Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Shaving
2.3. Plaque Psoriasis Induction
2.4. KYC Treatment
2.5. Ear Inflammation Quantification
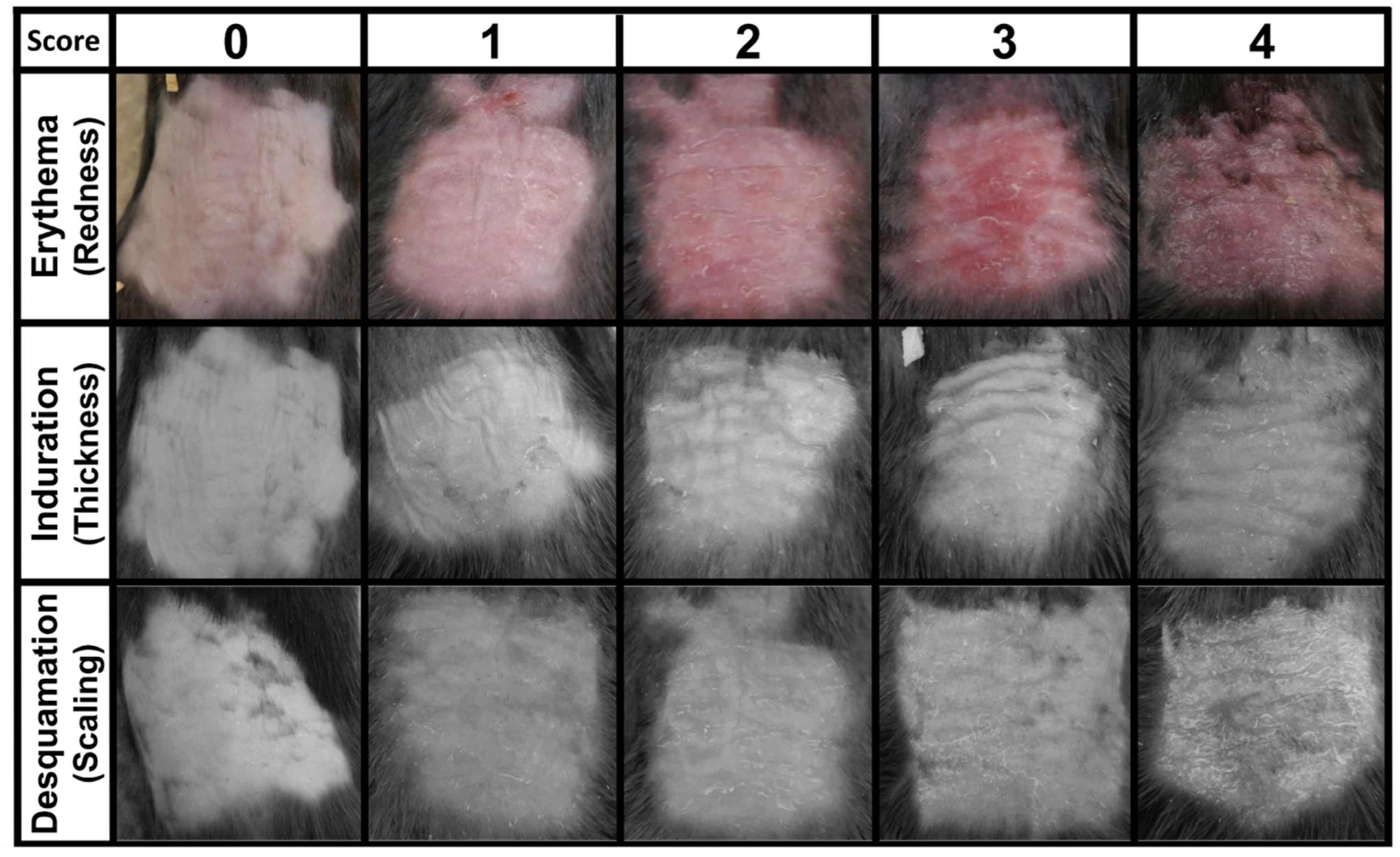
2.6. Plaque Psoriasis Severity Scoring
2.7. Histology
2.8. Statistical Analysis
3. Results
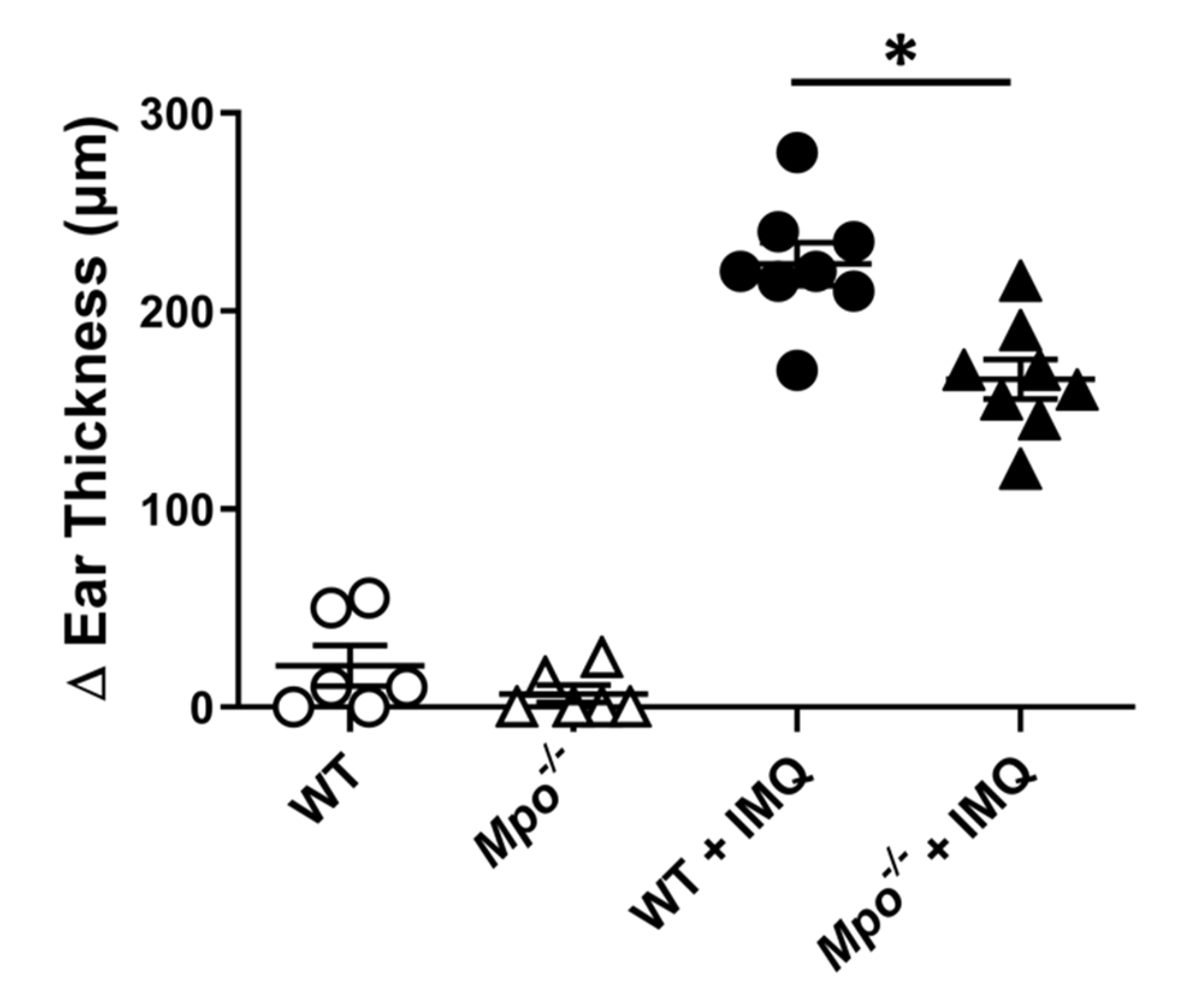
3.1. MPO Deficiency Attenuated Plaque Psoriasis
3.2. Development of a Mouse PASI Scoring System
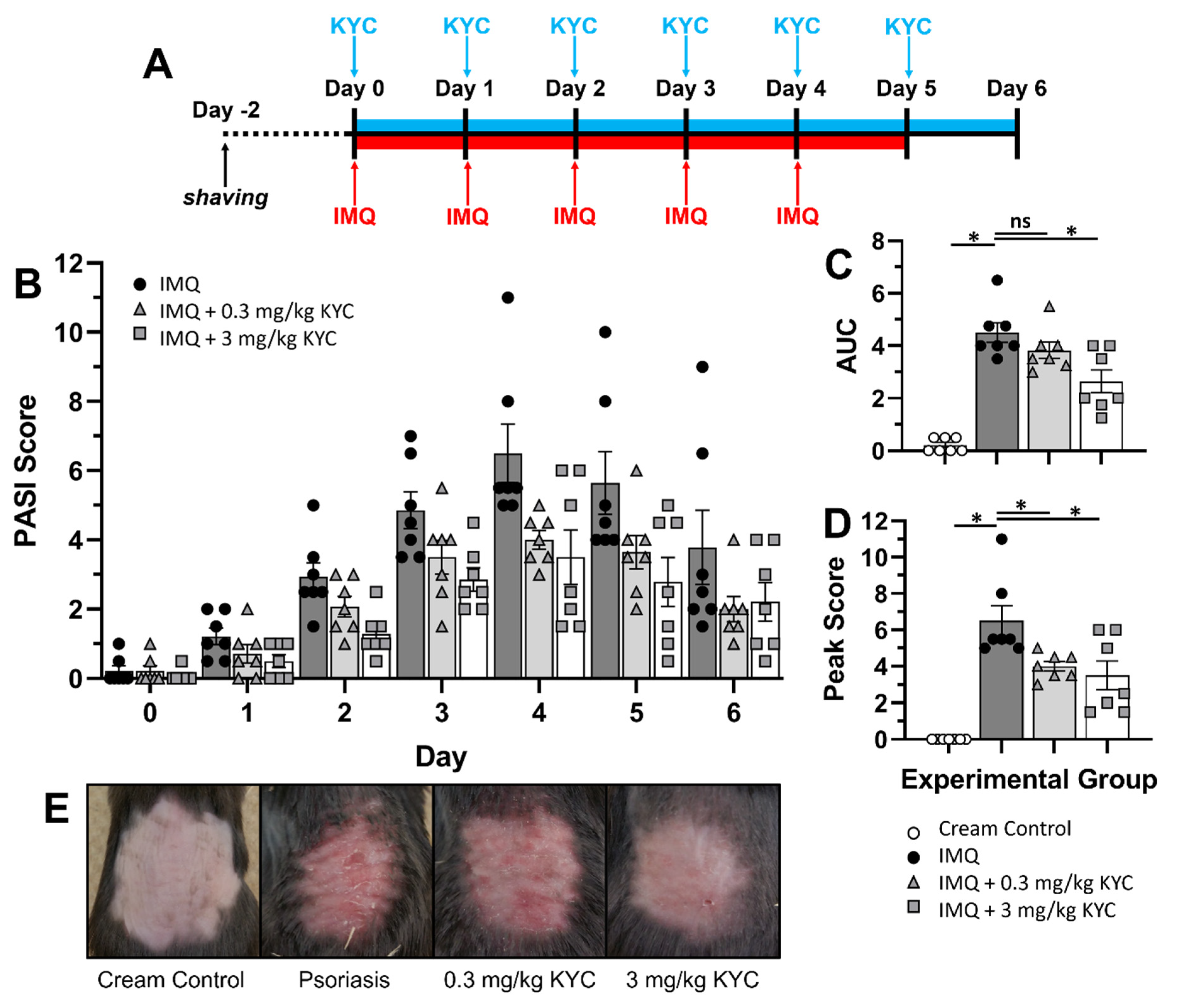
3.3. Systemic MPO Inhibition Attenuated Plaque Psoriasis
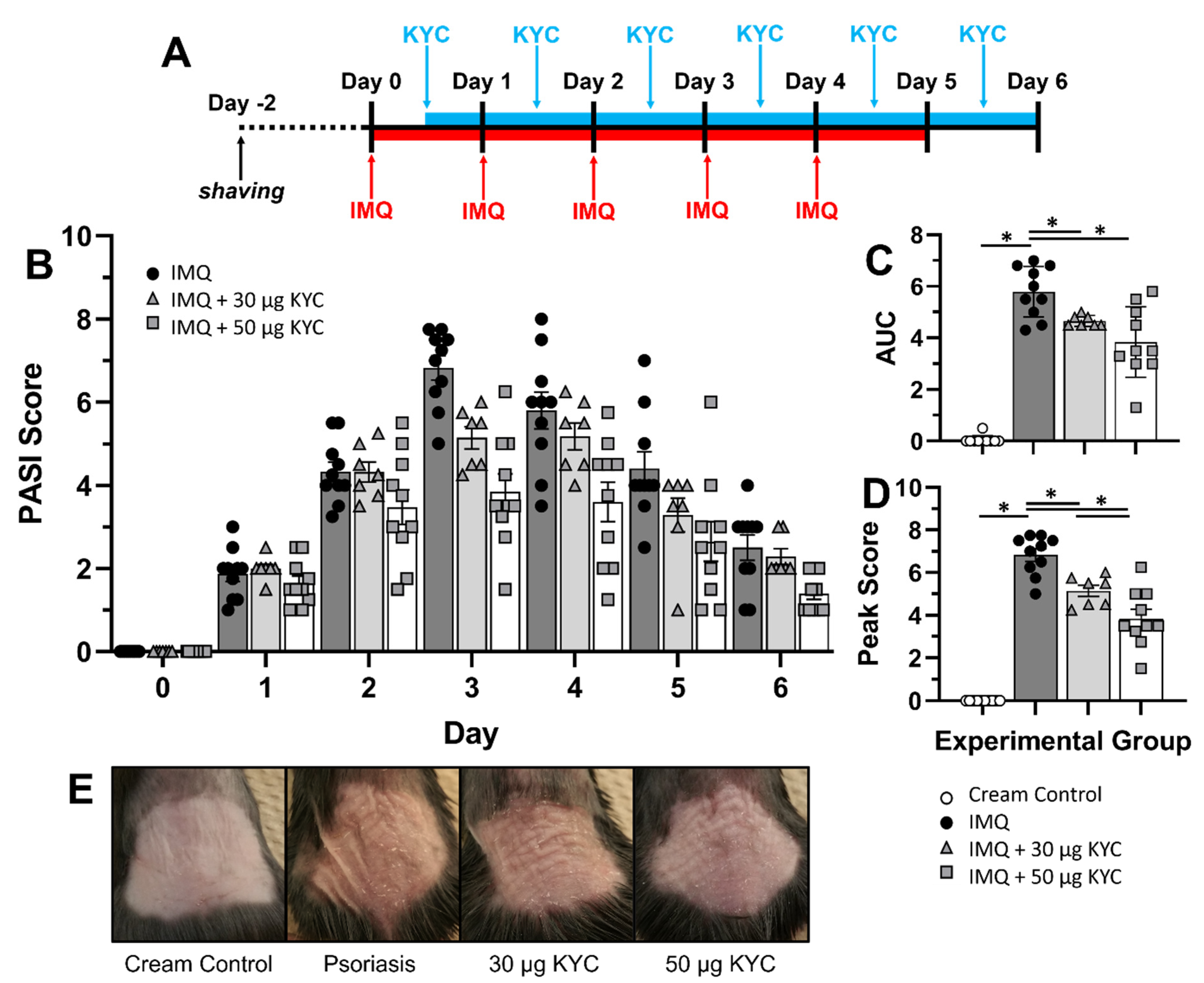
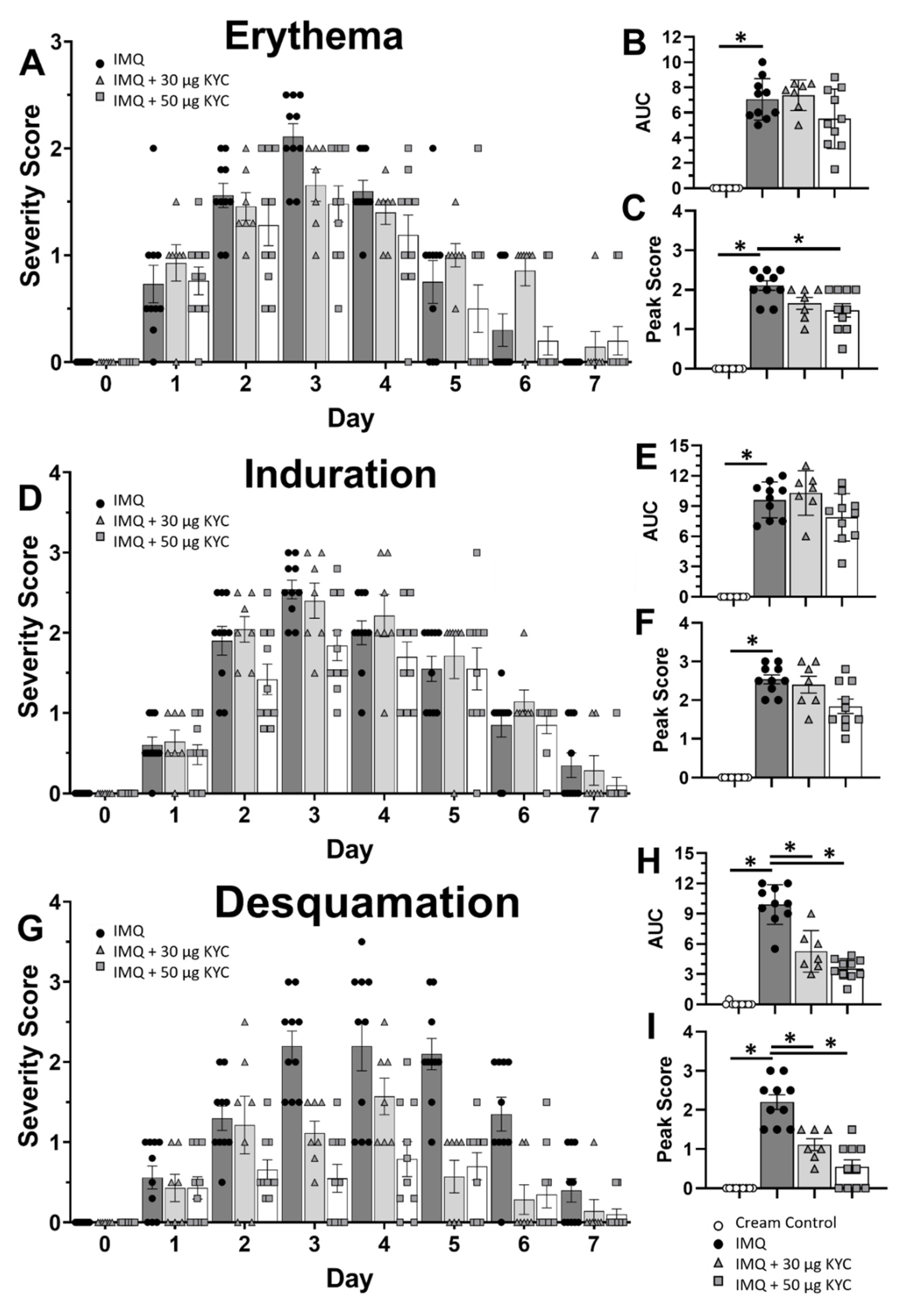
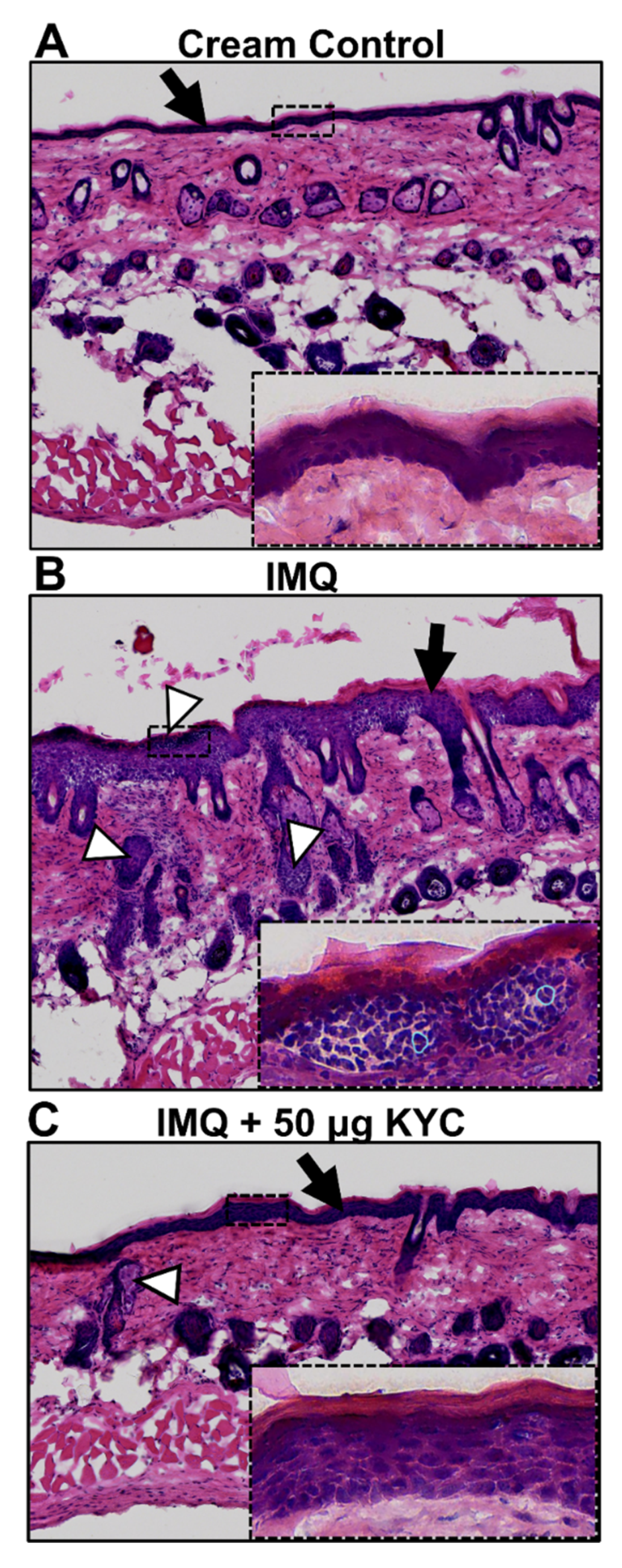
3.4. Topical Administration of KYC Attenuated Plaque Psoriasis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sterry, W. Psoriasis: Diagnosis and Management; Wiley: Hoboken, NJ, USA, 2014; pp. 55–75. [Google Scholar] [CrossRef]
- Terui, T.; Ozawa, M.; Tagami, H. Role of neutrophils in induction of acute inflammation in T-cell-mediated immune dermatosis, psoriasis: A neutrophil-associated inflammation-boosting loop. Exp. Dermatol. 2000, 9, 1–10. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Walt, J.M.; Ashcroft, D.M.; Flohr, C.; Naldi, L. The global state of psoriasis disease epidemiology: A workshop report. Br. J. Dermatol. 2007, 177, e4–e7. [Google Scholar] [CrossRef]
- Michalek, I.M.; Loring, B.; John, S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. 2017, 31, 205–212. [Google Scholar] [CrossRef]
- McClure, S.L.; Valentine, J.; Gordon, K.B. Comparative tolerability of systemic treatments for plaque-type psoriasis. Drug Saf. 2002, 25, 913–927. [Google Scholar] [CrossRef]
- Horn, E.J.; Fox, K.M.; Patel, V.; Kimball, A.B.; Gordon, K.B.; Lebwohl, M.G. Treatment Satisfaction and Health-Related Quality of Life among Individuals with Psoriasis: National Psoriasis Foundation Survey Findings; Psoriasis Forum; Sage: Los Angeles, CA, USA, 2008; Volume 14, pp. 27–34. [Google Scholar] [CrossRef]
