Potential Activity Mechanisms of Aesculus hippocastanum Bark: Antioxidant Effects in Chemical and Biological In Vitro Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Material and Preparation of the Extract
2.3. Phytochemical Characterization of the Extract
2.4. Antioxidant Activity in Chemical Models
2.5. Preparation of Plasma Samples
2.6. Antioxidant Activity in Human Plasma Model
2.7. Protection against Nitrative Modifications of Human Fibrinogen
2.8. Influence on Plasma Hemostasis Parameters
2.9. Statistical Analysis
3. Results
3.1. Extract Standardization
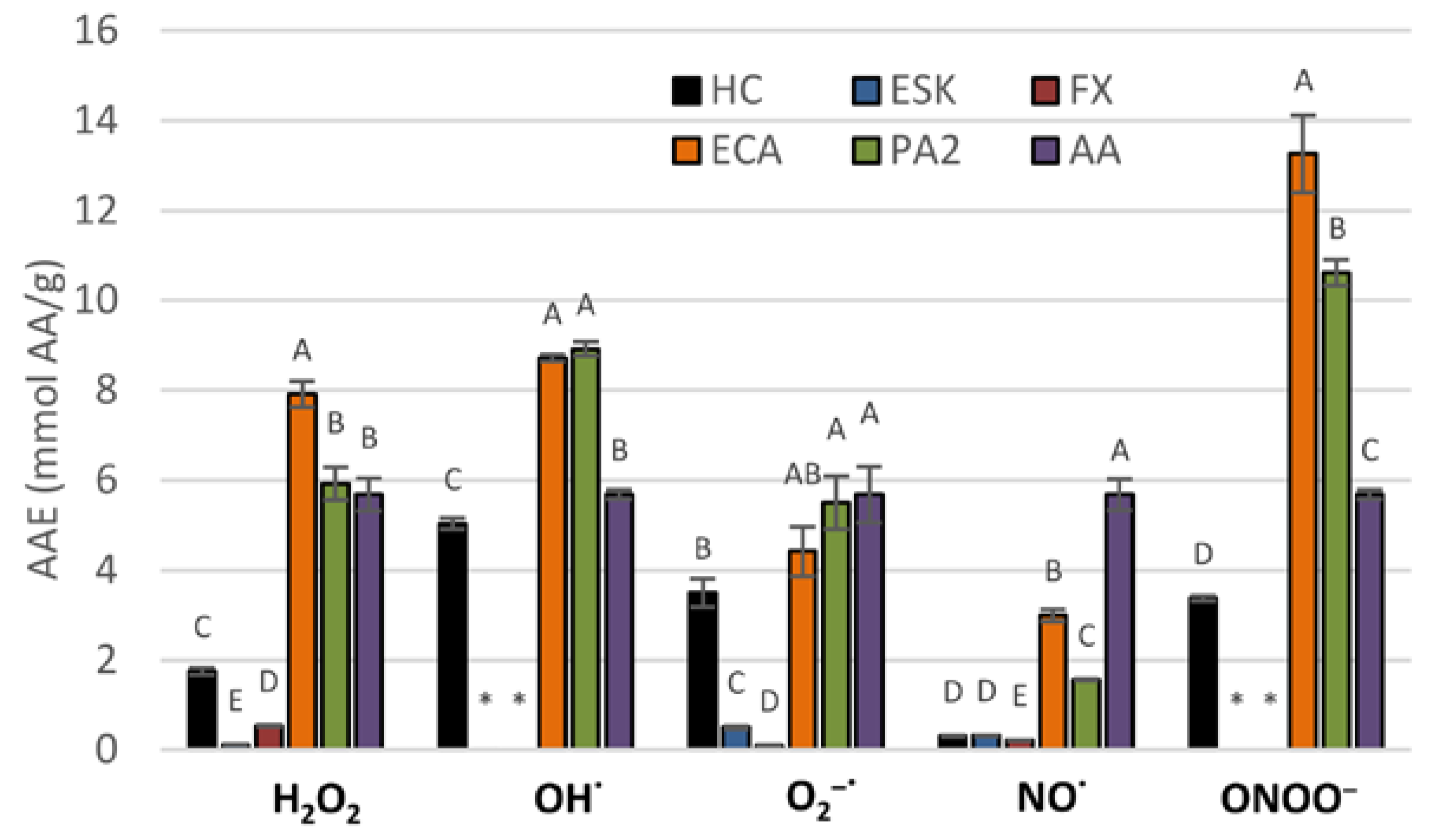
3.2. Antioxidant Activity in Chemical Models
3.3. Antioxidant Activity in Human Plasma Model
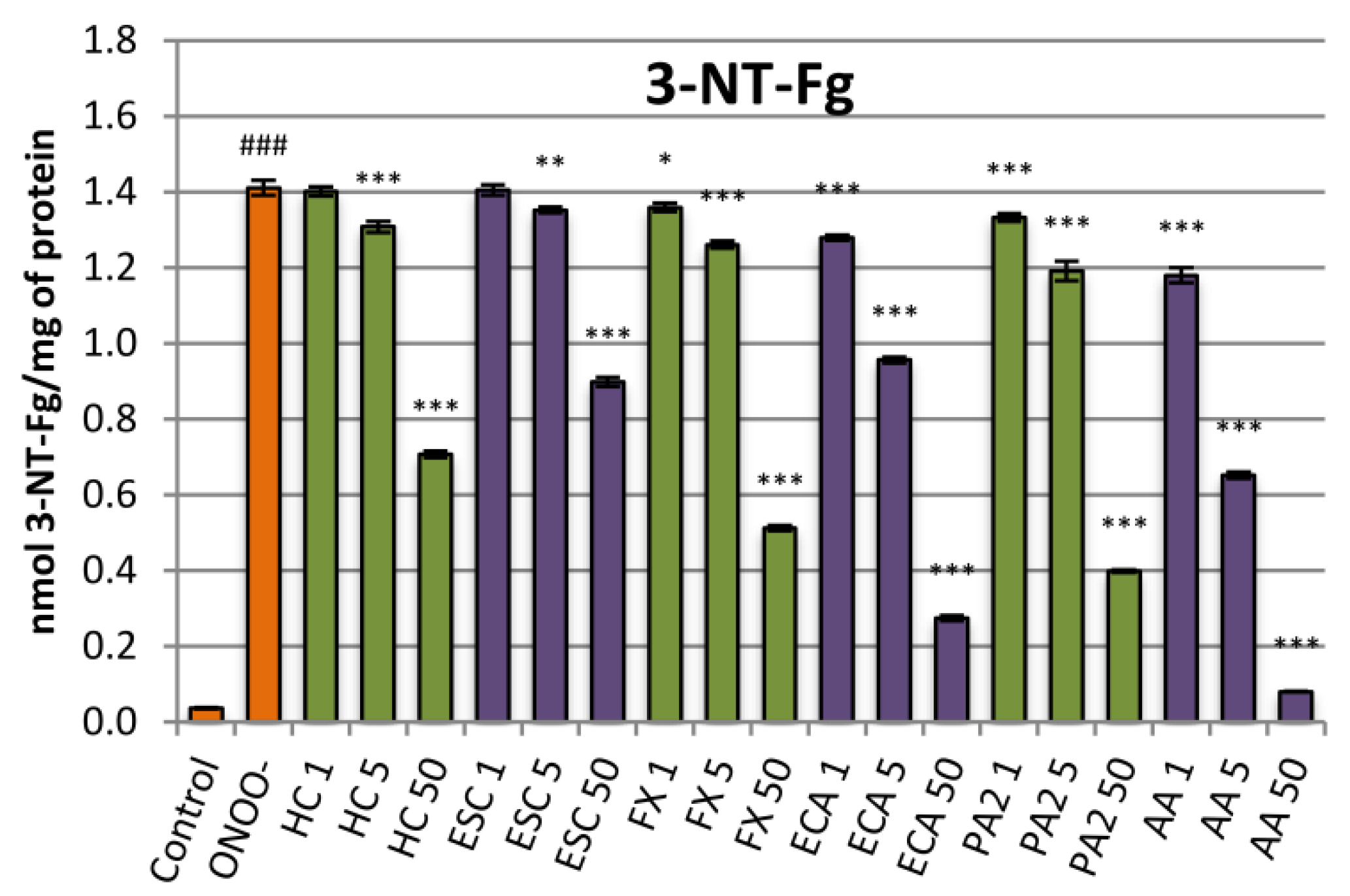
3.4. Influence on the ONOO−-Induced Formation of 3-Nitrotyrosine in Fibrinogen
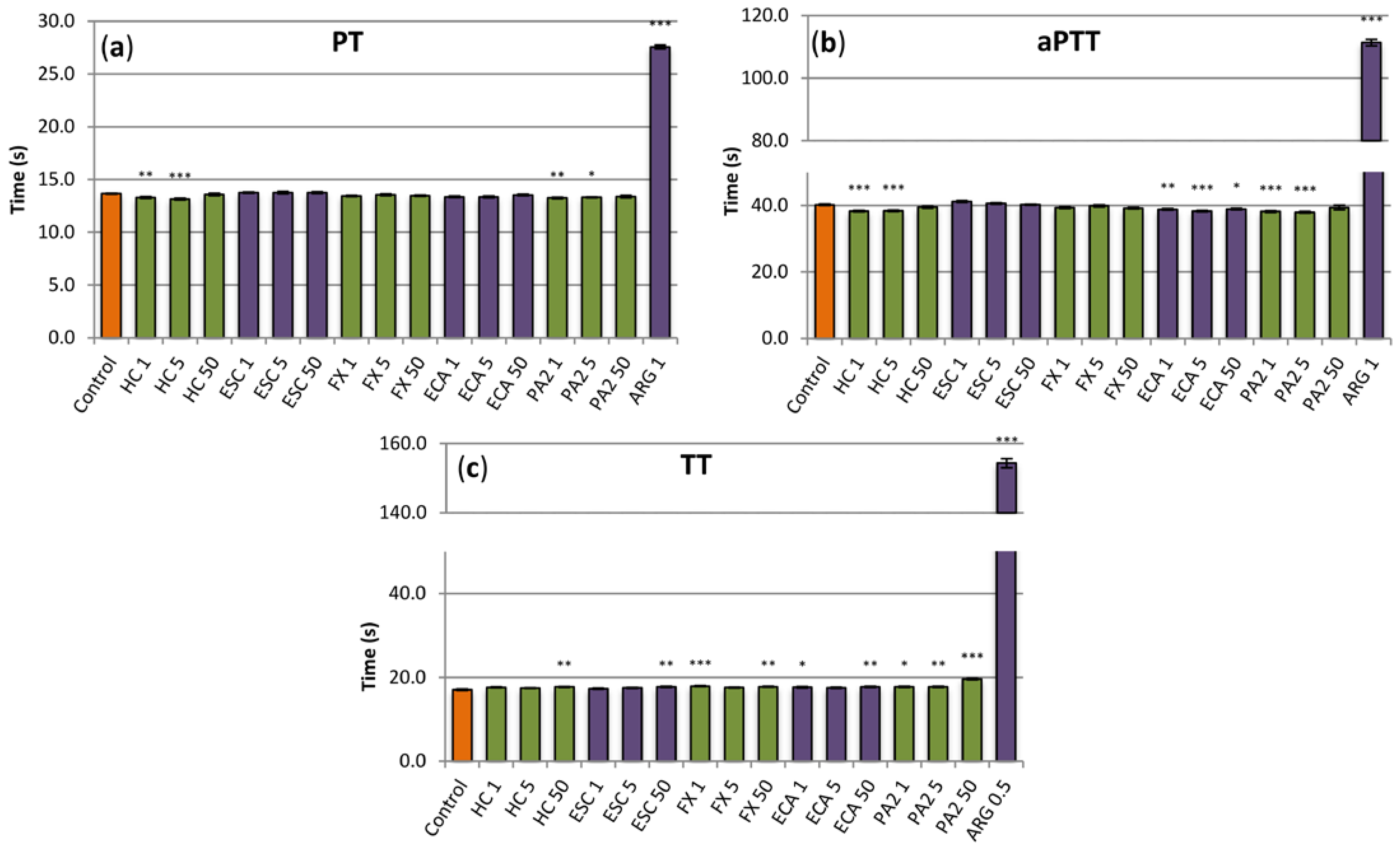
3.5. Influence on Plasma Hemostasis Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Li, S.; Lian, X.; Temple, A.; State, S.F.A. An overview of genus Aesculus L.: Ethnobotany, phytochemistry, and pharmacological activities. Pharm. Crop. 2010, 1, 24–51. [Google Scholar] [CrossRef] [Green Version]
- Comittee on Herbal Medicinal Products. Assessment Report on Aesculus hippocastanum L., Cortex; European Medicines Agency: London, UK, 2011.
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Owczarek, A.; Kłys, A.; Olszewska, M.A. A validated 1H qNMR method for direct and simultaneous quantification of esculin, fraxin and (–)-epicatechin in Hippocastani cortex. Talanta 2019, 192, 263–269. [Google Scholar] [CrossRef]
