Hypoxia Tolerance Declines with Age in the Absence of Methionine Sulfoxide Reductase (MSR) in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Stocks
2.2. Hypoxia Treatment
2.3. Monitoring Fly Movement
2.4. Statistical Analyses
3. Results
3.1. Characterization and Age-Grouping of Wild-Type and MSR-Deficient Strains
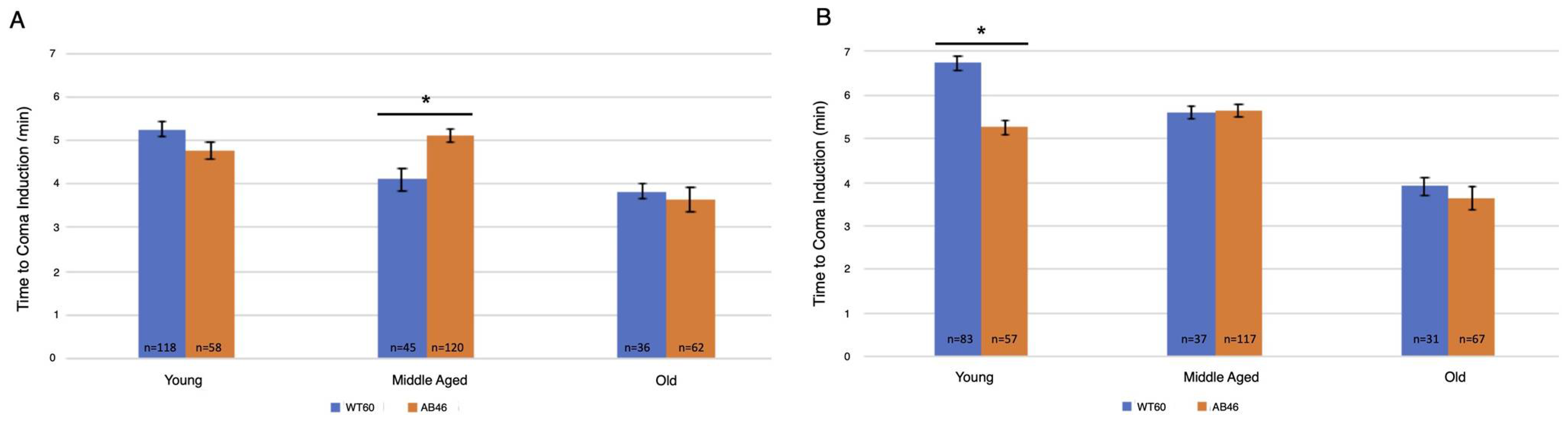
3.2. Rapid Induction of the Spreading Depression Coma
3.3. Moderate and Slow Induction of Hypoxic Coma
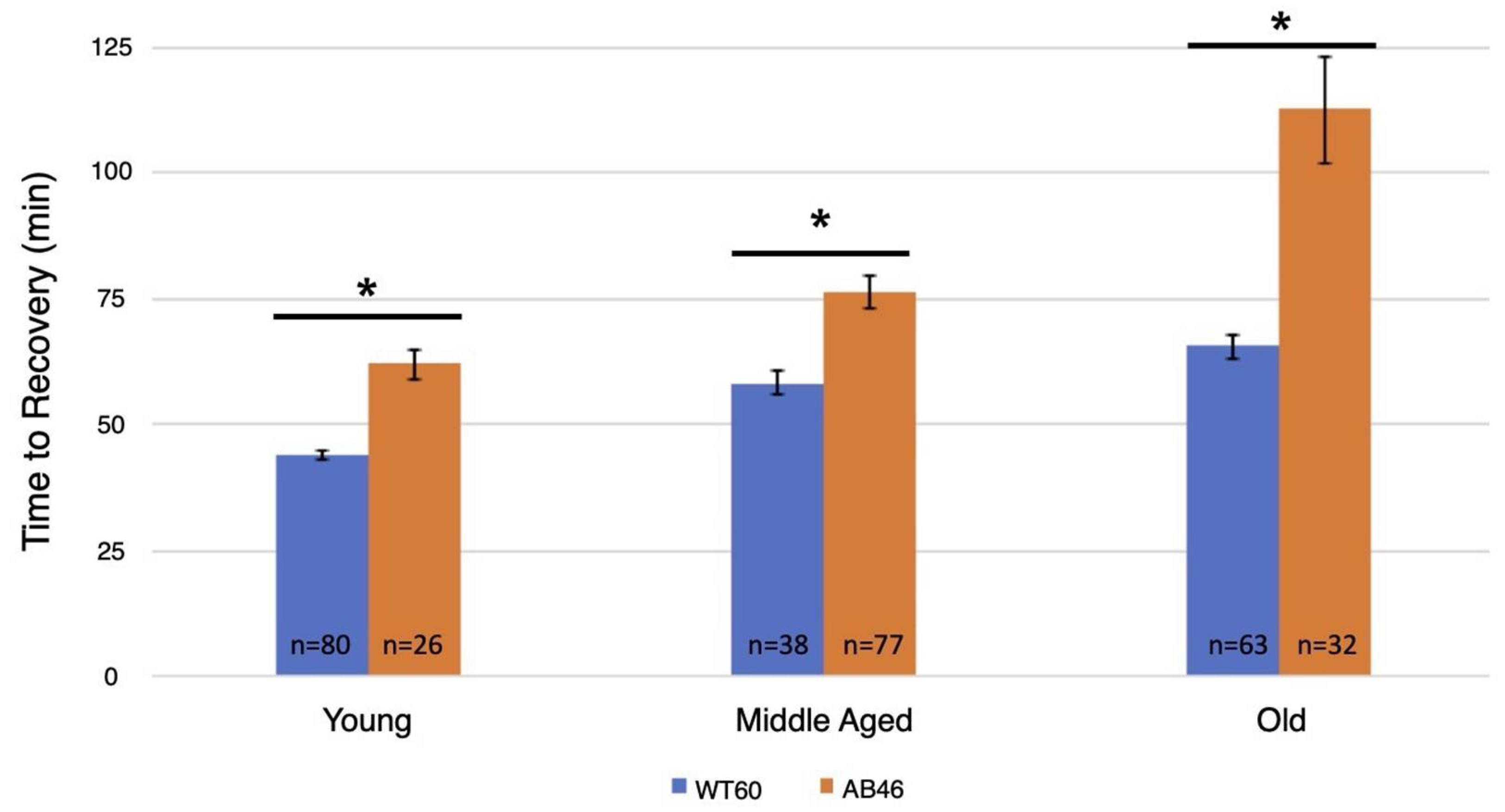
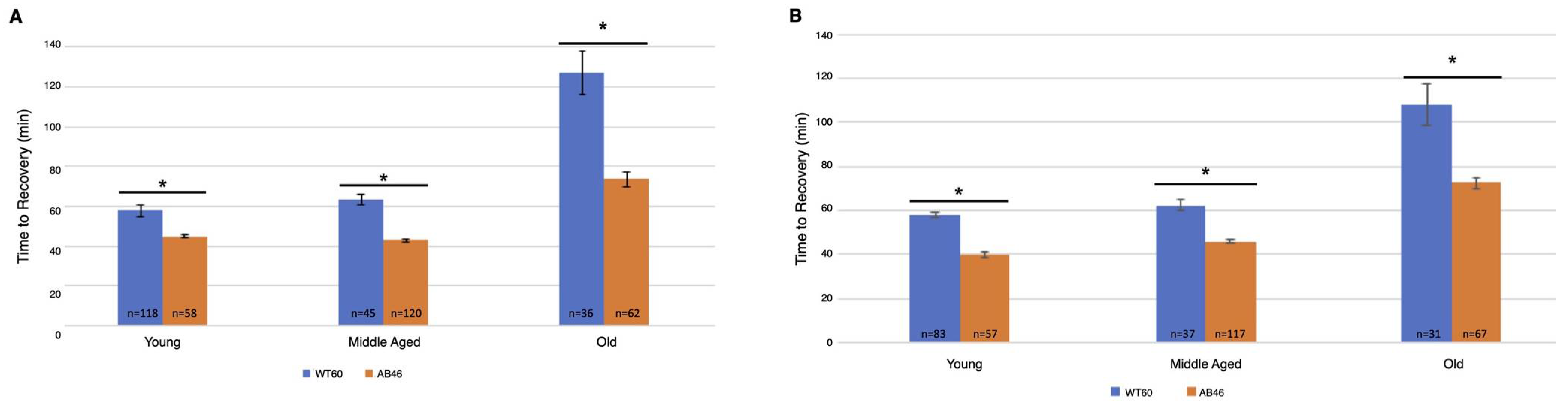
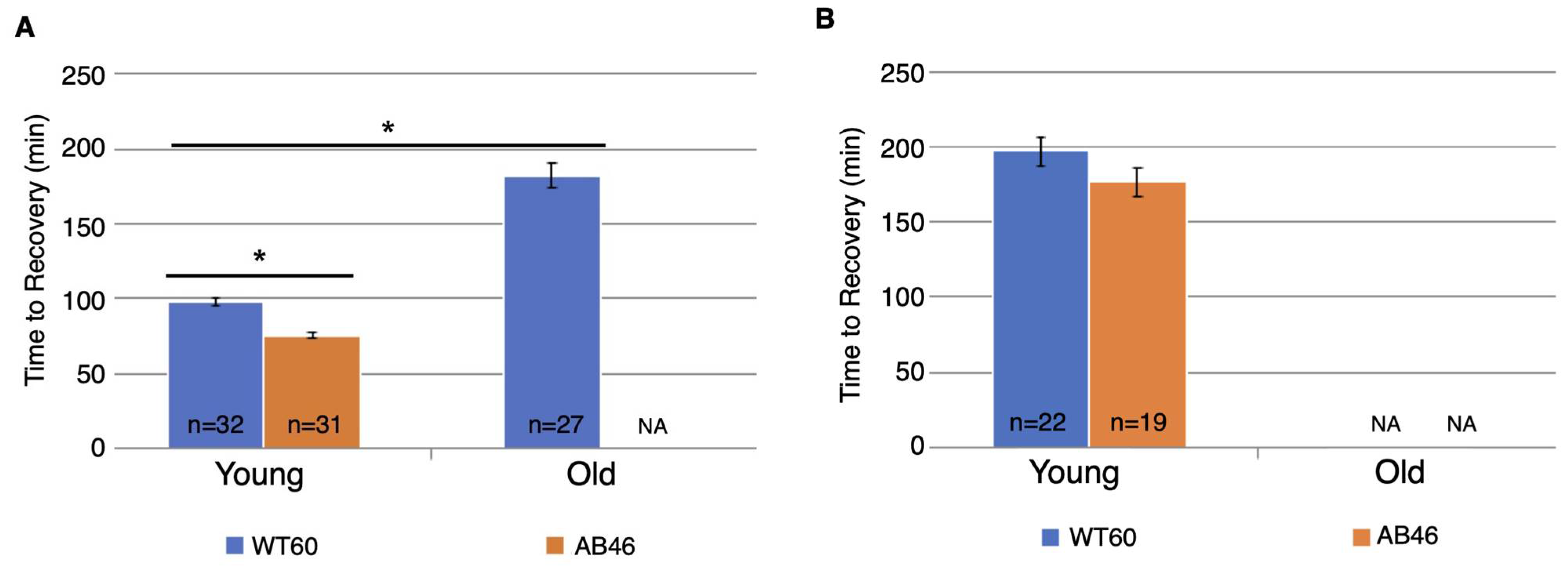
3.4. Recovery Following Moderate and Slow Induction of Hypoxia
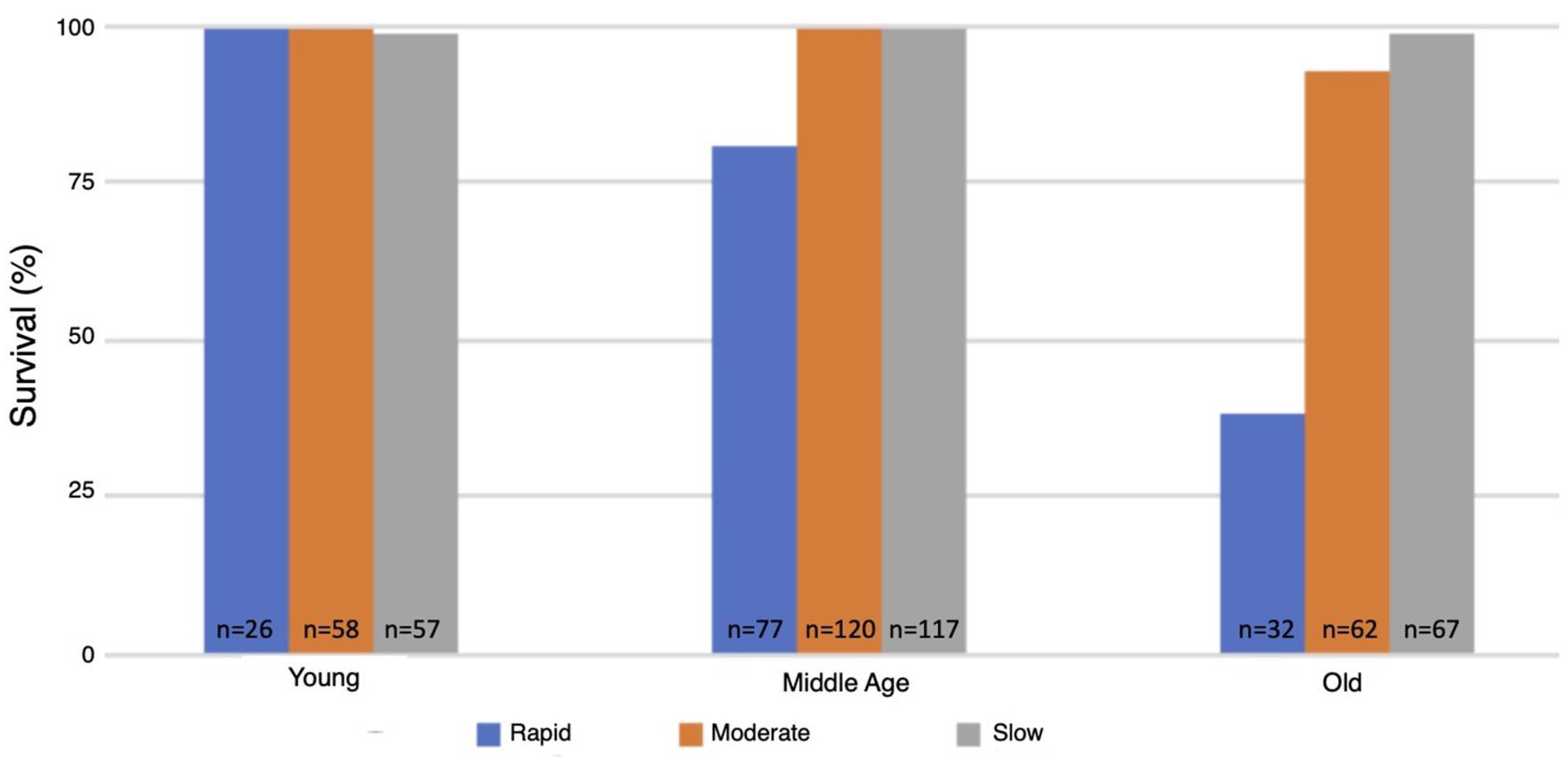
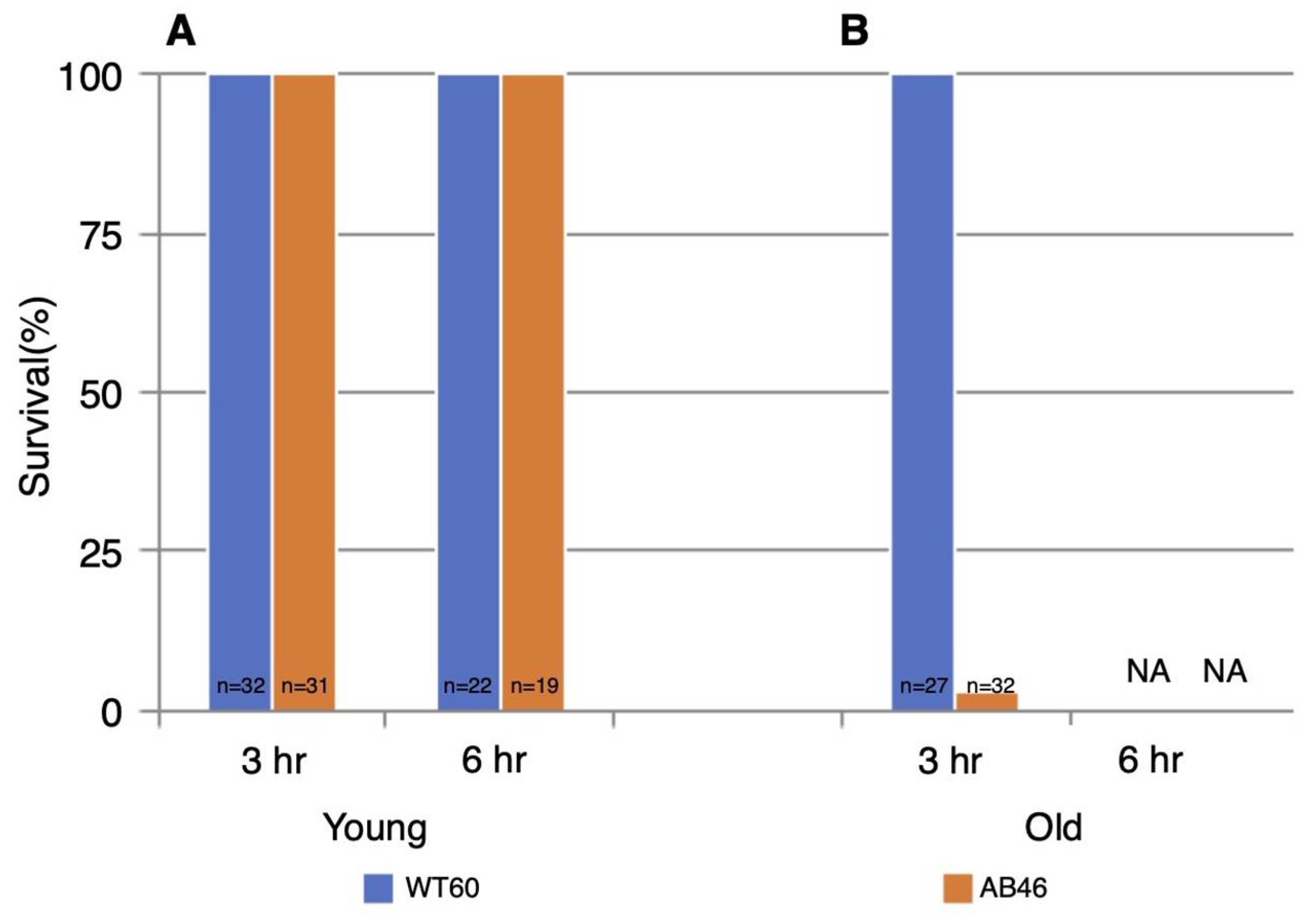
3.5. Survival Following One Hour of Hypoxia
3.6. Effects of Prolonged Hypoxia on Recovery and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, G.E.; Lutz, P.L. Anoxia tolerant brains. J. Cereb. Blood Flow Metab. 2004, 24, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spong, K.E.; Rodríguez, E.C.; Robertson, R.M. Spreading depolarization in the brain of Drosophila is induced by inhibition of the Na+/K+-ATPase and mitigated by a decrease in activity of protein kinase G. J. Neurophysiol. 2016, 116, 1152–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achilli, C.; Ciana, A.; Minetti, G. The discovery of methionine sulfoxide reductase enzymes: An historical account and future perspectives. BioFactors 2015, 41, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Vogt, W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.W.