- Bata-Csorgo, Z.; Hammerberg, C.; Voorhees, J.J.; Cooper, K.D. Flow cytometric identification of proliferative subpopulations within normal human epidermis and the localization of the primary hyperproliferative population in psoriasis. J. Exp. Med. 1993, 178, 1271–1281. [Google Scholar] [CrossRef]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Zaba, L.C.; Cardinale, I.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Novitskaya, I.; Khatcherian, A.; Bluth, M.J.; Lowes, M.A.; et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007, 204, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 Cytokines Stimulate CCL20 Expression in Keratinocytes In Vitro and In Vivo: Implications for Psoriasis Pathogenesis. J. Investig. Dermatol. 2009, 129, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Arany, I.; Apisarnthanarax, N.; Rajaraman, S.; Tyring, S.K.; Horikoshi, T.; Brysk, H.; Brysk, M.M. Response of keratinocytes from normal and psoriatic epidermis to interferon-γ differs in the expression of zinc-α2-glycoprotein and cathepsin D. FASEB J. 2000, 14, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, A.; Sugai, J.; Ohkido, M.; Ohtsuki, M.; Nakagawa, H.; Kitahara, H.; Tamaki, K.; Urabe, K.; Nakagawa, J.; Horikoshi, T.; et al. Cyclosporin in psoriasis: Continuous monotherapy versus intermittent long-term therapy. Eur. J. Dermatol. 1999, 9, 218–223. [Google Scholar]
- Baker, B.S.; Swain, A.F.; Griffiths, C.E.M.; Leonard, J.N.; Fry, L.; Valdimarsson, H. The Effects of topical treatment with steroids or dithranol on epidermal T lymphocytes and dendritic cells in psoriasis. Scand. J. Immunol. 1985, 22, 471–477. [Google Scholar] [CrossRef]
- Valdimarsson, H.; Bake, B.S.; Jónsdótdr, I.; Fry, L. Psoriasis: A disease of abnormal Keratinocyte proliferation induced by T lymphocytes. Immunol. Today 1986, 7, 256–259. [Google Scholar] [CrossRef]
- Dinant, H.J.; Van Kuijk, A.W.R.; Goedkoop, A.Y.; Kraan, M.C.; De Rie, M.A.; Dijkmans, B.A.C.; Vaishnaw, A.K.; Krueger, G.; Tak, P.P. Alefacept selectively reduces memory-effector (CD45RO+) T cells and improves clinical outcomes in psoriasis and psoriatic arthritis. J. Allergy Clin. Immun. 2002, 109, S256. [Google Scholar] [CrossRef]