- Owczarek, A.; Olszewska, M.A. Development and validation of UHPLC-PDA method for simultaneous determination of bioactive polyphenols of horse-chestnut bark using numerical optimization with MS Excel Solver. J. Pharm. Biomed. Anal. 2020, 190, 113544. [Google Scholar] [CrossRef]
- Bombardelli, E.; Morazzoni, P.; Griffini, A. Aesculus hippocastanum L. Fitoterapia 1996, 67, 483–511. [Google Scholar]
- Celep, A.G.S.; Yilmaz, S.; Coruh, N. Antioxidant capacity and cytotoxicity of Aesculus hippocastanum, on breast cancer MCF-7 cells. J. Food Drug Anal. 2012, 20. [Google Scholar] [CrossRef]
- Braga, P.C.; Marabini, L.; Wang, Y.Y.; Lattuada, N.; Calò, R.; Bertelli, A.; Falchi, M.; Dal Sasso, M.; Bianchi, T. Characterisation of the antioxidant effects of Aesculus hippocastanum L. bark extract on the basis of radical scavenging activity, the chemiluminescence of human neutrophil bursts and lipoperoxidation assay. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1–9. [Google Scholar] [PubMed]
- Wichtl, M. (Ed.) Herbal Drug and Phytopharmaceuticals. A Handbook for Practice on a Scientific Basis; Medpharm GmbH Scientific Publishers: Stuttgart, Germany, 2004. [Google Scholar]
- Bijak, M.; Kolodziejczyk-Czepas, J.; Ponczek, M.B.; Saluk, J.; Nowak, P. Protective effects of grape seed extract against oxidative and nitrative damage of plasma proteins. Int. J. Biol. Macromol. 2012, 51, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The Antioxidant Activity of Coumarins and Flavonoids. Mini-Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef]
