Nox4 Maintains Blood Pressure during Low Sodium Diet
Abstract
:1. Introduction
2. Material and Methods
2.1. Knockout Animals and Animal Procedure
2.2. RNAscope® In Situ Hybridization Combined with Immunofluorescence
2.3. Amplex Red Measurements from Renal Tissue
2.4. Blood Pressure Measurements
2.5. Clinical Chemistry
2.6. Statistics
3. Results
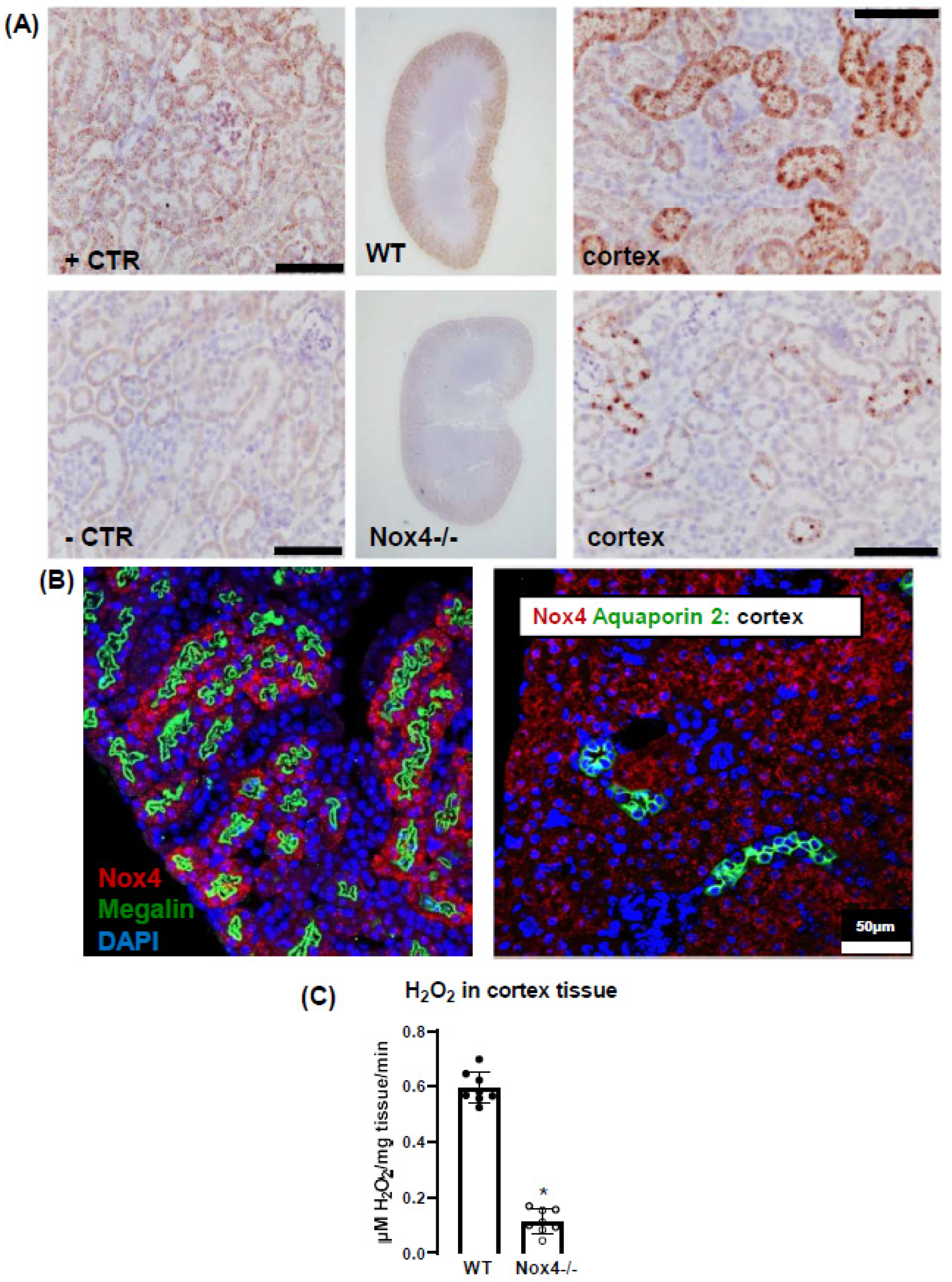
3.1. Nox4 Expression Is Restricted to Proximal Tubule Cells
3.2. Nox4 Contributes to H2O2 Production of the Renal Cortex
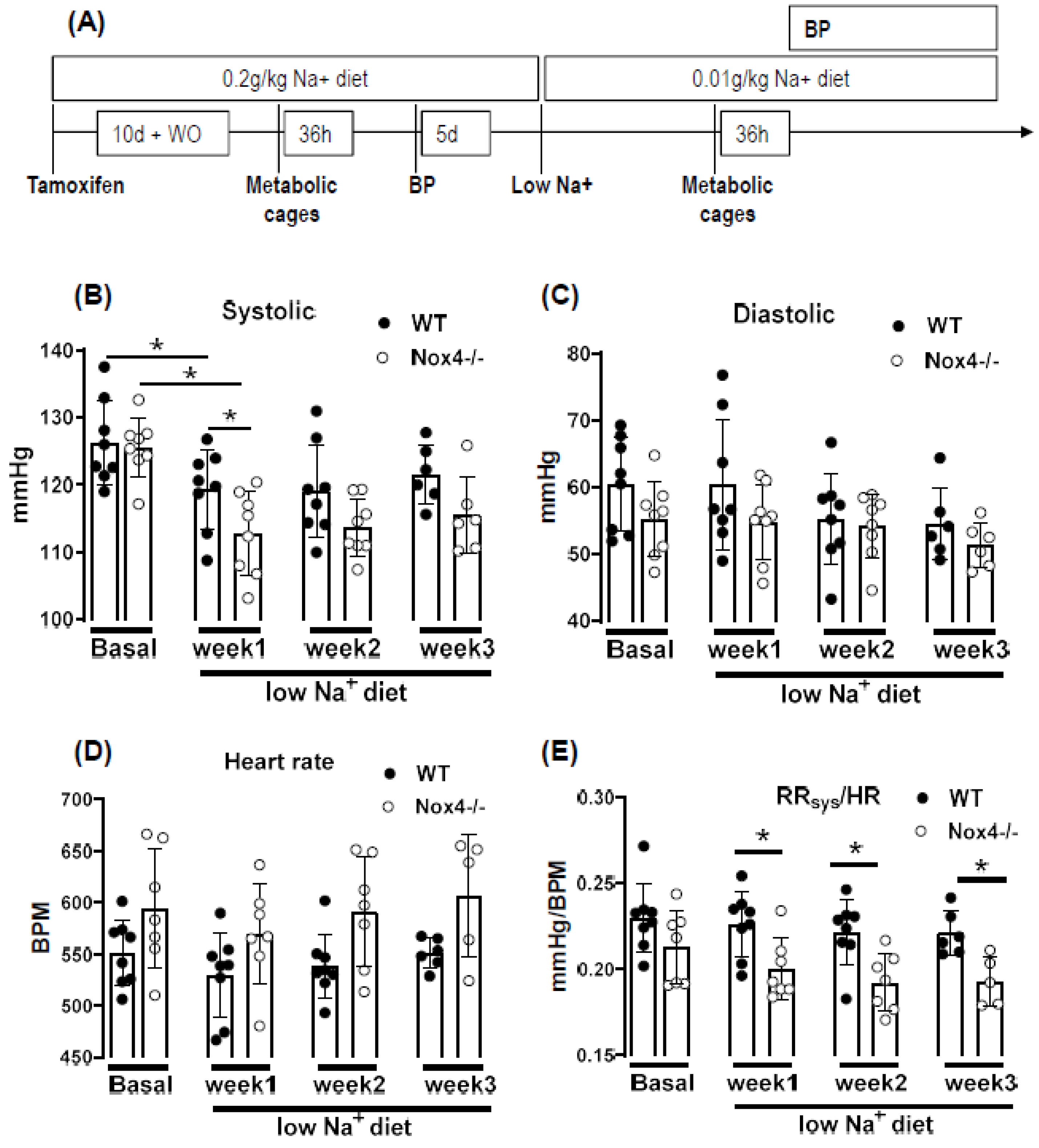
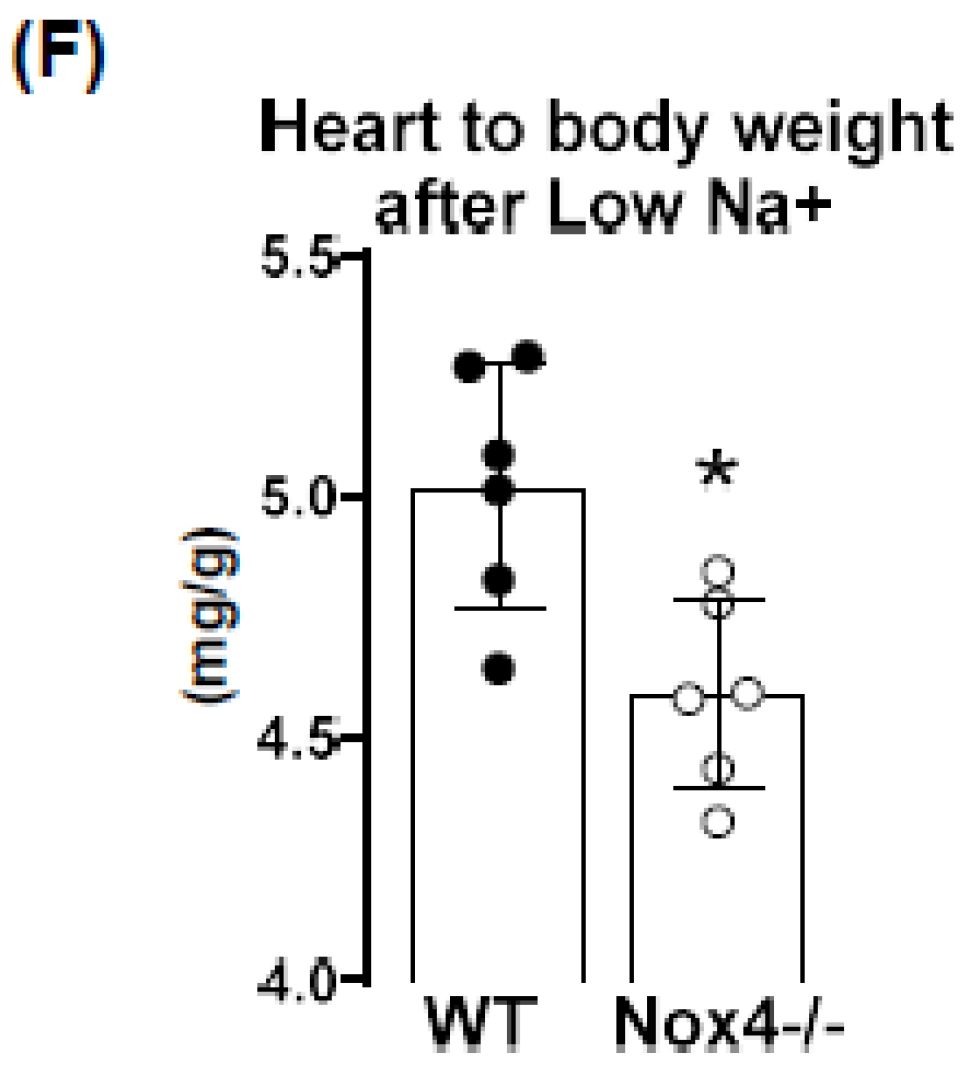
3.3. Knockout of Nox4 Lowers Blood Pressure and Cardiac Mass in Response to Low Sodium Diet
3.4. Knockout of Nox4 Does Not Alter Body Weight and Water Intake in Response to Low Sodium Diet
3.5. Knockout of Nox4 Does Not Affect Renal Clearance nor Plasma Level of Na+, Cl− and K+
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araujo, M.; Wilcox, C.S. Oxidative Stress in Hypertension: Role of the Kidney. Antioxidants Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef] [Green Version]
- Gill, P.S.; Wilcox, C.S. NADPH Oxidases in the Kidney. Antioxidants Redox Signal. 2006, 8, 1597–1607. [Google Scholar] [CrossRef]
- Chabrashvili, T.; Tojo, A.; Onozato, M.L.; Kitiyakara, C.; Quinn, M.; Fujita, T.; Welch, W.J.; Wilcox, C.S. Expression and Cellular Localization of Classic NADPH Oxidase Subunits in the Spontaneously Hypertensive Rat Kidney. Hypertension 2002, 39, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Sedeek, M.; Hébert, R.L.; Kennedy, C.R.; Burns, K.D.; Touyz, R.M. Molecular mechanisms of hypertension: Role of Nox family NADPH oxidases. Curr. Opin. Nephrol. Hypertens. 2009, 18, 122–127. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.; De Lucca Camargo, L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, A.; Skelton, M.M.; Zou, A.-P.; Cowley, A.W. Increased Renal Medullary H2O2 Leads to Hypertension. Hypertension 2003, 42, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, A.; Skelton, M.M.; Zou, A.