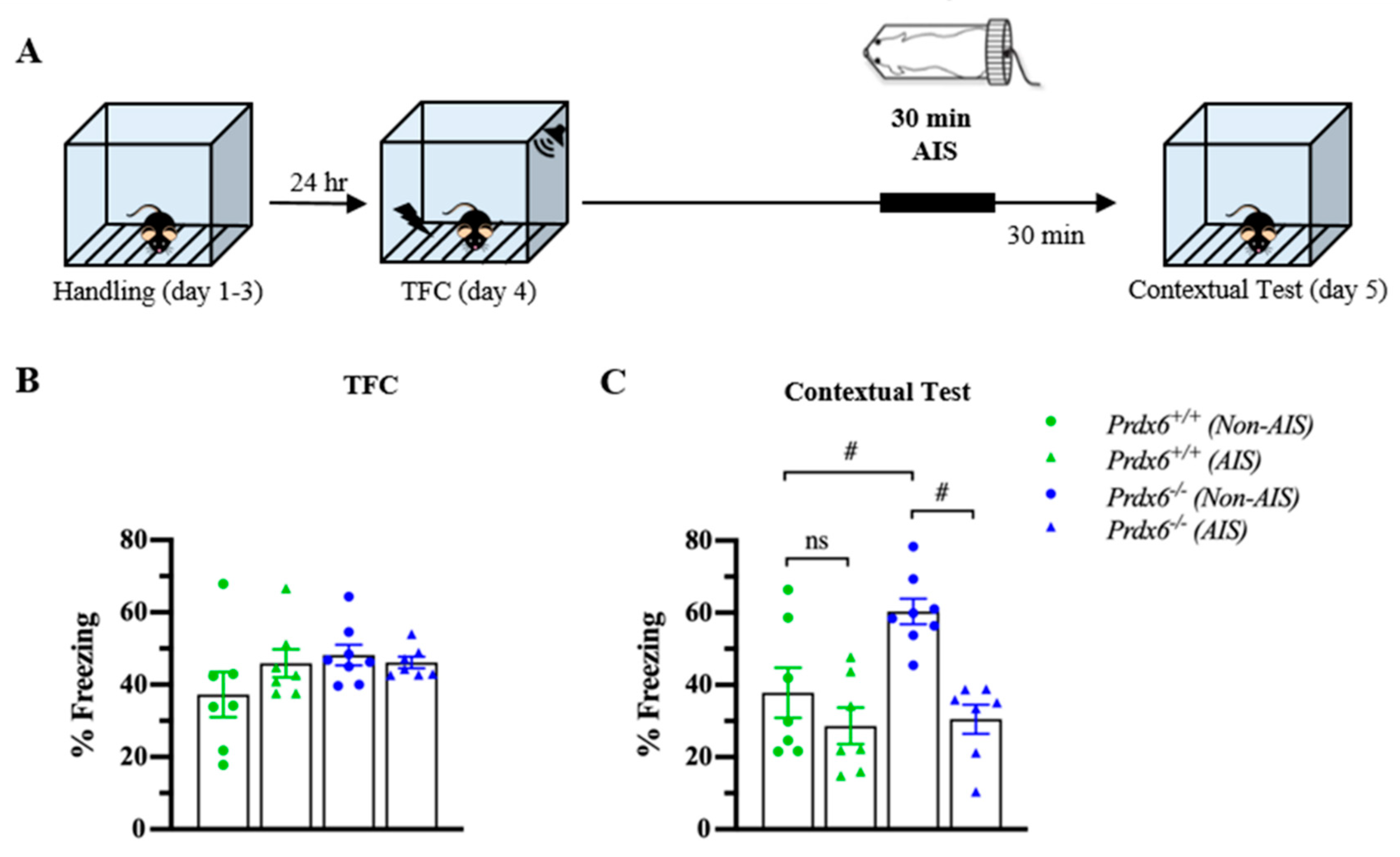
3.5. The Prdx6−/− Mice Demonstrated Lower Memory Retrieval to Context after AIS Compared with Non-AIS Prdx6−/− Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandi, C. Stress and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef]
- Uwaya, A.; Lee, H.; Park, J.; Lee, H.; Muto, J.; Nakajima, S.; Ohta, S.; Mikami, T. Acute immobilization stress following contextual fear conditioning reduces fear memory: Timing is essential. Behav. Brain Funct. 2016, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Hori, H.; Ozeki, Y.; Teraishi, T.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Suto, S.; Terada, S.; Higuchi, T.; Kunugi, H. Relationships between psychological distress, coping styles, and HPA axis reactivity in healthy adults. J. Psychiatr. Res. 2010, 44, 865–873. [Google Scholar] [CrossRef]
- Phillips, L.J.; McGorry, P.D.; Garner, B.; Thompson, K.N.; Pantelis, C.; Wood, S.J.; Berger, G. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: Implications for the development of psychotic disorders. Aust. N. Z. J. Psychiatry 2006, 40, 725–741. [Google Scholar] [CrossRef]
- Armario, A.; Marti, O.; Molina, T.; de Pablo, J.; Valdes, M. Acute stress markers in humans: Response of plasma glucose, cortisol and prolactin to two examinations differing in the anxiety they provoke. Psychoneuroendocrinology 1996, 21, 17–24. [Google Scholar] [CrossRef]
- Quijano, C.; Trujillo, M.; Castro, L.; Trostchansky, A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016, 8, 28–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, J.C.; Berry, J.E.; Lim, S.C.; Ong, S.K.; Kheirbek, M.A.; Hen, R. Contextual fear memory retrieval by correlated ensembles of ventral CA1 neurons. Nat. Commun. 2020, 11, 3492. [Google Scholar] [CrossRef] [PubMed]
- Rajasethupathy, P.; Sankaran, S.; Marshel, J.H.; Kim, C.K.; Ferenczi, E.; Lee, S.Y.; Berndt, A.; Ramakrishnan, C.; Jaffe, A.; Lo, M.; et al. Projections from neocortex mediate top-down control of memory retrieval. Nature 2015, 526, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedeva, Y.V.; Ji, S.G.; Yin, H.Z.; Weiss, J.H. Differential Vulnerability of CA1 versus CA3 Pyramidal Neurons After Ischemia: Possible Relationship to Sources of Zn2+ Accumulation and Its Entry into and Prolonged Effects on Mitochondria. J. Neurosci. 2017, 37, 726–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reul, J.M.; de Kloet, E.R. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology 1985, 117, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D. The Relationship between Acute Glucocorticoid Levels and Hippocampal Function Depends Upon Task Aversiveness and Memory Processing Stage. Nonlinearity Biol. Toxicol. Med. 2005, 3, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Revest, J.M.; Kaouane, N.; Mondin, M.; Le Roux, A.; Rouge-Pont, F.; Vallee, M.; Barik, J.; Tronche, F.; Desmedt, A.; Piazza, P.V. The enhancement of stress-related memory by glucocorticoids depends on synapsin-Ia/Ib. Mol. Psychiatry 2010, 15, 1140–1151. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, V.; Pulopulos, M.M.; Salvador, A. Acute psychosocial stress effects on memory performance: Relevance of age and sex. Neurobiol. Learn Mem. 2019, 157, 48–60. [Google Scholar] [CrossRef]