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Aledo, J.C. Methionine in proteins: The Cinderella of the proteinogenic amino acids. Protein Sci. 2019, 28, 1785–1796. [Google Scholar] [CrossRef]
- Morrissey, J.J.; Cupp, L.E.; Weissbach, H.; Brot, N. Synthesis of ribosomal proteins L7L12 in relaxed and stringent strains of Escherichia coli. J. Biol. Chem. 1976, 251, 5516–5521. [Google Scholar] [CrossRef]
- Brot, N.; Weissbach, L.; Werth, J.; Weissbach, H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc. Natl. Acad. Sci. USA 1981, 78, 2155–2158. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Marty, L.; Siala, W.; Schwarzländer, M.; Fricker, M.D.; Wirtz, M.; Sweetlove, L.J.; Meyer, Y.; Meyer, A.J.; Reichheld, J.-P.; Hell, R. The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 9109. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Chew, E.-H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef] [Green Version]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [Green Version]
- Stadtman, E.R.; Van Remmen, H.; Richardson, A.; Wehr, N.B.; Levine, R.L. Methionine oxidation and aging. Biochim. Biophys. Acta 2005, 1703, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Bruce, L.; Singkornrat, D.; Wilson, K.; Hausman, W.; Robbins, K.; Huang, L.; Foss, K.; Binninger, D. In Vivo Effects of Methionine Sulfoxide Reductase Deficiency in Drosophila melanogaster. Antioxidants 2018, 7, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weymouth, T.R. Transactions of the American Society of Mechanical Engineers. Am. Soc. Mech. Eng. 1912, 55, 197. [Google Scholar]