-P.; Roman, R.J.; Cowley, A.W. Increased Renal Medullary Oxidative Stress Produces Hypertension. Hypertension 2002, 39, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Kopkan, L.; Castillo, A.; Navar, L.G.; Majid, D.S.A. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am. J. Physiol. Physiol. 2006, 290, F80–F86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babelova, A.; Avaniadi, D.; Jung, O.; Fork, C.; Beckmann, J.; Kosowski, J.; Weissmann, N.; Anilkumar, N.; Shah, A.; Schaefer, L.; et al. Role of Nox4 in murine models of kidney disease. Free. Radic. Biol. Med. 2012, 53, 842–853. [Google Scholar] [CrossRef]
- Prior, K.-K.; Leisegang, M.S.; Josipovic, I.; Löwe, O.; Shah, A.M.; Weissmann, N.; Schröder, K.; Brandes, R.P. CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity. Redox Biol. 2016, 9, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.; Morel, F.; Brandes, R.P. The E-loop Is Involved in Hydrogen Peroxide Formation by the NADPH Oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löwe, O.; Rezende, F.; Heidler, J.; Wittig, I.; Helfinger, V.; Brandes, R.P.; Schröder, K. BIAM switch assay coupled to mass spectrometry identifies novel redox targets of NADPH oxidase 4. Redox Biol. 2019, 21, 101125. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.X.; Hafstad, A.D.; Beretta, M.; Zhang, M.; Molenaar, C.; Kopec, J.; Fotinou, D.; Murray, T.V.; Cobb, A.M.; Martin, D.; et al. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF 2α-mediated stress signaling. EMBO J. 2016, 35, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Wu, F.-R.; Wang, J.-N.; Gao, L.; Jiang, L.; Li, H.-D.; Ma, Q.; Liu, X.-Q.; Wei, B.; Zhou, L.; et al. Nox4 in renal diseases: An update. Free Radic. Biol. Med. 2018, 124, 466–472. [Google Scholar] [CrossRef]
- Holterman, C.; Read, N.C.; Kennedy, C.R.J. Nox and renal disease. Clin. Sci. 2014, 128, 465–481. [Google Scholar] [CrossRef]
- Thallas-Bonke, V.; Jandeleit-Dahm, K.A.; Cooper, M.E. Nox-4 and progressive kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 74–80. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, H.; Fang, Y.; Lin, P.; Lu, Z.; Zhang, P.; Jiao, X.; Teng, J.; Ding, X.; Dai, Y. Salvianolate ameliorates oxidative stress and podocyte injury through modulation of NOX4 activity in db/db mice. J. Cell. Mol. Med. 2021, 25, 1012–1023. [Google Scholar] [CrossRef]