- Watanabe, H.; Kawaguchi, M.; Fujishima, S.; Ogura, M.; Matsukura, S.; Takeuchi, H.; Ohba, M.; Sueki, H.; Kokubu, F.; Hizawa, N.; et al. Functional characterization of IL-17F as a selective neutrophil attractant in psoriasis. J. Investig. Dermatol. 2009, 129, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Cichon, I.; Santocki, M.; Ortmann, W.; Kolaczkowska, E. Neutrophil, methods and protocols. Methods Mol. Biol. 2019, 2087, 443–466. [Google Scholar] [CrossRef]
- Christoffersson, G.; Phillipson, M. The neutrophil: One cell on many missions or many cells with different agendas? Cell Tissue Res. 2018, 371, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.G. Human neutrophil degranulation. Methods Enzymol. 1988, 162, 538–551. [Google Scholar] [CrossRef]
- Aratani, Y.; Kura, F.; Watanabe, H.; Akagawa, H.; Takano, Y.; Suzuki, K.; Dinauer, M.C.; Maeda, N.; Koyama, H. In vivo role of myeloperoxidase for the host defense. Jpn. J. Infect. Dis. 2004, 57, S15. [Google Scholar] [PubMed]
- Chiang, C.C.; Cheng, W.J.; Korinek, M.; Lin, C.Y.; Hwang, T.L. Neutrophils in psoriasis. Front. Immunol. 2019, 10, 2376. [Google Scholar] [CrossRef]
- Shao, S.; Fang, H.; Dang, E.; Xue, K.; Zhang, J.; Li, B.; Qiao, H.; Cao, T.; Zhuang, Y.; Shen, S.; et al. Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front. Immunol. 2019, 10, 746. [Google Scholar] [CrossRef]
- Herster, F.; Bittner, Z.; Archer, N.; Dickhöfer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Löffler, M.W.; Kalbacher, H.; et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Kaminker, K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem. Biophys. 1962, 96, 465–467. [Google Scholar] [CrossRef]
- Hataishi, R.; Kobayashi, H.; Takahashi, Y.; Hirano, S.; Zapol, W.M.; Jones, R.C. Myeloperoxidase-associated tyrosine nitration after intratracheal administration of lipopolysaccharide in rats. Anesthesiology 2002, 97, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, N.; Forrer, P.; Casse, O.; Li, J.; Felmy, B.; Burgener, A.-V.; Ehrenfeuchter, N.; Hardt, W.-D.; Recher, M.; Hess, C.; et al. Myeloperoxidase targets oxidative host attacks to Salmonella and prevents collateral tissue damage. Nat. Microbiol. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Jong, N.W.M.; Ramyar, K.X.; Guerra, F.E.; Nijland, R.; Fevre, C.; Voyich, J.M.; McCarthy, A.; Garcia, B.L.; Van Kessel, K.P.M.; van Strijp, J.A.G.; et al. Immune evasion by a staphylococcal inhibitor of myeloperoxidase. Proc. Natl. Acad. Sci. USA 2017, 114, 9439–9444. [Google Scholar] [CrossRef] [PubMed]