- Haddad, G.G. Tolerance to low O2: Lessons from invertebrate genetic models. Exp. Physiol. 2006, 91, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, J.A.; O’Farrell, P.H. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 1999, 98, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.W.; Haddad, G.G. Review: Hypoxic and oxidative stress resistance in Drosophila melanogaster. Placenta 2011, 32 (Suppl. 2), S104–S108. [Google Scholar] [CrossRef] [Green Version]
- Perry, G.; Nunomura, A.; Hirai, K.; Zhu, X.; Perez, M.; Avila, J.; Castellani, R.J.; Atwood, C.S.; Aliev, G.; Sayre, L.M.; et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic. Biol. Med. 2002, 33, 1475–1479. [Google Scholar] [CrossRef]
- Robertson, R.M.; Dawson-Scully, K.D.; Andrew, R.D. Neural shutdown under stress: An evolutionary perspective on spreading depolarization. J. Neurophysiol. 2020, 123, 885–895. [Google Scholar] [CrossRef]
- Yermolaieva, O.; Xu, R.; Schinstock, C.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Hoshi, T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc. Natl. Acad. Sci. USA 2004, 101, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Weissbach, H.; Etienne, F.; Hoshi, T.; Heinemann, S.H.; Lowther, W.T.; Matthews, B.; St John, G.; Nathan, C.; Brot, N. Peptide methionine sulfoxide reductase: Structure, mechanism of action, and biological function. Arch. BioChem. Biophys. 