Vitamin A and E Homologues Impacting the Fate of Acrylamide in Equimolar Asparagine-Glucose Model System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Asparagine-Glucose Model System
2.3. Acrylamide Analysis
2.3.1. Sample Extraction
2.3.2. GC-MS Analysis
2.3.3. Quantification of Acrylamide
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Some Industrial Chemicals. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono60.pdf (accessed on 12 October 2018).
- Friedman, M. Acrylamide: Inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct. 2016, 6, 1752–1772. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Saeidnia, S.; Abdollahi, M. Role of antioxidants and phytochemicals on acrylamide mitigation from food and reducing its toxicity. J. Food Sci. Technol. 2015, 52, 3169–3186. [Google Scholar] [CrossRef] [Green Version]
- Sundram, K.; Sambanthamurthi, R.; Tan, A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Ubaldi, A.; Delbono, G.; Fusari, A.; Serventi, P. Quick HPLC method to determine vitamin E concentration in cow’s milk. Ann. Fac. Med. Vet. Di Parma 2005, 25, 101–110. [Google Scholar]
- Eqbal, D.; Halimah, A.S.; Aminah, A.; Halimah, M.; Gapor, M.T. Vitamin E and Beta Carotene Composition in Four Different Vegetable Oils. Am. J. Appl. Sci. 2011, 8, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Yap, S.C.; Choo, Y.M.; Ooi, C.K.; Ong, C.K.; Goh, S.H. Quantitative analysis of carotenes in the oil from different palm species. Elaeis 1991, 3, 309–318. [Google Scholar]
- Patrick, L. Beta carotene: The controversy continues. Altern. Med. Rev. 2000, 5, 530–545. [Google Scholar] [PubMed]
- Kushairi, A.; Ong-Abdullah, M.; Nambiappan, B.; Hishamuddin, E.; Bidin, M.N.I.Z.; Ghazali, R.; Subramaniam, V.; Sundram, S.; Parveez, G.K.A. Oil palm economic performance in Malaysia and r&d progress in 2018. J. Oil Palm Res. 2019, 31, 165–194. [Google Scholar] [CrossRef]
- Miskiewicz, K.; Nebesny, E.; Rosicka, J.; Zyzelewicz, D.; Budryn, G. The effects of baking conditions on acrylamide content in shortcrust cookies with added freeze-dried aqueous rosemary extract. J. Food Sci. Technol. 2018, 55, 4184–4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Chen, Y.; Zhang, Y.; Lu, B.; Jin, C.; Wu, X. Study on mitigation of acrylamide formation in cookies by 5 antioxidants. J. Food Sci. 2012, 77, C1144–C1149. [Google Scholar] [CrossRef]
