High-Intensity Interval Training and Moderate-Intensity Continuous Training Attenuate Oxidative Damage and Promote Myokine Response in the Skeletal Muscle of ApoE KO Mice on High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Determination of the Maximal Running Speed on Treadmill
2.3. Training Protocols
2.4. Assessment of Endurance Exercise Performance
2.5. Plasma Lipid Profiles
2.6. Real-time Quantitative PCR Analysis
2.7. Western Blotting
2.8. Reactive Oxygen Species (ROS) Generation
2.9. Glutathione Redox State and Protein Carbonyl Content of the Skeletal Muscle
2.10. Plasma Irisin and Muscle Musclin Concentration
2.11. Statistical Analysis
3. Results
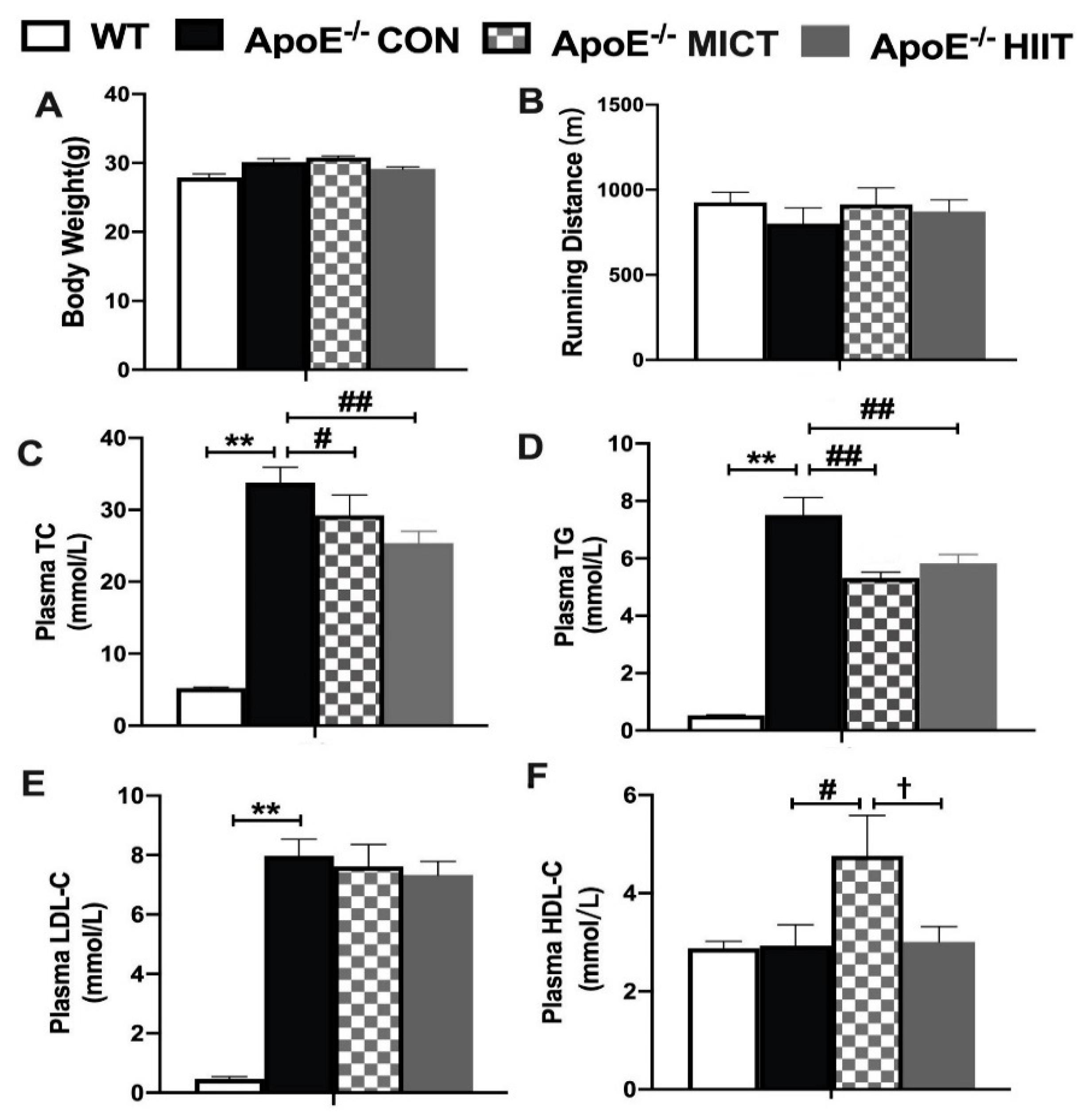
3.1. Body Weight, Running Distance, and Plasma Lipid Profiles
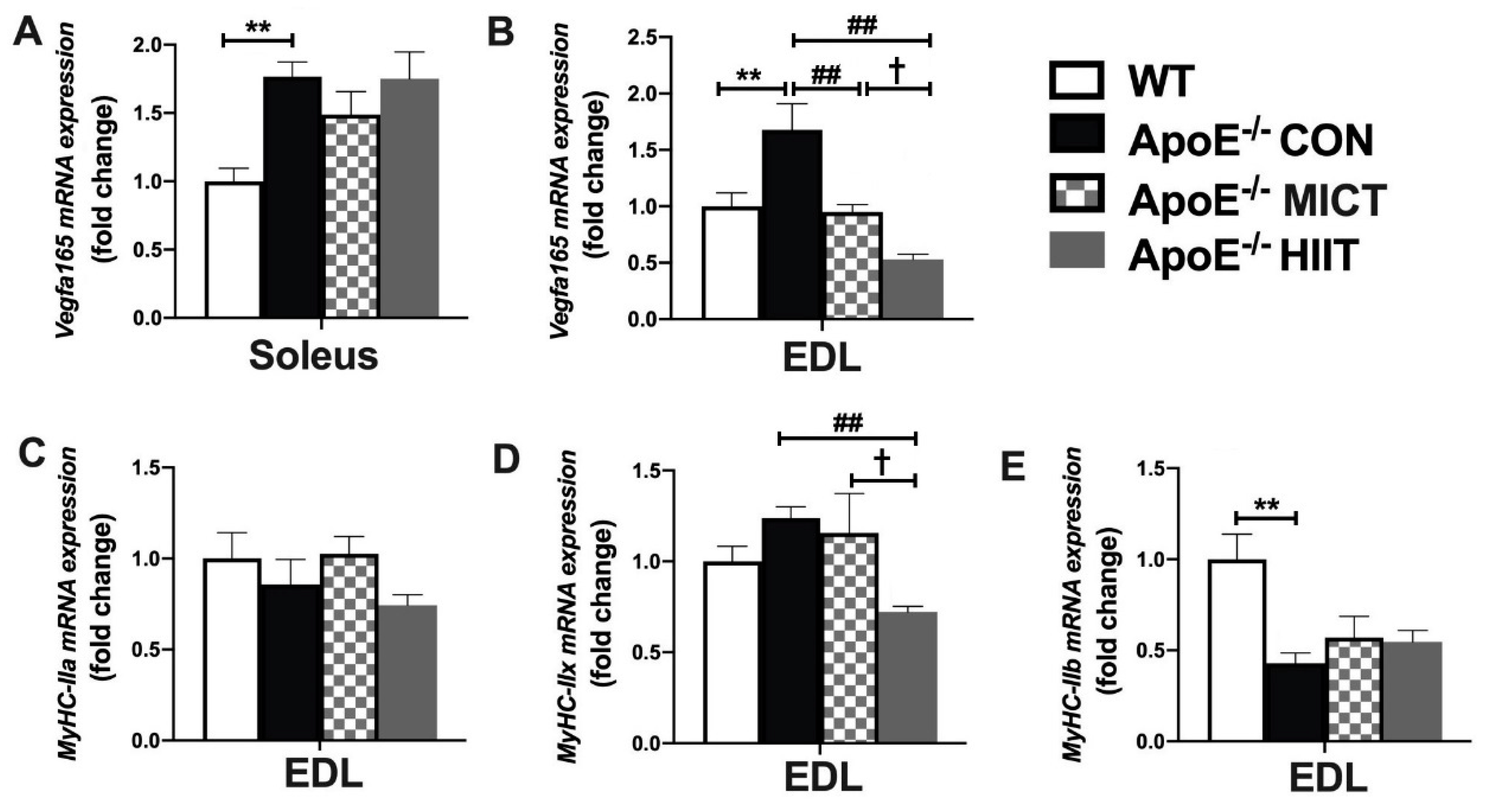
3.2. The mRNA Expression of Vegfa165, MyHC-IIa, MyHC-IIx, and MyHC-IIb in Soleus or EDL
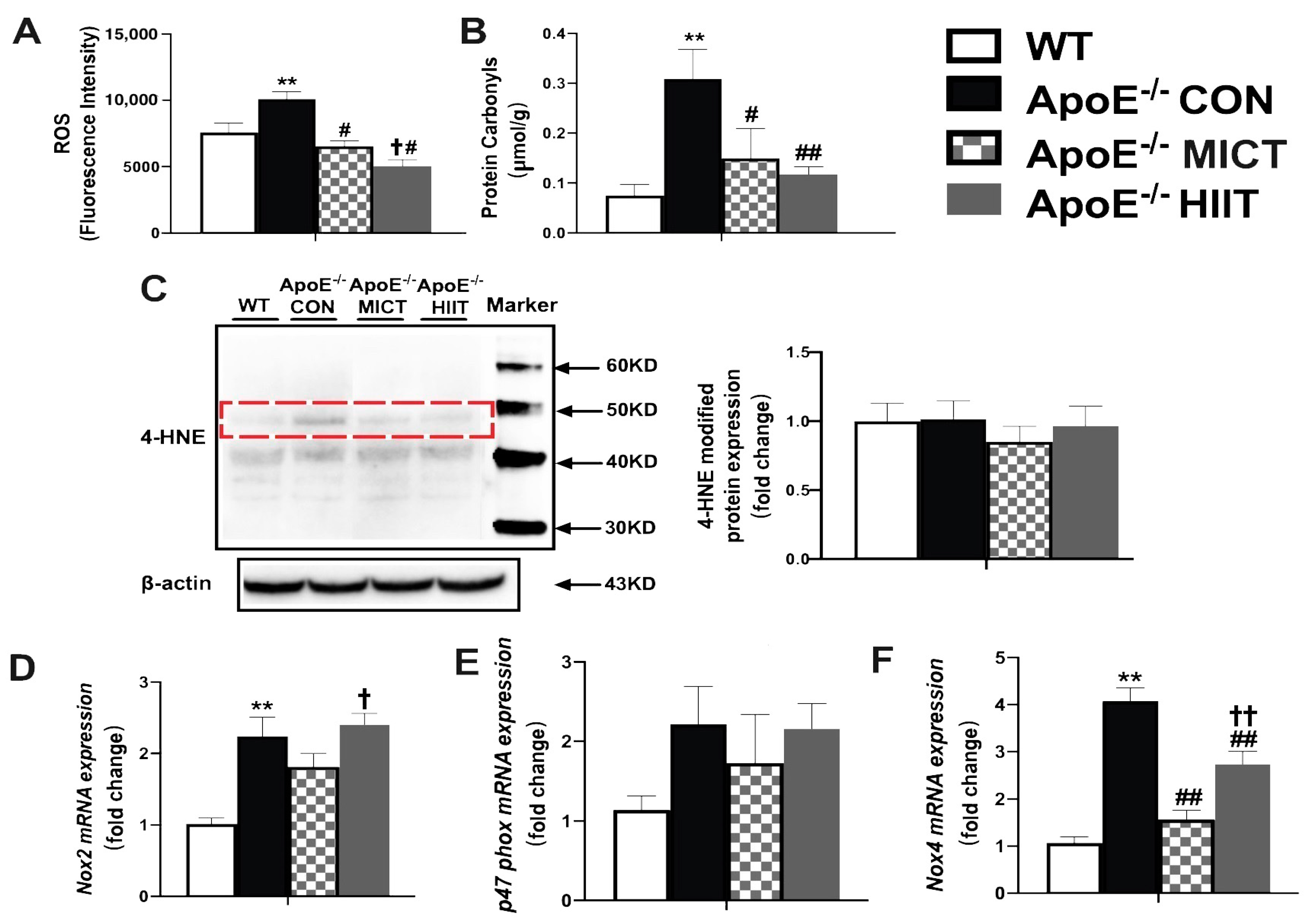
3.3. Muscle ROS, Protein Carbonyl, 4-HNE Modified Proteins, and the mRNA Expression Levels of Nox2, p47phox and Nox4
3.4. The mRNA Expression of Genes Involved in the Production of GSH, GSH, GSSG Levels, and GSH/GSSG Ratio
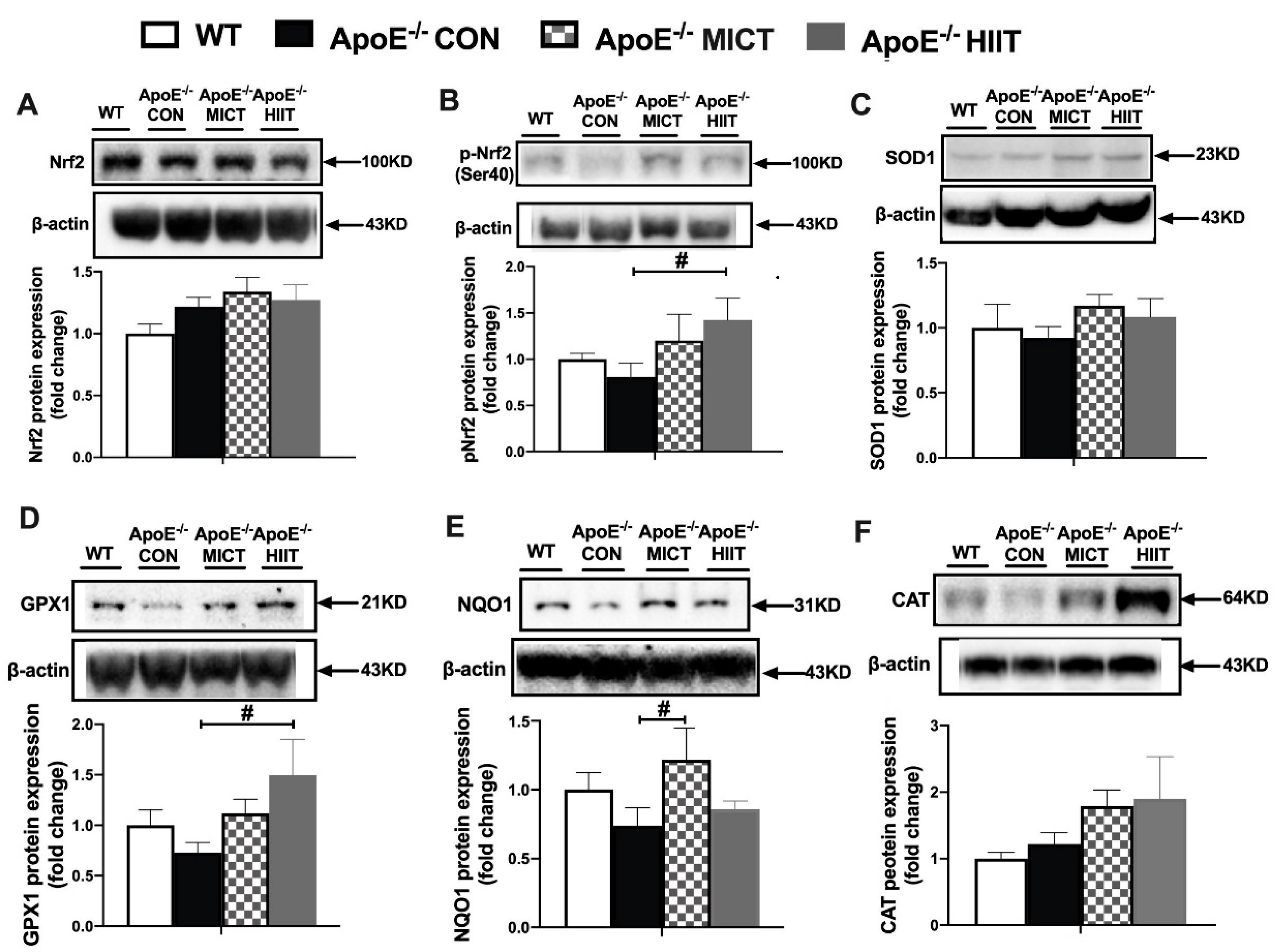
3.5. The Protein Expression of Nrf2, p-Nrf2 (ser40), and Antioxidants
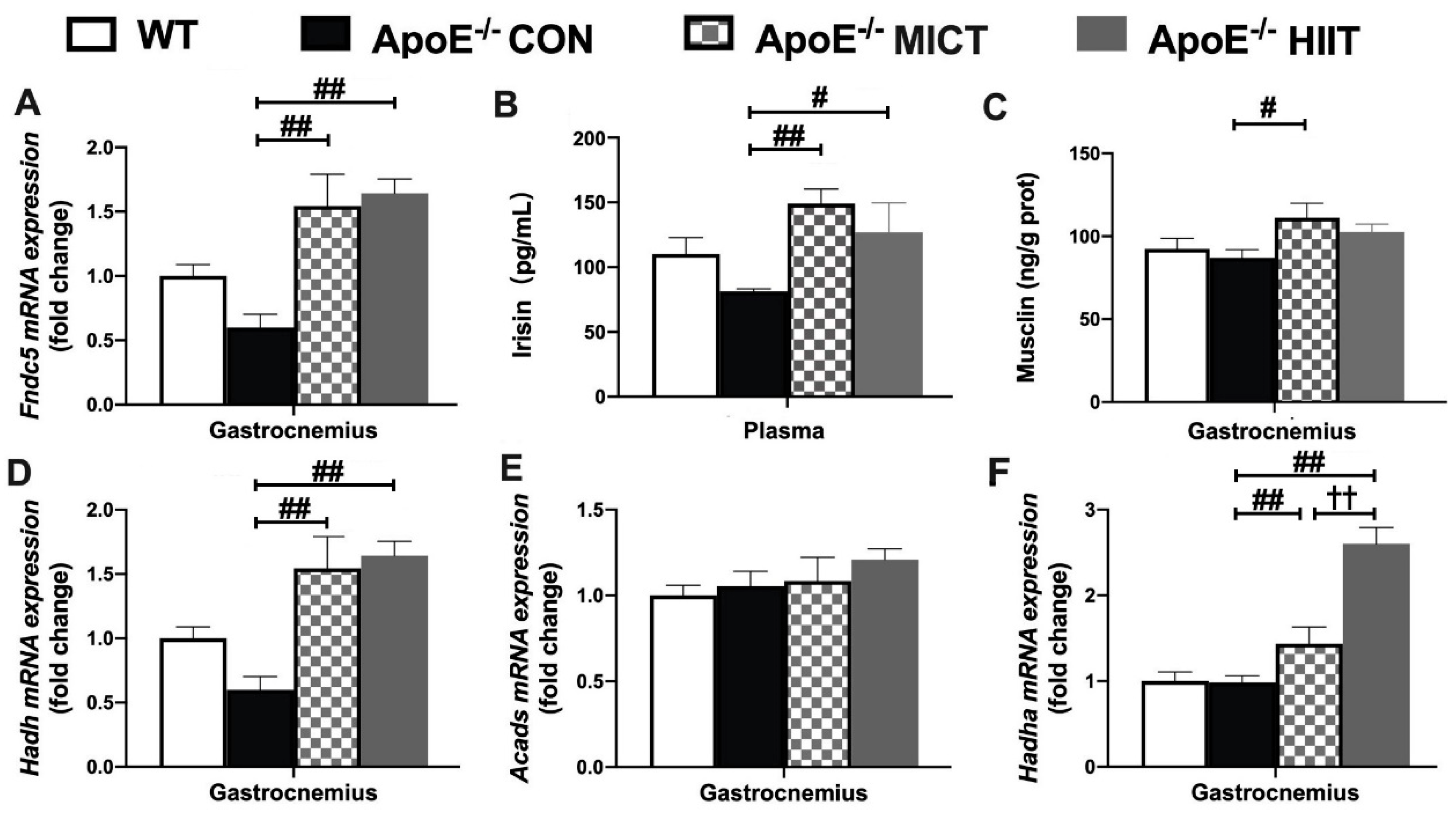
3.6. The mRNA Expression of Fndc5, Hadh, Acads, and Hadha in Gastrocnemius, Plasma Irisin Level, as Well as Muscle Musclin Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aggarwal, S.; Loomba, R.S.; Arora, R. Preventive aspects in peripheral artery disease. Ther. Adv. Cardiovasc. Dis. 2012, 6, 53–70. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [Green Version]