2002, 397, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Vaish, M.; Johansson, T.R.; Baum, K.R.; Ring, R.P.; Singh, S.; Shukla, S.K.; Moskovitz, J. Significance of four methionine sulfoxide reductases in Staphylococcus aureus. PLoS ONE 2015, 10, e0117594. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.; Gasch, A.P.; Rutherford, J.C.; Kim, H.Y.; Gladyshev, V.N. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc. Natl. Acad. Sci. USA 2004, 101, 7999–8004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minniti, A.N.; Cataldo, R.; Trigo, C.; Vasquez, L.; Mujica, P.; Leighton, F.; Inestrosa, N.C.; Aldunate, R. Methionine sulfoxide reductase A expression is regulated by the DAF-16/FOXO pathway in Caenorhabditis elegans. Aging Cell 2009, 8, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Bar-Noy, S.; Williams, W.M.; Requena, J.; Berlett, B.S.; Stadtman, E.R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 12920–12925. [Google Scholar] [CrossRef] [Green Version]
- Salmon, A.B.; Pérez, V.I.; Bokov, A.; Jernigan, A.; Kim, G.; Zhao, H.; Levine, R.L.; Richardson, A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J. 2009, 23, 3601–3608. [Google Scholar] [CrossRef] [Green Version]
- Vijg, J.; Kennedy, B.K. The Essence of Aging. Gerontology 2016, 62, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, H.; Tang, X.D.; Chen, M.-L.; Joiner, M.-L.A.; Sun, G.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Iverson, L.; Wu, C.