- Duric, K.; Kovac-Besovic, E.E.; Niksic, H.; Muratovic, S.; Sofic, E. Anticoagulant activity of some artemisia dracunculus leaf extracts. Bosn. J. Basic Med. Sci. 2015, 15, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinegre, T.; Teissandier, D.; Milenkovic, D.; Morand, C.; Lebreton, A. Epicatechin influences primary hemostasis, coagulation and fibrinolysis. Food Funct. 2019, 10, 7291–7298. [Google Scholar] [CrossRef]
- Kesieme, E.; Kesieme, C.; Jebbin, N.; Irekpita, E.; Dongo, A. Deep vein thrombosis: A clinical review. J. Blood Med. 2011, 2, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Rutkowska, M.; Michel, P.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. New insights into antioxidant activity of Prunus spinosa flowers: Extracts, model polyphenols and their phenolic metabolites in plasma towards multiple in vivo-relevant oxidants. Phytochem. Lett. 2019, 30, 288–295. [Google Scholar] [CrossRef]
- Czerwinska, M.; Kiss, A.; Naruszewicz, M. A comparative study of the effects of oleuropein and its dialdehydic form (oleacein) on human neutrophil oxidative burst. Planta Med. 2010. [Google Scholar] [CrossRef]
- Krzyzanowska-Kowalczyk, J.; Kolodziejczyk-Czepas, J.; Kowalczyk, M.; Pecio, Ł.; Nowak, P.; Stochmal, A. Yunnaneic Acid B, a Component of Pulmonaria officinalis Extract, Prevents Peroxynitrite-Induced Oxidative Stress in Vitro. J. Agric. Food Chem. 2017. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa L. flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijak, M.; Nowak, P.; Borowiecka, M.; Ponczek, M.B.; Zbikowska, H.M.; Wachowicz, B. Protective effects of (-)-epicatechin against nitrative modifications of fibrinogen. Thromb. Res. 2012, 130, e123–e128. [Google Scholar] [CrossRef]