- Weiss, D.J.; Casale, G.P.; Koutakis, P.; Nella, A.A.; Swanson, S.A.; Zhu, Z.; Miserlis, D.; Johanning, J.M.; Pipinos, I.I. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J. Transl. Med. 2013, 11, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pipinos, I.I.; Judge, A.R.; Selsby, J.T.; Zhu, Z.; Swanson, S.A.; Nella, A.A.; Dodd, S.L. The myopathy of peripheral arterial occlusive disease: Part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc. Endovasc. Surg. 2007, 41, 481–489. [Google Scholar] [CrossRef]
- Vasquez, E.C.; Peotta, V.A.; Gava, A.L.; Pereira, T.M.; Meyrelles, S.S. Cardiac and vascular phenotypes in the apolipoprotein E-deficient mouse. J. Biomed. Sci. 2012, 19, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfyri, P.; Matsakas, A. Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: Emphasis on apolipoprotein E deficiency and peripheral arterial disease. J. Biomed. Sci. 2017, 24, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef] [Green Version]
- Pellegrin, M.; Bouzourène, K.; Aubert, J.F.; Bielmann, C.; Gruetter, R.; Rosenblatt-Velin, N.; Poitry-Yamate, C.; Mazzolai, L. Impact of aerobic exercise type on blood flow, muscle energy metabolism, and mitochondrial biogenesis in experimental lower extremity artery disease. Sci. Rep. 2020, 10, 14048. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Groussard, C.; Maillard, F.; Vazeille, E.; Barnich, N.; Sirvent, P.; Otero, Y.F.; Combaret, L.; Madeuf, E.; Sourdrille, A.; Delcros, G.; et al. Tissue-Specific Oxidative Stress Modulation by Exercise: A Comparison between MICT and HIIT in an Obese Rat Model. Oxid. Med. Cell. Longev. 2019, 2019, 1965364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisløff, U.; Coombes, J.S.; Rognmo, Ø. CrossTalk proposal: High intensity interval training does have a role in risk reduction or treatment of disease. J. Physiol. 2015, 593, 5215–5217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mury, P.; Chirico, E.N.; Mura, M.; Millon, A.; Canet-Soulas, E.; Pialoux, V. Oxidative Stress and Inflammation, Key Targets of Atherosclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sports Med. 2018, 48, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Daiber, A.; Dopheide, J.F.; Münzel, T.; Espinola-Klein, C. Peripheral artery disease, redox signaling, oxidative stress—Basic and clinical aspects. Redox Biol. 2017, 12, 787–797. [Google Scholar] [CrossRef]
- Pipinos, I.I.; Swanson, S.A.; Zhu, Z.; Nella, A.A.; Weiss, D.J.; Gutti, T.L.; McComb, R.D.; Baxter, B.T.; Lynch, T.G.; Casale, G.P. Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R290–R296. [Google Scholar] [CrossRef] [PubMed]
- Pipinos, I.I.; Judge, A.R.; Zhu, Z.; Selsby, J.T.; Swanson, S.A.; Johanning, J.M.; Baxter, B.T.; Lynch, T.G.; Dodd, S.L. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic. Biol. Med. 2006, 41, 262–269. [Google Scholar] [CrossRef]