- Kamarudin, S.A.; Jinap, S.; Sukor, R.; Foo, S.P.; Sanny, M. Effect of fat-soluble anti-oxidants in vegetable oils on acrylamide concentrations during deep-fat frying of French fries. Malays. J. Med Sci. 2018, 25, 128–139. [Google Scholar] [CrossRef]
- Kuek, S.L.; Ahmad Tarmizi, A.H.; Abd Razak, R.A.; Jinap, S.; Norliza, S.; Sanny, M. Contribution of lipid towards acrylamide formation during intermittent frying of French fries. Food Control 2020, 118, 107430. [Google Scholar] [CrossRef]
- Jin, C.; Wu, X.; Zhang, Y. Relationship between antioxidants and acrylamide formation: A review. Food Res. Int. 2013, 51, 611–620. [Google Scholar] [CrossRef]
- Tareke, E. Identification and Origin of Potential Background Carcinogens: Endogenous Isoprene and Oxi-ranes, Dietary Acrylamide. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2003. [Google Scholar]
- Ahmad, S.N.S.; Tarmizi, A.H.A.; Razak, R.A.A.; Jinap, S.; Norliza, S.; Sulaiman, R.; Sanny, M. Selection of vegetable oils and frying cycles influencing acrylamide formation in the intermittently fried beef nuggets. Foods 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Cheng, K.W.; Jiang, Y.; Lin, Z.X.; Shi, J.J.; Ou, S.Y.; Chen, F.; Wang, M. Inhibition of acrylamide formation by vitamins in model reactions and fried potato strips. Food Chem. 2009, 116, 34–39. [Google Scholar] [CrossRef]
- Kocadagli, T.; Goncuoglu, T.N.; Hamzalioglu, A.; Gokmen, V. In depth study of acrylamide formation in coffee during roasting: Role of sucrose decomposition and lipid oxidation. Food Funct. 2012, 3, 970–975. [Google Scholar] [CrossRef]
- Ou, S.; Shi, J.; Huang, C.; Zhang, G.; Teng, J.; Jiang, Y.; Yang, B. Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mater. 2010, 182, 863–868. [Google Scholar] [CrossRef]
- Knol, J.J.; Van Loon, W.A.M.; Linssen, J.P.H.; Ruck, A.L.; Van Boekel, M.A.J.S.; Voragen, A.G.J. Toward a kinetic model for acrylamide formation in a glucose-asparagine reaction system. J. Agric. Food Chem. 2005, 53, 6133–6139. [Google Scholar] [CrossRef]