-F.; et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA 2002, 99, 2748–2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CABREIRO, F.; PICOT, C.R.; FRIGUET, B.; PETROPOULOS, I. Methionine Sulfoxide Reductases. Ann. N. Y. Acad. Sci. 2006, 1067, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Di Lisa, F. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection. J. Mol. Cell Cardiol. 2015, 78, 100–106. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [Green Version]
- Loor, G.; Kondapalli, J.; Iwase, H.; Chandel, N.S.; Waypa, G.B.; Guzy, R.D.; Vanden Hoek, T.L.; Schumacker, P.T. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim. Biophys. Acta 2011, 1813, 1382–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.O.; Zweier, J.L.; Sollott, S.J. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Hochachka, P.W.; Owen, T.G.; Allen, J.F.; Whittow, G.C. Multiple end products of anaerobiosis in diving vertebrates. Comp. Biochem. Physiol. Part B Comp. Biochem. 1975, 50, 17–22. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Mustafa, T. Invertebrate Facultative Anaerobiosis. Science 1972, 178, 1056. [Google Scholar] [CrossRef]
- Pell, V.R.; Spiroski, A.M.; Mulvey, J.; Burger, N.; Costa, A.S.H.; Logan, A.; Gruszczyk, A.V.; Rosa, T.; James, A.M.; Frezza, C.; et al. Ischemic preconditioning protects against cardiac ischemia reperfusion injury without affecting succinate accumulation or oxidation. J. Mol. Cell Cardiol. 