- Hart, C.R.; Layec, G.; Trinity, J.D.; Kwon, O.S.; Zhao, J.; Reese, V.R.; Gifford, J.R.; Richardson, R.S. Increased skeletal muscle mitochondrial free radical production in peripheral arterial disease despite preserved mitochondrial respiratory capacity. Exp. Physiol. 2018, 103, 838–850. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef]
- Hidalgo, C.; Sánchez, G.; Barrientos, G.; Aracena-Parks, P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J. Biol. Chem. 2006, 281, 26473–26482. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.H.; Gallin, J.I.; Holland, S.M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 1995, 182, 751–758. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfyri, P.P.; Yuldasheva, N.Y.; Tzimou, A.; Giallourou, N.; Crispi, V.; Aburima, A.; Beltran-Alvarez, P.; Patel, K.; Mougios, V.; Swann, J.R.; et al. Attenuation of oxidative stress-induced lesions in skeletal muscle in a mouse model of obesity-independent hyperlipidaemia and atherosclerosis through the inhibition of Nox2 activity. Free Radic. Biol. Med. 2018, 129, 504–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K. The Physiology of Optimizing Health with a Focus on Exercise as Medicine. Annu. Rev. Physiol. 2019, 81, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Lavier, J.; Beaumann, M.; Ménetrey, S.; Mazzolai, L.; Peyter, A.C.; Pellegrin, M.; Millet, G.P. Supramaximal Intensity Hypoxic Exercise and Vascular Function Assessment in Mice. J. Vis. Exp. 2019, 145, e58708. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, S.; Yan, L.; Wei, H.; Wang, J.; Yu, S.; Kong, A.T.; Zhang, Y. Hypoxia preconditioning promotes endurance exercise capacity of mice by activating skeletal muscle Nrf2. J. Appl. Physiol. 2019, 127, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cai, Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-γ and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 2019, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartelt, A.; Orlando, P.; Mele, C.; Ligresti, A.; Toedter, K.; Scheja, L.; Heeren, J.; Di Marzo, V. Altered endocannabinoid signalling after a high-fat diet in Apoe(-/-) mice: Relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia 2011, 54, 2900–2910. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Perrard, X.D.; Perrard, J.L.; Mukherjee, A.; Rosales, C.; Chen, Y.; Smith, C.W.; Pownall, H.J.; Ballantyne, C.M.; Wu, H. ApoE and the role of very low density lipoproteins in adipose tissue inflammation. Atherosclerosis 2012, 223, 342–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Kim, K.; Park, E.; Lee, J.; Markofski, M.M.; Marrelli, S.P.; Park, Y. Exercise ameliorates endoplasmic reticulum stress-mediated vascular dysfunction in mesenteric arteries in atherosclerosis. Sci. Rep. 2018, 8, 7938. [Google Scholar] [CrossRef]
- Powers, S.K.; Criswell, D.; Lawler, J.; Ji, L.L.; Martin, D.; Herb, R.A.; Dudley, G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol. 1994, 266, R375–R380. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Goto, M.; Terada, S.; Kato, M.; Katoh, M.; Yokozeki, T.; Tabata, I.; Shimokawa, T. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem. Biophys. Res. Commun. 2000, 274, 350–354. [Google Scholar] [CrossRef]