- Odobasic, D.; Yang, Y.; Mulijadi, R.C.; O’Sullivan, K.M.; Kao, W.; Smith, M.; Morand, E.F.; Holdsworth, S.R. Endogenous myeloperoxidase is a mediator of joint inflammation and damage in experimental arthritis. Arthritis Rheumatol. 2014, 66, 907–917. [Google Scholar] [CrossRef]
- Sugiyama, S.; Okada, Y.; Sukhova, G.K.; Virmani, R.; Heinecke, J.W.; Libby, P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 2001, 158, 879–891. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Rodriguez, D.; Russo, M.; Campa, A. Macrophage activation includes high intracellular myeloperoxidase activity. Biochem. Bioph. Res. Commun. 2002, 292, 869–873. [Google Scholar] [CrossRef]
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of oxidative stress in various stages of psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392. [Google Scholar] [CrossRef]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef]
- Zhang, H.; Jing, X.; Shi, Y.; Xu, H.; Du, J.; Guan, T.; Weihrauch, D.; Jones, D.W.; Wang, W.; Gourlay, D.; et al. N-acetyl lysyltyrosylcysteine amide inhibits myeloperoxidase, a novel tripeptide inhibitor 1 [S]. J. Lipid Res. 2013, 54, 3016–3029. [Google Scholar] [CrossRef] [PubMed]
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cell Immunol. 2017, 317, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ray, A.; Miller, N.M.; Hartwig, D.; Pritchard, K.A.; Dittel, B.N. Inhibition of myeloperoxidase at the peak of experimental autoimmune encephalomyelitis restores blood-brain barrier integrity and ameliorates disease severity. J. Neurochem. 2016, 136, 826–836. [Google Scholar] [CrossRef]
- Strzepa, A.; Gurski, C.J.; Dittel, L.J.; Szczepanik, M.; Pritchard, A.K.; Dittel, B.N. Neutrophil-derived myeloperoxidase facilitates both the induction and elicitation phases of contact hypersensitivity. Front. Immunol. 2021, 11, 608871. [Google Scholar] [CrossRef]