- Kang, S.W.; Baines, I.C.; Rhee, S.G. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J. Biol. Chem. 1998, 273, 6303–6311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Lee, H.P.; Lee, D.H.; Jo, M.; Shin, D.H.; Yoon, D.Y.; Han, S.B.; Hong, J.T. PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. Free Radic. Biol. Med. 2014, 69, 367–376. [Google Scholar] [CrossRef]
- Arriga, R.; Pacifici, F.; Capuani, B.; Coppola, A.; Orlandi, A.; Scioli, M.G.; Pastore, D.; Andreadi, A.; Sbraccia, P.; Tesauro, M.; et al. Peroxiredoxin 6 Is a Key Antioxidant Enzyme in Modulating the Link between Glycemic and Lipogenic Metabolism. Oxidative Med. Cell. Longev. 2019, 2019, 9685607. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Phasuk, S.; Pairojana, T.; Suresh, P.; Yang, C.H.; Roytrakul, S.; Huang, S.P.; Chen, C.C.; Pakaprot, N.; Chompoopong, S.; Nudmamud-Thanoi, S.; et al. Enhanced contextual fear memory in peroxiredoxin 6 knockout mice is associated with hyperactivation of MAPK signaling pathway. Mol. Brain 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Kekesi, K.A.; Juhasz, G.; Simor, A.; Gulyassy, P.; Szego, E.M.; Hunyadi-Gulyas, E.; Darula, Z.; Medzihradszky, K.F.; Palkovits, M.; Penke, B.; et al. Altered functional protein networks in the prefrontal cortex and amygdala of victims of suicide. PLoS ONE 2012, 7, e50532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, I.; Fisher, A.B.; Christofidou-Solomidou, M.; Gao, L.; Tao, J.Q.; Sorokina, E.M.; Lien, Y.C.; Bates, S.R.; Feinstein, S.I. Keratinocyte growth factor and glucocorticoid induction of human peroxiredoxin 6 gene expression occur by independent mechanisms that are synergistic. Antioxid. Redox Signal. 2014, 20, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Pak, J.H.; Gonzales, L.W.; Feinstein, S.I.; Fisher, A.B. Regulation of 1-cys peroxiredoxin expression in lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 2002, 27, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota, Y.; Acar, N.; Tranguch, S.; Burnum, K.E.; Xie, H.; Kodama, A.; Osuga, Y.; Ustunel, I.; Friedman, D.B.; Caprioli, R.M.; et al. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc. Natl. Acad. Sci. USA 2010, 107, 15577–15582. [Google Scholar] [CrossRef] [Green Version]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martins-de-Souza, D.; Guest, P.C.; Harris, L.W.; Vanattou-Saifoudine, N.; Webster, M.J.; Rahmoune, H.; Bahn, S. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl. Psychiatry 2012, 2, e87. [Google Scholar] [CrossRef] [Green Version]