- Miura, S.; Kawanaka, K.; Kai, Y.; Tamura, M.; Goto, M.; Shiuchi, T.; Minokoshi, Y.; Ezaki, O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 2007, 148, 3441–3448. [Google Scholar] [CrossRef] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Miura, K.; Arima, H.; Fujiyoshi, A.; Kadota, A.; Kadowaki, S.; Zaid, M.; Miyagawa, N.; Satoh, A.; Kunimura, A.; et al. Relationship of serum irisin levels to prevalence and progression of coronary artery calcification: A prospective, population-based study. Int. J. Cardiol. 2018, 267, 177–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Q.; Zhou, Z.; Song, H.; Zhang, Y.; Wu, F.; Jiang, M.; Wang, F.; Zhang, W.; Li, L.; et al. Protective Effect of Irisin on Atherosclerosis via Suppressing Oxidized Low Density Lipoprotein Induced Vascular Inflammation and Endothelial Dysfunction. PLoS ONE 2016, 11, e0158038. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M.; et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, H.; Matsuda, M.; Yamada, Y.; Kawai, K.; Suzuki, E.; Makishima, M.; Kitamura, T.; Shimomura, I. Musclin, a novel skeletal muscle-derived secretory factor. J. Biol. Chem. 2004, 279, 19391–19395. [Google Scholar] [CrossRef] [Green Version]
- Bastu, E.; Zeybek, U.; Gurel Gurevin, E.; Yüksel Ozgor, B.; Celik, F.; Okumus, N.; Demiral, I.; Dural, O.; Celik, C.; Bulut, H.; et al. Effects of Irisin and Exercise on Metabolic Parameters and Reproductive Hormone Levels in High-Fat