- Yu, G.; Liang, Y.; Huang, Z.; Jones, D.W.; Pritchard, K.A.; Zhang, H. Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J. Neuroinflamm. 2016, 13, 119. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Weihrauch, D.; Jones, D.W.; Jing, X.; Shi, Y.; Gourlay, D.; Oldham, K.T.; Hillery, C.A.; Pritchard, K.A. Inhibition of myeloperoxidase decreases vascular oxidative stress and increases vasodilatation in sickle cell disease mice 1 [S]. J. Lipid Res. 2013, 54, 3009–3015. [Google Scholar] [CrossRef]
- Swindell, W.R.; Michaels, K.A.; Sutter, A.J.; Diaconu, D.; Fritz, Y.; Xing, X.; Sarkar, M.K.; Liang, Y.; Tsoi, A.; Gudjonsson, J.E.; et al. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med. 2017, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Van Der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Woods, V.E.; Epstein, H. Three parameters affecting interlitter variations. Dev. Psychobiol. 1979, 12, 317–328. [Google Scholar] [CrossRef]
- Graham, P.H.; Plant, N.; Graham, J.L.; Browne, L.; Borg, M.; Capp, A.; Delaney, G.P.; Harvey, J.; Kenny, L.; Francis, M.; et al. A paired, double-blind, randomized comparison of a moisturizing durable barrier cream to 10% glycerine cream in the prophylactic management of postmastectomy irradiation skin care: Trans Tasman Radiation Oncology Group (TROG) 04.01. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 45–50. [Google Scholar] [CrossRef]
- Zabalawi, M.; Bharadwaj, M.; Horton, H.; Cline, M.; Willingham, M.; Thomas, M.J.; Sorci-Thomas, M.G. Inflammation and skin cholesterol in LDLr−/−, apoA-I−/− mice: Link between cholesterol homeostasis and self-tolerance? J. Lipid Res. 2007, 48, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Zabalawi, M.; Bhat, S.; Loughlin, T.; Thomas, M.J.; Alexander, E.; Cline, M.; Bullock, B.; Willingham, M.; Sorci-Thomas, M.G. Induction of fatal inflammation in LDL receptor and ApoA-I double-knockout mice fed dietary fat and cholesterol. Am. J. Pathol. 2003, 163, 1201–1213. [Google Scholar] [CrossRef]