2018, 123, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Caplan, S.L.; Milton, S.L.; Dawson-Scully, K. A cGMP-dependent protein kinase (PKG) controls synaptic transmission tolerance to acute oxidative stress at the Drosophila larval neuromuscular junction. J. Neurophysiol. 2013, 109, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T. Radical medicine: Treating ageing to cure disease. Nat. Rev. Mol. Cell Biol. 2005, 6, 971–976. [Google Scholar] [CrossRef]
- Rodgers, C.I.; Armstrong, G.A.; Shoemaker, K.L.; LaBrie, J.D.; Moyes, C.D.; Robertson, R.M. Stress preconditioning of spreading depression in the locust CNS. PLoS ONE 2007, 2, e1366. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, E.C.; Robertson, R.M. Protective effect of hypothermia on brain potassium homeostasis during repetitive anoxia in Drosophila melanogaster. J. Exp. Biol. 2012, 215, 4157–4165. [Google Scholar] [CrossRef] [Green Version]
- Van Voorhies, W.A. Metabolic function in Drosophila melanogaster in response to hypoxia and pure oxygen. J. Exp. Biol. 2009, 212, 3132–3141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hylland, P.; Milton, S.; Pek, M.; Nilsson, G.E.; Lutz, P.L. Brain Na+/K+-ATPase activity in two anoxia tolerant vertebrates: Crucian carp and freshwater turtle. Neurosci. Lett. 1997, 235, 89–92. [Google Scholar] [CrossRef]
- Harrison, J.F.; Haddad, G.G. Effects of oxygen on growth and size: Synthesis of molecular, organismal, and evolutionary studies with Drosophila melanogaster. Annu. Rev. Physiol. 2011, 73, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Cudic, P.; Joshi, N.; Sagher, D.; Williams, B.T.; Stawikowski, M.J.; Weissbach, H. Identification of activators of methionine sulfoxide reductases A and B. BioChem. Biophys. Res. Commun. 2016, 469, 863–867. [Google Scholar] [CrossRef] [Green Version]