- Rajaram, R.D.; Dissard, R.; Faivre, A.; Ino, F.; Delitsikou, V.; Jaquet, V.; Cagarelli, T.; Lindenmeyer, M.; Jansen-Duerr, P.; Cohen, C.; et al. Tubular NOX4 expression decreases in chronic kidney disease but does not modify fibrosis evolution. Redox Biol. 2019, 26, 101234. [Google Scholar] [CrossRef]
- Shi, Q.; Lee, D.-Y.; Féliers, D.; Abboud, H.E.; Bhat, M.A.; Gorin, Y. Interplay between RNA-binding protein HuR and Nox4 as a novel therapeutic target in diabetic kidney disease. Mol. Metab. 2020, 36, 100968. [Google Scholar] [CrossRef] [PubMed]
- Jha, J.C.; Thallas-Bonke, V.; Banal, C.; Gray, S.P.; Chow, B.S.M.; Ramm, G.; Quaggin, S.E.; Cooper, M.E.; Schmidt, H.H.H.W.; Jandeleit-Dahm, K.A. Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia 2016, 59, 379–389. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-H.; Quach, T.; Saito, R.; Pham, J.; Sharma, K. Metabolomics Reveals a Key Role for Fumarate in Mediating the Effects of NADPH Oxidase 4 in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2015, 27, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Thallas-Bonke, V.; Tan, S.M.; Lindblom, R.S.; Snelson, M.; Granata, C.; Jha, J.C.; Sourris, K.C.; Laskowski, A.; Watson, A.; Tauc, M.; et al. Targeted deletion of nicotinamide adenine dinucleotide phosphate oxidase 4 from proximal tubules is dispensable for diabetic kidney disease development. Nephrol. Dial. Transplant. 2021, 36, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W.; Abe, M.; Mori, T.; O’Connor, P.M.; Ohsaki, Y.; Zheleznova, N.N. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am. J. Physiol. Physiol. 