- Wohn, C.T.; Pantelyushin, S.; Ober-Blöbaum, J.L.; Clausen, B.E. Aldara-induced psoriasis-like skin inflammation: Isolation and characterization of cutaneous dendritic cells and innate lymphocytes. Methods Mol Biol. 2014, 1193, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Swanson, N.; Abramovits, W.; Berman, B.; Kulp, J.; Rigel, D.S.; Levy, S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: Results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J. Am. Acad. Dermatol. 2010, 62, 582–590. [Google Scholar] [CrossRef]
- Garzorz-Stark, N.; Lauffer, F.; Krause, L.; Thomas, J.; Atenhan, A.; Franz, R.; Roenneberg, S.; Boehner, A.; Jargosch, M.; Batra, R.; et al. Toll-like receptor 7/8 agonists stimulate plasmacytoid dendritic cells to initiate TH17-deviated acute contact dermatitis in human subjects. J. Allergy Clin. Immun. 2018, 141, 1320–1333.e11. [Google Scholar] [CrossRef]
- Afifi, L.; Shankle, L.; Armstrong, A.W.; Boas, M.; Bridges, A.; Chiguil, V.; Frank, D.; Duffin, K.C.; Fielding, E.; Fleischmann, R.; et al. National psoriasis foundation priorities for patient-centered research: Proceedings from the 2016 conference. J. Psoriasis Psoriatic Arthritis 2017, 2, 73–80. [Google Scholar] [CrossRef]
- Fleischer, A.B.; Rapp, S.R.; Reboussin, D.M.; Vanarthos, J.C.; Feldman, S.R. Patient measurement of psoriasis disease severity with a structured instrument. J. Investig. Dermatol. 1994, 102, 967–969. [Google Scholar] [CrossRef][Green Version]
- Youn, S.W.; Choi, C.W.; Kim, B.R.; Chae, J.B. Reduction of inter-rater and intra-rater variability in psoriasis area and severity index assessment by photographic training. Ann. Dermatol. 2015, 27, 557–562. [Google Scholar] [CrossRef]
- Pflumio, F.; Fonteneau, P.; Gavériaux, C.; Cammisuli, S.; Loor, F. The C57BL/6 nude, beige mouse: A model of combined T cell and NK effector cell immunodeficiency. Cell Immunol. 1989, 120, 218–229. [Google Scholar] [CrossRef]
- Nehls, M.; Pfeifer, D.; Schorpp, M.; Hedrich, H.; Boehm, T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 1994, 372, 103–107. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Murine models of psoriasis and their usefulness for drug discovery. Expert Opin. Drug Dis. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Farber, E.M.; Wilkinson, D.I.; Trozak, D.J. Clinical testing of new drugs for lesional skin diseases such as psoriasis A new delivery system. Skin Res. Technol. 1995, 1, 41–43. [Google Scholar] [CrossRef]