| Age Group | Wild-Type | MSR-Deficient |
|---|---|---|
| Young | 20–25 Days Old | 5–10 Days Old |
| Middle Age | 40–45 Days Old | 30–35 Days Old |
| Old | 60–65 Days Old | 40–45 Days Old |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suthakaran, N.; Chandran, S.; Iacobelli, M.; Binninger, D. Hypoxia Tolerance Declines with Age in the Absence of Methionine Sulfoxide Reductase (MSR) in Drosophila melanogaster. Antioxidants 2021, 10, 1135. https://doi.org/10.3390/antiox10071135
Suthakaran N, Chandran S, Iacobelli M, Binninger D. Hypoxia Tolerance Declines with Age in the Absence of Methionine Sulfoxide Reductase (MSR) in Drosophila melanogaster. Antioxidants. 2021; 10(7):1135. https://doi.org/10.3390/antiox10071135
Chicago/Turabian StyleSuthakaran, Nirthieca, Sanjana Chandran, Michael Iacobelli, and David Binninger. 2021. "Hypoxia Tolerance Declines with Age in the Absence of Methionine Sulfoxide Reductase (MSR) in Drosophila melanogaster" Antioxidants 10, no. 7: 1135. https://doi.org/10.3390/antiox10071135
APA StyleSuthakaran, N., Chandran, S., Iacobelli, M., & Binninger, D. (2021). Hypoxia Tolerance Declines with Age in the Absence of Methionine Sulfoxide Reductase (MSR) in Drosophila melanogaster. Antioxidants, 10(7), 1135. https://doi.org/10.3390/antiox10071135