- Martins-de-Souza, D.; Gattaz, W.F.; Schmitt, A.; Novello, J.C.; Marangoni, S.; Turck, C.W.; Dias-Neto, E. Proteome analysis of schizophrenia patients Wernicke’s area reveals an energy metabolism dysregulation. BMC Psychiatry 2009, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.X.; Han, H.L.; Tian, M.; Cao, J.; Xiu, J.B.; Song, N.N.; Huang, Y.; Xu, T.L.; Ding, Y.Q.; Xu, L. Enhanced contextual fear memory in central serotonin-deficient mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11981–11986. [Google Scholar] [CrossRef] [Green Version]
- Phasuk, S.; Jasmin, S.; Pairojana, T.; Chang, H.K.; Liang, K.C.; Liu, I.Y. Lack of the peroxiredoxin 6 gene causes impaired spatial memory and abnormal synaptic plasticity. Mol. Brain 2021, 14, 72. [Google Scholar] [CrossRef]
- Wang, X.; Phelan, S.A.; Forsman-Semb, K.; Taylor, E.F.; Petros, C.; Brown, A.; Lerner, C.P.; Paigen, B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J. Biol. Chem. 2003, 278, 25179–25190. [Google Scholar] [CrossRef] [Green Version]
- Zha, X.; Wu, G.; Zhao, X.; Zhou, L.; Zhang, H.; Li, J.; Ma, L.; Zhang, Y. PRDX6 Protects ARPE-19 Cells from Oxidative Damage via PI3K/AKT Signaling. Cell Physiol. Biochem. 2015, 36, 2217–2228. [Google Scholar] [CrossRef]
- Jin, H.L.; Choi, Y.; Jeong, K.W. Crosstalk between Aryl Hydrocarbon Receptor and Glucocorticoid Receptor in Human Retinal Pigment Epithelial Cells. Int. J. Endocrinol. 2017, 2017, 5679517. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Shylaja, H.; Sandeep Rao, K.S.; Santhosh Kumar, V.R.; Jagadeesh, M. Hesperidin ameliorates immobilization-stress-induced behavioral and biochemical alterations and mitochondrial dysfunction in mice by modulating nitrergic pathway. ISRN Pharmacol. 2012, 2012, 479570. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Goyal, R. Quercetin protects against acute immobilization stress-induced behaviors and biochemical alterations in mice. J. Med. Food 2008, 11, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Niemeyer, H.; Engel, S.; Cwik, J.C.; Laufer, S.; Klusmann, H.; Knaevelsrud, C. HPA axis regulation in posttraumatic stress disorder: A meta-analysis focusing on potential moderators. Neurosci. Biobehav. Rev. 2019, 100, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul. Med. 2014, 87, 3–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinner, V.L.; Het, S.; Wolf, O.T. Emotion regulation: Exploring the impact of stress and sex. Front. Behav. Neurosci. 2014, 8, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.M.; Yun, S.J.; Nam, K.N.; Kang, C.; Won, R.; Lee, E.H. Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can. J. Physiol. Pharmacol. 2009, 87, 440–447. [Google Scholar] [CrossRef]
- Sanner, B.M.; Meder, U.; Zidek, W.; Tepel, M. Effects of glucocorticoids on generation of reactive oxygen species in platelets. Steroids 2002, 67, 715–719. [Google Scholar] [CrossRef]
- Kasahara, E.; Inoue, M. Cross-talk between HPA-axis-increased glucocorticoids and mitochondrial stress determines immune responses and clinical manifestations of patients with sepsis. Redox Rep. 2015, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Chhunchha, B.; Kubo, E.; Fatma, N.; Singh, D.P. Sumoylation-deficient Prdx6 gains protective function by amplifying enzymatic activity and stability and escapes oxidative stress-induced aberrant Sumoylation. Cell Death Dis. 2017, 8, e2525. [Google Scholar] [CrossRef] [Green Version]