- Sanny, M.; Jinap, S.; Bakker, E.J.; Van Boekel, M.A.J.S.; Luning, P.A. Possible causes of variation in acrylamide concentration in French fries prepared in food service establishments: An observational study. Food Chem. 2012, 132, 134–143. [Google Scholar] [CrossRef]
- Bowry, V.; Stocker, R. Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J. Am. Chem. Soc. 1993, 115, 6029–6044. [Google Scholar] [CrossRef]
- Miller, E.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.; Appel, L.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietjens, I.M.C.M.; Boersma, M.G.; Haan, L.D.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.P.; Van Zanden, J.J.; Woude, H.V.D.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Palozza, P. Prooxidant Actions of Carotenoids in Biologic Systems. Nutr. Rev. 1998, 56, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Ingold, K.U. beta-Carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- Yasuhara, A.; Tanaka, Y.; Hengel, M.; Shibamoto, T. Gas chromatographic investigation of acrylamide formation in browning model systems. J. Agric. Food Chem. 2003, 51, 3999–4003. [Google Scholar] [CrossRef]
- Kotsiou, K.; Tasioula-Margari, M.; Kukurova, K.; Ciesarova, Z. Impact of oregano and virgin olive oil phenolic compounds on acrylamide content in a model system and fresh potatoes. Food Chem. 2010, 123, 1149–1155. [Google Scholar] [CrossRef]
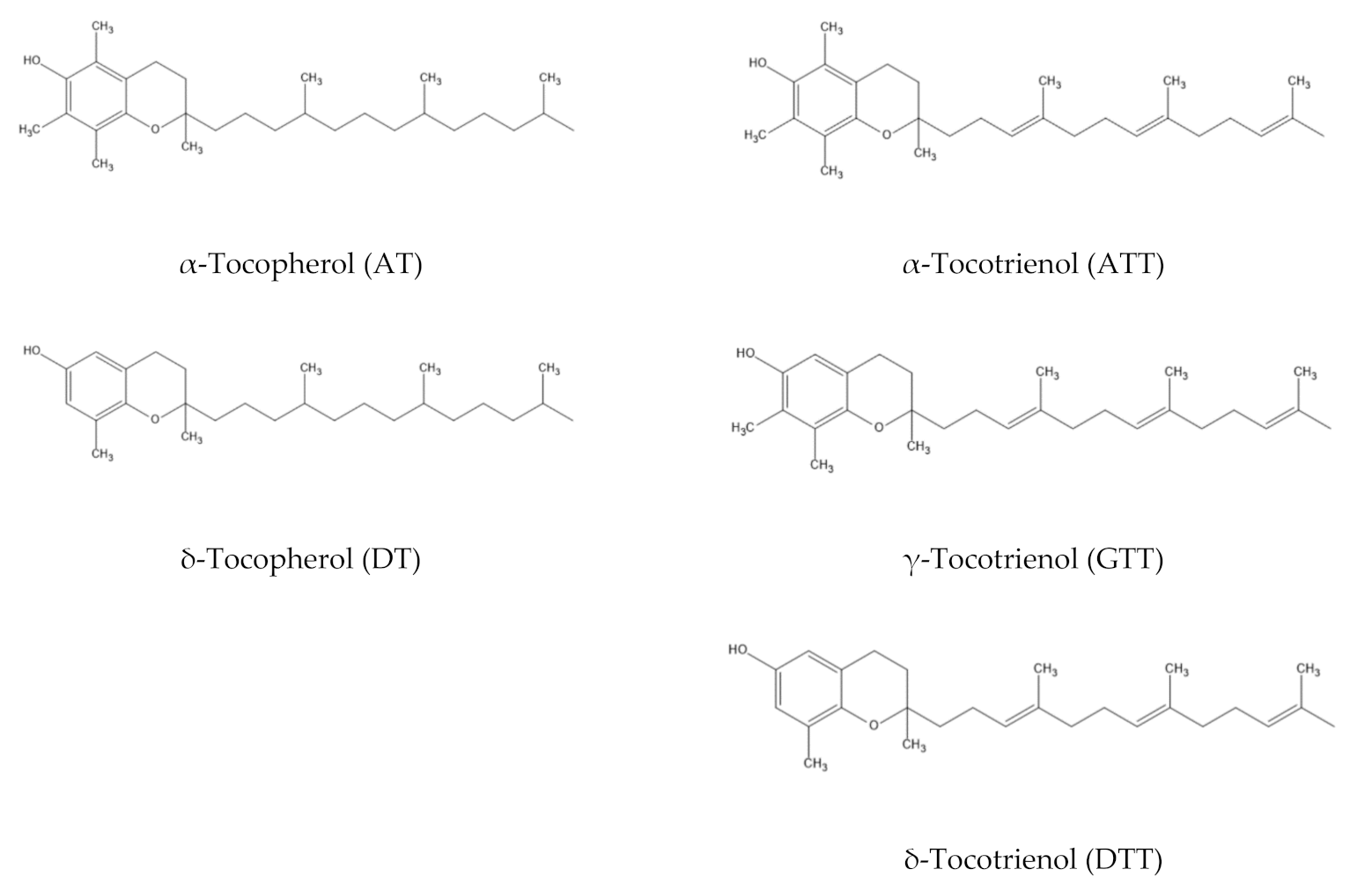
- Inoue, T.; Honma, S.; Otsuka, Y.; Nakane, T.; Takano, A.; Gamo, Y.; Hashidume, F.; Yuasa, H.; Nakanishi, Y.; Hirahara, Y. Distribution of Eight Vitamin E Homologs Found in 81 Plants Using LC-MS. J. Nutr. Food Sci. 2017, 7. [Google Scholar] [CrossRef]
- Hedegaard, R.V.; Granby, K.; Frandsen, H.; Thygesen, J.; Skibsted, L.H. Acrylamide in bread. Effect of prooxidants and antioxidants. Eur. Food Res. Technol. 2008, 227, 519–525. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, X.; Zhao, S.; Zhang, Y. Antioxidant-capacity-based models for the prediction of acrylamide reduction by flavonoids. Food Chem. 2015, 168, 90–99. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Ke, J.; Corke, H. Evaluation of the effect of plant extracts and phenolic compounds on reduction of acrylamide in an asparagine/glucose model system by RP-HPLC-DAD. J. Sci. Food Agric. 2009, 89, 1674–1681. [Google Scholar] [CrossRef]