- Hagg, T. Continuous central nervous system infusion with Alzet osmotic pumps. Methods Neurosci. 1994, 21, 201–213. [Google Scholar] [CrossRef]
- Fara, J.W. Osmotic delivery systems for research. Methods Enzymol. 1985, 112, 470–484. [Google Scholar] [CrossRef]
- Volkman, R.; Ben-Zur, T.; Kahana, A.; Garty, B.Z.; Offen, D. Myeloperoxidase deficiency inhibits cognitive decline in the 5XFAD mouse model of Alzheimer’s disease. Front. Neurosci. 2019, 13, 990. [Google Scholar] [CrossRef]
- Allen, R.C.; Stephens, J.T. Myeloperoxidase selectively binds and selectively kills microbes. Infect Immun. 2011, 79, 474–485. [Google Scholar] [CrossRef]
- Britigan, B.E.; Ratcliffe, H.R.; Buettner, G.R.; Rosen, G.M. Binding of myeloperoxidase to bacteria: Effect on hydroxyl radical formation and susceptibility to oxidant-mediated killing. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1996, 1290, 231–240. [Google Scholar] [CrossRef]
- Kamili, Q.U.A.; Menter, A. Topical treatment of psoriasis. Curr. Probl. Dermatol. 2009, 38, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, P.C.M.; Van de Vissers, W.H.P.M. The topical treatment of psoriasis. Skin Pharmacol. Physiol. 2003, 16, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.L.; Kimball, A.; Papp, K.A.; Yeilding, N.; Guzzo, C.; Wang, Y.; Li, S.; Dooley, L.T.; Gordon, K.B. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008, 371, 1665–1674. [Google Scholar] [CrossRef]
- Leonardi, C.L.; Matheson, R.; Zachariae, C.; Cameron, G.; Li, L.; Edson-Heredia, E.; Braun, D.; Banerjee, S. Anti–interleukin-17 monoclonal antibody Ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 2012, 366, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Goodarzi, H.; Garcia, M.S.; Raychaudhuri, S.P.; Wehrli, L.N.; Ono, Y.; Maverakis, E. Biologic therapies in the treatment of psoriasis: A comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clin. Rev. Allergy Immunol. 2013, 44, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Pilon, D.; Teeple, A.; Zhdanava, M.; Ladouceur, M.; Cheung, H.C.; Muser, E.; Lefebvre, P. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J. Med. Econ. 2018, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
| Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Erythema (redness) | Fleshy pink. (Minor cuts appear dark but discreet) | Minor reddening across surface. | Medium red across surface. | Medium red across surface with dark red patches. | Dark red |
| Induration (thickness) | No skin thickening. Flesh should be loose and unwrinkled | Visible skin puckering; thickness unchanged when pinched (~1 mm) | Skin thickness of 1–2 mm when pinched; visible ridges in some areas. | Skin thickness of >2mm when pinched; visible ridges are loose. | Skin thickness of >2 mm without pinching; visible ridges are tight. |
| Desquamation (scaling) | No skin flaking. | Minor dry spots without flaking. | Dry spots across a majority of the skin; flaking along crevices | Moderate flaking across a large surface area. | Moderate flaking across a large surface area; severe flaking along crevices |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neu, S.D.; Strzepa, A.; Martin, D.; Sorci-Thomas, M.G.; Pritchard, K.A., Jr.; Dittel, B.N. Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice. Antioxidants 2021, 10, 1338. https://doi.org/10.3390/antiox10091338
Neu SD, Strzepa A, Martin D, Sorci-Thomas MG, Pritchard KA Jr., Dittel BN. Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice. Antioxidants. 2021; 10(9):1338. https://doi.org/10.3390/antiox10091338
Chicago/Turabian StyleNeu, Savannah D., Anna Strzepa, Dustin Martin, Mary G. Sorci-Thomas, Kirkwood A. Pritchard, Jr., and Bonnie N. Dittel. 2021. "Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice" Antioxidants 10, no. 9: 1338. https://doi.org/10.3390/antiox10091338
APA StyleNeu, S. D., Strzepa, A., Martin, D., Sorci-Thomas, M. G., Pritchard, K. A., Jr., & Dittel, B. N. (2021). Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice. Antioxidants, 10(9), 1338. https://doi.org/10.3390/antiox10091338