2015, 308, F179–F197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathkolb, B.; Hans, W.; Prehn, C.; Fuchs, H.; Gailus-Durner, V.; Aigner, B.; Adamski, J.; Wolf, E.; de Angelis, M.H. Clinical Chemistry and Other Laboratory Tests on Mouse Plasma or Serum. Curr. Protoc. Mouse Biol. 2013, 3, 69–100. [Google Scholar] [CrossRef] [PubMed]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Fórró, L.; Schlegel, W.; Krause, K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Cowley, J.A.W.; Yang, C.; Zheleznova, N.N.; Staruschenko, A.; Kurth, T.; Rein, L.; Kumar, V.; Sadovnikov, K.; Dayton, A.; Hoffman, M.; et al. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension 2016, 67, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Shrestha, R.; Qiu, C.; Kondo, A.; Huang, S.; Werth, M.; Li, M.; Barasch, J.; Suszták, K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 2018, 360, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Khodo, S.N.; Dizin, E.; Sossauer, G.; Szanto, I.; Martin, P.-Y.; Feraille, E.; Krause, K.H.; De Seigneux, S. NADPH-Oxidase 4 Protects against Kidney Fibrosis during Chronic Renal Injury. J. Am. Soc. Nephrol. 2012, 23, 1967–1976. [Google Scholar] [CrossRef] [Green Version]
- Eshbach, M.L.; Sethi, R.; Avula, R.; Lamb, J.; Hollingshead, D.J.; Finegold, D.N.; Locker, J.D.; Chandran, U.R.; Weisz, O.A. The transcriptome of the Didelphis virginiana opossum kidney OK proximal tubule cell line. Am. J. Physiol. Physiol. 2017, 313, F585–F595. [Google Scholar] [CrossRef]
- McDonough, A.A. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R851–R861. [Google Scholar] [CrossRef] [Green Version]
- Satriano, J.; Wead, L.; Cardús, A.; Deng, A.; Boss, G.R.; Thomson, S.C.; Blantz, R.C. Regulation of ecto-5′-nucleotidase by NaCl and nitric oxide: Potential roles in tubuloglomerular feedback and adaptation. Am. J. Physiol. Physiol. 2006, 291, F1078–F1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infanger, D.W.; Cao, X.; Butler, S.D.; Burmeister, M.A.; Zhou, Y.; Stupinski, J.A.; Sharma, R.V.; Davisson, R.L. Silencing Nox4 in the Paraventricular Nucleus Improves Myocardial Infarction–Induced Cardiac Dysfunction by Attenuating Sympathoexcitation and Periinfarct Apoptosis. Circ. Res. 2010, 106, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-A.; Hess, D.T.; Nogueira, L.; Yong, S.; Bowles, D.E.; Eu, J.; Laurita, K.R.; Meissner, G.; Stamler, J.S. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl. Acad. Sci. USA 2011, 108, 16098–16103. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, A.M.; Thompson, M.D.; Bolotina, V.M.; Tong, X.; Cohen, R.A. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic. Biol. Med. 2012, 53, 2327–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, R.; Jiang, H.; Sun, B.; Wu, X.; Li, W.; Zhu, S.; Liao, C.; Zhong, Z.; Chen, J. Advanced oxidation protein products sensitized the transient receptor potential vanilloid 1 via NADPH oxidase 1 and 4 to cause mechanical hyperalgesia. Redox Biol. 2016, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-S.; Lee, S.-H.; Huang, H.-S.; Chen, Y.-S.; Ma, M.-C. H2O2 generated by NADPH oxidase 4 contributes to transient receptor potential vanilloid 1 channel-mediated mechanosensation in the rat kidney. Am. J. Physiol. Physiol. 2015, 309, F369–F376. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Fu, X.; Tian, L.; Chen, Y.; Yang, K.; Chen, X.; Zhang, J.; Lu, W.; Wang, J. NOX4 Mediates BMP4-Induced Upregulation of TRPC1 and 6 Protein Expressions in Distal Pulmonary Arterial Smooth Muscle Cells. PLoS ONE 2014, 9, e107135. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Anderson, M.; Dryer, S.E. Insulin increases surface expression of TRPC6 channels in podocytes: Role of NADPH oxidases and reactive oxygen species. Am. J. Physiol. Physiol. 2012, 302, F298–F307. [Google Scholar] [CrossRef] [Green Version]
- Clempus, R.E.; Sorescu, D.; Dikalova, A.E.; Pounkova, L.; Jo, P.; Sorescu, G.P.; Schmidt, H.H.H.; Lassègue, B.; Griendling, K.K. Nox4 Is Required for Maintenance of the Differentiated Vascular Smooth Muscle Cell Phenotype. Arter. Thromb. Vasc. Biol. 2007, 27, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Hilenski, L.L.; Clempus, R.E.; Quinn, M.; Lambeth, J.D.; Griendling, K.K. Distinct Subcellular Localizations of Nox1 and Nox4 in Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2004, 24, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Martín, A.S.; Valdivia, A.; Martin-Garrido, A.; Griendling, K.K. Redox-Sensitive Regulation of Myocardin-Related Transcription Factor (MRTF-A) Phosphorylation via Palladin in Vascular Smooth Muscle Cell Differentiation Marker Gene Expression. PLoS ONE 2016, 11, e0153199. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garrido, A.; Brown, D.I.; Lyle, A.N.; Dikalova, A.; Seidel-Rogol, B.; Lassègue, B.; Martín, A.S.; Griendling, K.K. NADPH oxidase 4 mediates TGF-β-induced smooth muscle α-actin via p38MAPK and serum response factor. Free Radic. Biol. Med. 2011, 50, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panico, C.; Luo, Z.; Damiano, S.; Artigiano, F.; Gill, P.; Welch, W.J. Renal Proximal Tubular Reabsorption Is Reduced In Adult Spontaneously Hypertensive Rats. Hypertension 2009, 54, 1291–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yan, Y.; Liu, L.; Xie, Z.; Malhotra, D.; Joe, B.; Shapiro, J.I. Impairment of Na/K-ATPase Signaling in Renal Proximal Tubule Contributes to Dahl Salt-sensitive Hypertension. J. Biol. Chem. 2011, 286, 22806–22813. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezende, F.; Malacarne, P.F.; Müller, N.; Rathkolb, B.; Hrabě de Angelis, M.; Schröder, K.; P Brandes, R. Nox4 Maintains Blood Pressure during Low Sodium Diet. Antioxidants 2021, 10, 1103. https://doi.org/10.3390/antiox10071103
Rezende F, Malacarne PF, Müller N, Rathkolb B, Hrabě de Angelis M, Schröder K, P Brandes R. Nox4 Maintains Blood Pressure during Low Sodium Diet. Antioxidants. 2021; 10(7):1103. https://doi.org/10.3390/antiox10071103
Chicago/Turabian StyleRezende, Flávia, Pedro Felipe Malacarne, Niklas Müller, Birgit Rathkolb, Martin Hrabě de Angelis, Katrin Schröder, and Ralf P Brandes. 2021. "Nox4 Maintains Blood Pressure during Low Sodium Diet" Antioxidants 10, no. 7: 1103. https://doi.org/10.3390/antiox10071103
APA StyleRezende, F., Malacarne, P. F., Müller, N., Rathkolb, B., Hrabě de Angelis, M., Schröder, K., & P Brandes, R. (2021). Nox4 Maintains Blood Pressure during Low Sodium Diet. Antioxidants, 10(7), 1103. https://doi.org/10.3390/antiox10071103