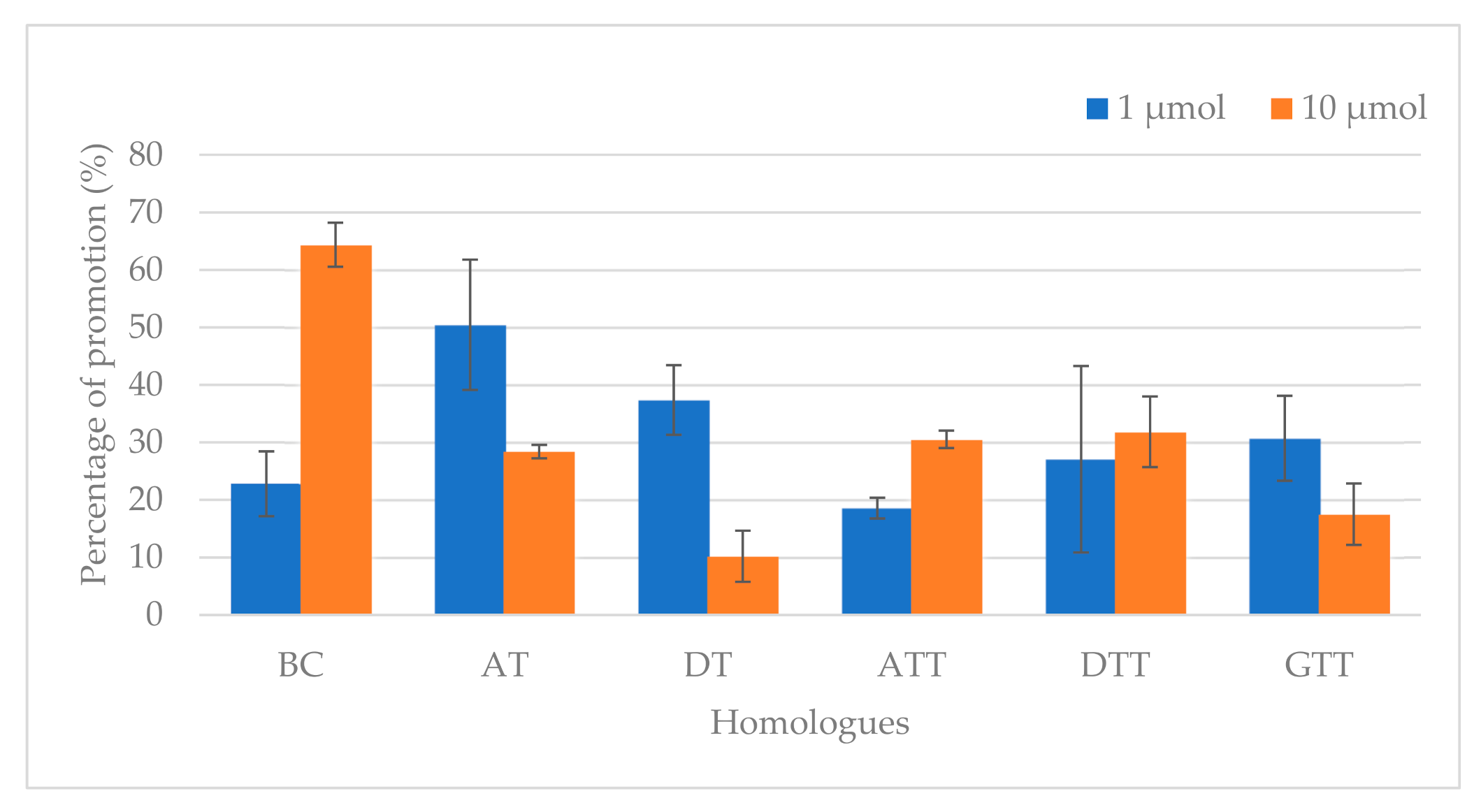
| Acrylamide Concentration (µg/kg) | ||
|---|---|---|
| Control 1 | 2250 ± 82 C [0 ppm] | 2250 ± 82 E [0 ppm] |
| Homologue Levels | 1 µmol | 10 µmol |
| BC | 2763 ± 95 BCb [537 2 ppm] | 3698 ± 73 Aa [5370 ppm] |
| AT | 3385 ± 42 Aa [431 ppm] | 2889 ± 45 BCb [4310 ppm] |
| DT | 3091 ± 93 ABa [403 ppm] | 2480 ± 81 DEb [4030 ppm] |
| ATT | 2668 ± 37 BCa [425 ppm] | 2937 ± 37 BCa [4250 ppm] |
| DTT | 2859 ± 47 BCa [397 ppm] | 2967 ± 21 Ba [3970 ppm] |
| GTT | 2941 ± 69 ABa [411 ppm] | 2644 ± 98 CDb [4110 ppm] |
| Homologue | Beta | Intercept | 1R2 | p-Value |
|---|---|---|---|---|
| BC | 91.7 | 2264 | 0.359 | 0.155 |
| AT | −83.0 | 3468 | 0.652 | p ≤ 0.05 |
| DT | −71.6 | 3162 | 0.930 | p ≤ 0.05 |
| ATT | 47.8 | 2460 | 0.584 | 0.133 |
| DTT | 40.6 | 2560 | 0.857 | 0.074 |
| GTT | −33.0 | 2974 | 0.626 | 0.061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuek, S.L.; Tarmizi, A.H.A.; Abd Razak, R.A.; Jinap, S.; Sanny, M. Vitamin A and E Homologues Impacting the Fate of Acrylamide in Equimolar Asparagine-Glucose Model System. Antioxidants 2021, 10, 993. https://doi.org/10.3390/antiox10070993
Kuek SL, Tarmizi AHA, Abd Razak RA, Jinap S, Sanny M. Vitamin A and E Homologues Impacting the Fate of Acrylamide in Equimolar Asparagine-Glucose Model System. Antioxidants. 2021; 10(7):993. https://doi.org/10.3390/antiox10070993
Chicago/Turabian StyleKuek, Su Lee, Azmil Haizam Ahmad Tarmizi, Raznim Arni Abd Razak, Selamat Jinap, and Maimunah Sanny. 2021. "Vitamin A and E Homologues Impacting the Fate of Acrylamide in Equimolar Asparagine-Glucose Model System" Antioxidants 10, no. 7: 993. https://doi.org/10.3390/antiox10070993
APA StyleKuek, S. L., Tarmizi, A. H. A., Abd Razak, R. A., Jinap, S., & Sanny, M. (2021). Vitamin A and E Homologues Impacting the Fate of Acrylamide in Equimolar Asparagine-Glucose Model System. Antioxidants, 10(7), 993. https://doi.org/10.3390/antiox10070993