- Acar, N.; Soylu, H.; Edizer, I.; Ozbey, O.; Er, H.; Akkoyunlu, G.; Gemici, B.; Ustunel, I. Expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and peroxiredoxin 6 (Prdx6) proteins in healthy and pathologic placentas of human and rat. Acta Histochem. 2014, 116, 1289–1300. [Google Scholar] [CrossRef]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [Green Version]
- Daverey, A.; Agrawal, S.K. Regulation of Prdx6 by Nrf2 Mediated Through aiPLA2 in White Matter Reperfusion Injury. Mol. Neurobiol. 2021, 58, 1275–1289. [Google Scholar] [CrossRef]
- Kratschmar, D.V.; Calabrese, D.; Walsh, J.; Lister, A.; Birk, J.; Appenzeller-Herzog, C.; Moulin, P.; Goldring, C.E.; Odermatt, A. Suppression of the Nrf2-dependent antioxidant response by glucocorticoids and 11beta-HSD1-mediated glucocorticoid activation in hepatic cells. PLoS ONE 2012, 7, e36774. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Feinstein, S.I.; Fisher, A.B. Peroxiredoxin 6 as an antioxidant enzyme: Protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J. Cell. Biochem. 2008, 104, 1274–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.P.; Chhunchha, B.; Fatma, N.; Kubo, E.; Singh, S.P.; Singh, D.P. Delivery of a protein transduction domain-mediated Prdx6 protein ameliorates oxidative stress-induced injury in human and mouse neuronal cells. Am. J. Physiol. Cell Physiol. 2016, 310, C1–C16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gero, D.; Szabo, C. Glucocorticoids Suppress Mitochondrial Oxidant Production via Upregulation of Uncoupling Protein 2 in Hyperglycemic Endothelial Cells. PLoS ONE 2016, 11, e0154813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejean, C.; Courtin, J.; Rozeske, R.R.; Bonnet, M.C.; Dousset, V.; Michelet, T.; Herry, C. Neuronal Circuits for Fear Expression and Recovery: Recent Advances and Potential Therapeutic Strategies. Biol Psychiatry 2015, 78, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Shackman, A.J.; Fox, A.S. Contributions of the Central Extended Amygdala to Fear and Anxiety. J. Neurosci. 2016, 36, 8050–8063. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Brown, A.C.; Mattek, A.M.; Chavez, S.J.; Taylor, J.M.; Palmer, A.L.; Wu, Y.C.; Whalen, P.J. The Inverse Relationship between the Microstructural Variability of Amygdala-Prefrontal Pathways and Trait Anxiety Is Moderated by Sex. Front. Syst. Neurosci 2016, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid. Med. Cell Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008, 11, 851–876. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.W.; Jaaro-Peled, H.; Shahani, N.; Sedlak, T.W.; Zoubovsky, S.; Burruss, D.; Emiliani, F.; Sawa, A.; Gallagher, M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc. Natl. Acad. Sci. USA 2013, 110, 12462–12467. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, M.R.; Silvestrin, R.B.; Mello, E.S.T.; Moreira, J.C. Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: Effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology 2007, 28, 1191–1199. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, J.E.; Diaz, J.R.; Marta-Ariza, M.; Lizinczyk, A.M.; Franco, L.A.; Sadowski, M.J. Peroxiredoxin 6 mediates protective function of astrocytes in Abeta proteostasis. Mol. Neurodegener. 2020, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A(2) activities. Antioxid. Redox Signal 2011, 15, 831–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlatkovic, J.; Todorovic, N.; Boskovic, M.; Pajovic, S.B.; Demajo, M.; Filipovic, D. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol. Cell Biochem. 2014, 393, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Manzanares, P.A.; Isoardi, N.A.; Carrer, H.F.; Molina, V.A. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J. Neurosci. 2005, 25, 8725–8734. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ko, M.J.; Gonzales, E.L.; Kang, R.J.; Kim, D.G.; Kim, Y.; Seung, H.; Oh, H.A.; Eun, P.H.; Shin, C.Y. Social support rescues acute stress-induced cognitive impairments by modulating ERK1/2 phosphorylation in adolescent mice. Sci. Rep. 2018, 8, 12003. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fan, Y.X.; Wang, W.; Tang, Y.Y. Effects of acute restraint stress on different components of memory as assessed by object-recognition and object-location tasks in mice. Behav. Brain Res. 2012, 227, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, L.; Weber, G.E.; Parfitt, G.M.; Paese, K.; Goncalves, C.O.; Serodre, T.M.; Furtado, C.A.; Santos, A.P.; Monserrat, J.M.; Barros, D.M. PEGylated carbon nanotubes impair retrieval of contextual fear memory and alter oxidative stress parameters in the rat hippocampus. Biomed. Res. Int. 2015, 2015, 104135. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phasuk, S.; Varinthra, P.; Nitjapol, A.; Bandasak, K.; Liu, I.Y. Peroxiredoxin 6 Knockout Mice Demonstrate Anxiety Behavior and Attenuated Contextual Fear Memory after Receiving Acute Immobilization Stress. Antioxidants 2021, 10, 1416. https://doi.org/10.3390/antiox10091416
Phasuk S, Varinthra P, Nitjapol A, Bandasak K, Liu IY. Peroxiredoxin 6 Knockout Mice Demonstrate Anxiety Behavior and Attenuated Contextual Fear Memory after Receiving Acute Immobilization Stress. Antioxidants. 2021; 10(9):1416. https://doi.org/10.3390/antiox10091416
Chicago/Turabian StylePhasuk, Sarayut, Peeraporn Varinthra, Andaman Nitjapol, Korakod Bandasak, and Ingrid Y. Liu. 2021. "Peroxiredoxin 6 Knockout Mice Demonstrate Anxiety Behavior and Attenuated Contextual Fear Memory after Receiving Acute Immobilization Stress" Antioxidants 10, no. 9: 1416. https://doi.org/10.3390/antiox10091416
APA StylePhasuk, S., Varinthra, P., Nitjapol, A., Bandasak, K., & Liu, I. Y. (2021). Peroxiredoxin 6 Knockout Mice Demonstrate Anxiety Behavior and Attenuated Contextual Fear Memory after Receiving Acute Immobilization Stress. Antioxidants, 10(9), 1416. https://doi.org/10.3390/antiox10091416