- Kolodziejczyk, J.; Olas, B.; Wachowicz, B.; Szajwaj, B.; Stochmal, A.; Oleszek, W. Clovamide-rich extract from Trifolium pallidum reduces oxidative stress-induced damage to blood platelets and plasma. J. Physiol. Biochem. 2011, 67, 391–399. [Google Scholar] [CrossRef]
- Nowak, P.; Zbikowska, H.M.; Ponczek, M.; Kolodziejczyk, J.; Wachowicz, B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: Functional consequences. Thromb. Res. 2007, 121, 163–174. [Google Scholar] [CrossRef]
- Buetler, T.M.; Krauskopf, A.; Ruegg, U.T. Role of superoxide as a signaling molecule. News Physiol. Sci. 2004, 19, 120–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretón-Romero, R.; Lamas, S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014, 2, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Krzyściak, W.; Kózka, M. Generation of reactive oxygen species by a sufficient, insufficient and varicose vein wall. Acta Biochim. Pol. 2011, 58, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wali, M.A.; Suleiman, S.A.; Kadoumi, O.F.; Nasr, M.A. Superoxide radical concentration and superoxide dismutase (SOD) enzyme activity in varicose veins. Ann. Thorac. Cardiovasc. Surg. 2002, 8, 286–290. [Google Scholar]
- Lubos, E.; Handy, D.E.; Loscalzo, J. Role of oxidative stress and nitric oxide in atherothrombosis. Front. Biosci. 2008, 13, 5323–5344. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Abraham, K.; Pfister, M.; Wöhrlin, F.; Lampen, A. Relative bioavailability of coumarin from cinnamon and cinnamon-containing foods compared to isolated coumarin: A four-way crossover study in human volunteers. Mol. Nutr. Food Res. 2011, 55, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Karatepe, O.; Unal, O.; Ugurlucan, M.; Kemik, A.; Karahan, S.; Aksoy, M.; Kurtoglu, M. The impact of valvular oxidative stress on the development of venous stasis ulcer valvular oxidative stress and venous ulcers. Angiology 2010, 61, 283–288. [Google Scholar] [CrossRef]
- Horecka, A.; Biernacka, J.; Hordyjewska, A.; Dąbrowski, W.; Terlecki, P.; Zubilewicz, T.; Musik, I.; Kurzepa, J. Antioxidative mechanism in the course of varicose veins. Phlebology 2018, 33, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Condezo-Hoyos, L.; Rubio, M.; Arribas, S.M.; España-Caparrós, G.; Rodríguez-Rodríguez, P.; Mujica-Pacheco, E.; González, M.C. A plasma oxidative stress global index in early stages of chronic venous insufficiency. J. Vasc. Surg. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saribal, D.; Kanber, E.M.; Hocaoglu-Emre, F.S.; Akyolcu, M.C. Effects of the oxidative stress and genetic changes in varicose vein patients. Phlebology 2019, 34, 406–413. [Google Scholar] [CrossRef]
- Glowinski, J.; Glowinski, S. Generation of reactive oxygen metabolites by the varicose vein wall. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riazanov, D.Y.; Mamunchak, O.V. Dynamics of endothelial dysfunction indices before and after operation in patients with varicose disease of the lower extremities. Zaporozhye Med. J. 2019, 21, 499–503. [Google Scholar] [CrossRef]
- Peluffo, G.; Radi, R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc. Res. 2007, 75, 291–302. [Google Scholar] [CrossRef]