Diet-Induced Obese Female Mice. Reprod. Sci. 2018, 25, 281–291. [Google Scholar] [CrossRef]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jähn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. β-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef] [Green Version]

| Gene Name | Gene ID | Forward Primer | Reverse Primer |
|---|---|---|---|
| Gclm | 14630 | 5′-AGGAGCTTCGGGACTGTATCC-3′ | 5′-GGGACATGGTGCATTCCAAAA-3′ |
| Gss | 14854 | 5′-CAAAGCAGGCCATAGACAGGG-3′ | 5′-AAAAGCGTGAATGGGGCATAC-3′ |
| Gclc | 14629 | 5′-GGGGTGACGAGGTGGAGTA-3′ | 5′-GTTGGGGTTTGTCCTCTCCC-3′ |
| Gsr | 14782 | 5′-CACGGCTATGCAACATTCGC-3′ | 5′-GTGTGGAGCGGTAAACTTTTTC-3′ |
| Nox2 | 13058 | 5′-TGAATGCCAGAGTCGGGATT-3′ | 5′-CGAGTCACGGCCACATACA-3′ |
| p47phox | 17969 | 5′-ACACCTTCATTCGCCATATTGC-3′ | 5′-TCGGTGAATTTTCTGTAGACCAC-3′ |
| Nox4 | 50490 | 5′-TCCATCAAGCCAAGATTCTGAG-3′ | 5′-GGTTTCCAGTCATCCAGTAGAG-3′ |
| Fndc5 | 384061 | 5′-TTGCCATCTCTCAGCAGAAGA-3′ | 5′-GGCCTGCACATGGACGATA-3′ |
| Acads | 11409 | 5′-GACTGGCGACGGTTACACA-3′ | 5′-GGCAAAGTCACGGCATGTC-3′ |
| Hadha | 97212 | 5′-TGCATTTGCCGCAGCTTTAC-3′ | 5′-GTTGGCCCAGATTTCGTTCA-3′ |
| Hadh | 15107 | 5′-TGCATTTGCCGCAGCTTTAC-3′ | 5′-GTTGGCCCAGATTTCGTTCA-3′ |
| Vegfa165 | 22339 | 5′-TGCAGGCTGCTGTAACGATG-3′ | 5′-GAACAAGGCTCACAGTGATTTTCT-3′ |
| MHC-IIa | 17886 | 5′-CAGCTGCACCTTCTCGTTTG-3′ | 5′-CCCGAAAACGGCCATCT-3′ |
| MHC-IIx | 17879 | 5′-GGACCCACGGTCGAAGTTG-3′ | 5′-CCCGAAAACGGCCATCT-3′ |