- Pannala, A.; Rice-Evans, C.A.; Halliwell, B.; Singh, S. Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochem. Biophys. Res. Commun. 1997, 232, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Cuker, A.; Mills, A.; Lightfoot, R.; Fan, Y.; Wilson Tang, W.H.; Hazen, S.L.; Ischiropoulos, H. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic. Biol. Med. 2012, 53, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Parastatidis, I.; Thomson, L.; Burke, A.; Chernysh, I.; Nagaswami, C.; Visser, J.; Stamer, S.; Liebler, D.C.; Koliakos, G.; Heijnen, H.F.G.; et al. Fibrinogen β-chain tyrosine nitration is a prothrombotic risk factor. J. Biol. Chem. 2008, 283, 33846–33853. [Google Scholar] [CrossRef] [Green Version]
- Vadseth, C.; Souza, J.M.; Thomson, L.; Seagraves, A.; Nagaswami, C.; Scheiner, T.; Torbet, J.; Vilaire, G.; Bennett, J.S.; Murciano, J.C.; et al. Pro-thrombotic State Induced by Post-translational Modification of Fibrinogen by Reactive Nitrogen Species. J. Biol. Chem. 2004, 279, 8820–8826. [Google Scholar] [CrossRef] [Green Version]
- Antignani, P.L. Medical Treatment of Chronic Venous Disease. SM J. Pharmacol. Ther. 2017, 3, 1015. [Google Scholar]
- Chamara, A.M.R.; Thiripuranathar, G. Assessement of haemostatic activity of medicinal palnts using in vitro methods: A concise review. IOSR J. Pharm. Biol. Sci. 2020, 15, 26–34. [Google Scholar]
- Jung, J.C.; Park, O.S. Synthetic approaches and biological activities of 4-hydroxycoumarin derivatives. Molecules 2009, 14, 4790–4803. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ma, H.; Yang, N.; Tang, Y.; Guo, J.; Tao, W.; Duan, J. A series of natural flavonoids as thrombin inhibitors: Structure-activity relationships. Thromb. Res. 2010, 126, e365–e378. [Google Scholar] [CrossRef] [PubMed]