| MHC-IIb | 77579 | 5′-CAATCAGGAACCTTCGGAACAC-3′ | 5′-GTCCTGGCCTCTGAGAGCAT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lavier, J.; Hua, W.; Wang, Y.; Gong, L.; Wei, H.; Wang, J.; Pellegrin, M.; Millet, G.P.; Zhang, Y. High-Intensity Interval Training and Moderate-Intensity Continuous Training Attenuate Oxidative Damage and Promote Myokine Response in the Skeletal Muscle of ApoE KO Mice on High-Fat Diet. Antioxidants 2021, 10, 992. https://doi.org/10.3390/antiox10070992
Wang L, Lavier J, Hua W, Wang Y, Gong L, Wei H, Wang J, Pellegrin M, Millet GP, Zhang Y. High-Intensity Interval Training and Moderate-Intensity Continuous Training Attenuate Oxidative Damage and Promote Myokine Response in the Skeletal Muscle of ApoE KO Mice on High-Fat Diet. Antioxidants. 2021; 10(7):992. https://doi.org/10.3390/antiox10070992
Chicago/Turabian StyleWang, Linjia, Jessica Lavier, Weicheng Hua, Yangwenjie Wang, Lijing Gong, Hao Wei, Jianxiong Wang, Maxime Pellegrin, Grégoire P. Millet, and Ying Zhang. 2021. "High-Intensity Interval Training and Moderate-Intensity Continuous Training Attenuate Oxidative Damage and Promote Myokine Response in the Skeletal Muscle of ApoE KO Mice on High-Fat Diet" Antioxidants 10, no. 7: 992. https://doi.org/10.3390/antiox10070992
APA StyleWang, L., Lavier, J., Hua, W., Wang, Y., Gong, L., Wei, H., Wang, J., Pellegrin, M., Millet, G. P., & Zhang, Y. (2021). High-Intensity Interval Training and Moderate-Intensity Continuous Training Attenuate Oxidative Damage and Promote Myokine Response in the Skeletal Muscle of ApoE KO Mice on High-Fat Diet. Antioxidants, 10(7), 992. https://doi.org/10.3390/antiox10070992