- Neiva, T.J.C.; Morais, L.; Polack, M.; Simões, C.M.O.; D’Amico, E.A. Effects of catechins on human blood platelet aggregation and lipid peroxidation. Phyther. Res. 1999, 13, 597–600. [Google Scholar] [CrossRef]
- Bijak, M.; Ziewiecki, R.; Saluk, J.; Ponczek, M.; Pawlaczyk, I.; Krotkiewski, H.; Wachowicz, B.; Nowak, P. Thrombin inhibitory activity of some polyphenolic compounds. Med. Chem. Res. 2014, 23, 2324–2337. [Google Scholar] [CrossRef] [Green Version]
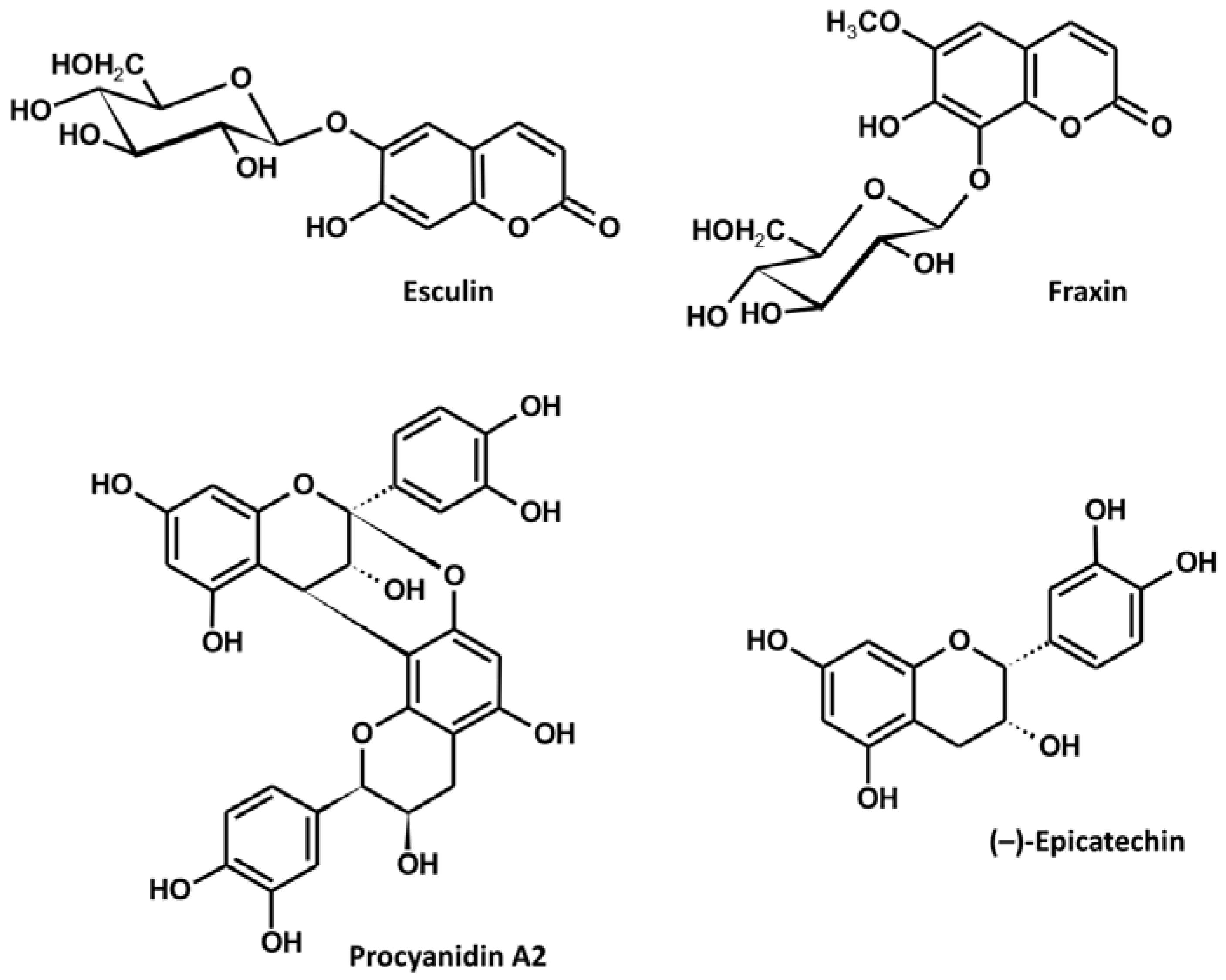


| No. | Analyte | Content (mg/g) |
|---|---|---|
| 1 | Esculin | 175.23 ± 2.32 |
| 2 | Isoscopolin | 3.63 ± 0.16 |
| 3 | Scopolin | 2.83 ± 0.07 |
| 4 | Esculetin | 10.03 ± 0.10 |
| 5 | Fraxin | 73.69 ± 0.99 |
| Total coumarins | 265.42 ± 3.30 | |
| 6 | (‒)-Epicatechin | 63.17 ± 0.73 |
| 7 | Proanthocyanidin dimer B-type | 14.14 ± 0.77 |
| 8 | Proanthocyanidin trimer A-type | 5.34 ± 0.72 |
| 9 | Procyanidin A2 | 55.91 ± 0.35 |
| Total flavan-3-ols | 138.56 ± 2.38 | |
| Total phenolics | 403.98 ± 3.86 |
| Analyte | SC50 (µg/mL) | ||||
|---|---|---|---|---|---|
| H2O2 | OH• | O2•− | NO• | ONOO− | |
| A. hippocastanum extract | 38.03 ± 1.74 C | 172.27 ± 4.57 C | 9.96 ± 0.90 B | 8.74 ± 0.35 D | 140.44 ± 2.37 D |
| Esculin | 574.43 ± 23.63 E | >1000 | 69.27 ± 5.35 C | 8.56 ± 0.10 D | >1200 |
| Fraxin | 124.71 ± 2.75 D | >1000 | 358.06 ± 31.37 D | 12.90 ± 0.21 E | >200 |
| (–)-Epicatechin | 8.41 ± 0.30 A | 99.53 ± 0.85 A | 7.89 ± 0.99 AB | 0.91 ± 0.04 B | 35.70 ± 2.32 A |
| Procyanidin A2 | 11.23 ± 0.68 B | 97.26 ± 1.74 A | 6.33 ± 0.68 A | 1.75 ± 0.02 C | 44.61 ± 1.19 B |
| Ascorbic acid | 11.71 = 0.76 B | 152.59 ± 2.79 B | 6.13 ± 0.67 A | 0.48 ± 0.03 A | 83.34 ± 1.50 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarek, A.; Kolodziejczyk-Czepas, J.; Woźniak-Serwata, J.; Magiera, A.; Kobiela, N.; Wąsowicz, K.; Olszewska, M.A. Potential Activity Mechanisms of Aesculus hippocastanum Bark: Antioxidant Effects in Chemical and Biological In Vitro Models. Antioxidants 2021, 10, 995. https://doi.org/10.3390/antiox10070995
Owczarek A, Kolodziejczyk-Czepas J, Woźniak-Serwata J, Magiera A, Kobiela N, Wąsowicz K, Olszewska MA. Potential Activity Mechanisms of Aesculus hippocastanum Bark: Antioxidant Effects in Chemical and Biological In Vitro Models. Antioxidants. 2021; 10(7):995. https://doi.org/10.3390/antiox10070995
Chicago/Turabian StyleOwczarek, Aleksandra, Joanna Kolodziejczyk-Czepas, Joanna Woźniak-Serwata, Anna Magiera, Natalia Kobiela, Katarzyna Wąsowicz, and Monika Anna Olszewska. 2021. "Potential Activity Mechanisms of Aesculus hippocastanum Bark: Antioxidant Effects in Chemical and Biological In Vitro Models" Antioxidants 10, no. 7: 995. https://doi.org/10.3390/antiox10070995
APA StyleOwczarek, A., Kolodziejczyk-Czepas, J., Woźniak-Serwata, J., Magiera, A., Kobiela, N., Wąsowicz, K., & Olszewska, M. A. (2021). Potential Activity Mechanisms of Aesculus hippocastanum Bark: Antioxidant Effects in Chemical and Biological In Vitro Models. Antioxidants, 10(7), 995. https://doi.org/10.3390/antiox10070995






