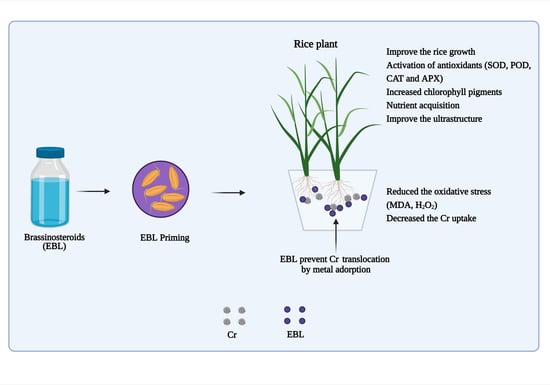
Seed Priming with Brassinosteroids Alleviates Chromium Stress in Rice Cultivars via Improving ROS Metabolism and Antioxidant Defense Response at Biochemical and Molecular Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Materials and Brassinosteroids (EBL) Preparation
2.2. Seed Priming and Germination Test
2.3. Plant Growth Conditions
2.4. Experimental Design and Treatment Pattern
2.5. Plant Growth Investigation
2.6. Measurement of Chlorophyll Pigments
2.7. Measurement of Cr Contents
2.8. Transmission Electron Microscopy Analysis
2.9. Investigation of Malondialdehyde (MDA) Contents and H2O2 Production
2.10. Measurement of Antioxidant Enzyme Activity
2.11. RNA Extraction and Gene Expression Analysis
2.12. Statistical Analysis
3. Results
3.1. Determination of the Significant Effect of Cr (VI) on Seed Vigor and Plant Development
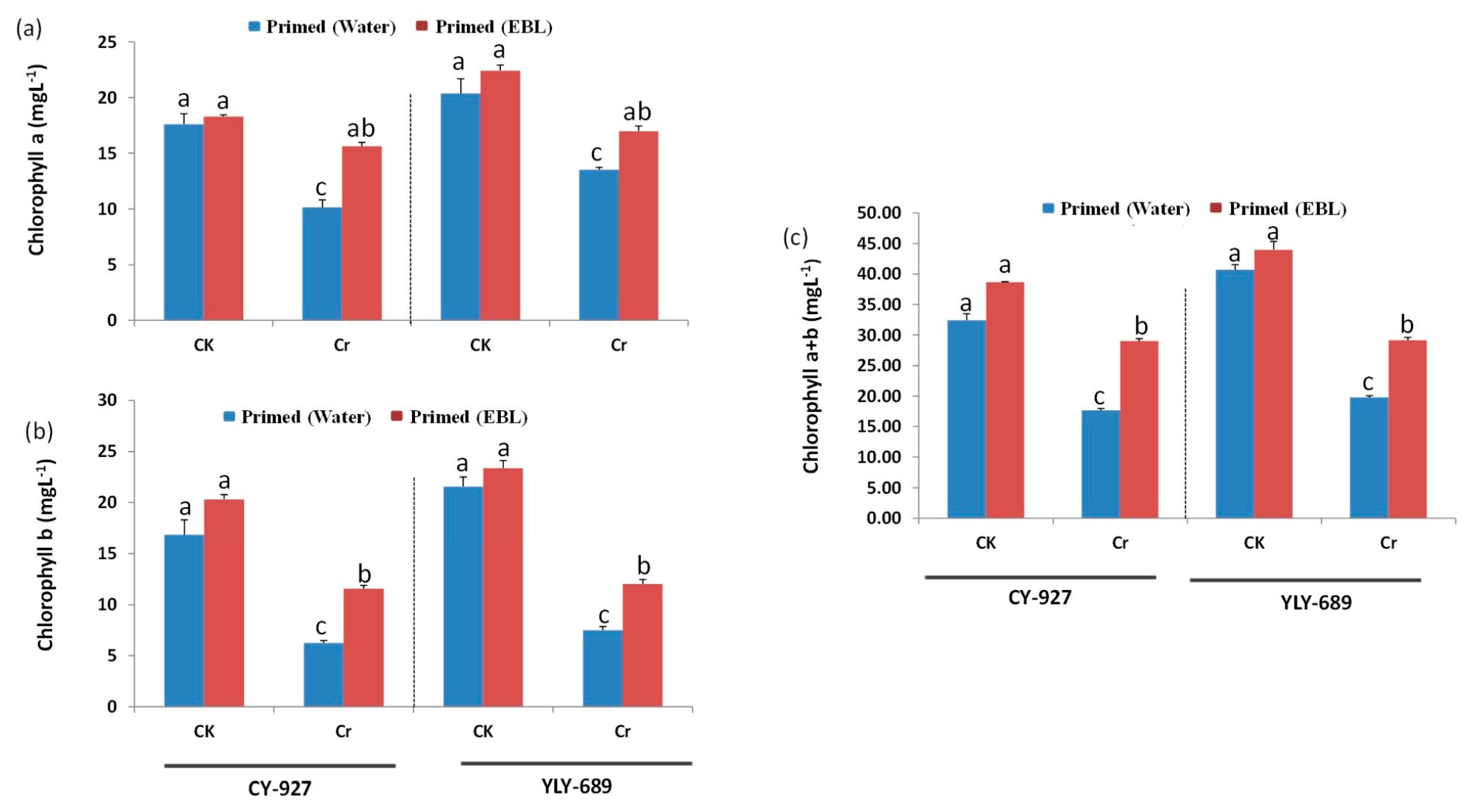
3.2. Seed Priming with EBL Significantly Enhanced Photosynthetic Pigments under Cr Stress
3.3. Accumulation of Cr Contents Was Reduced Significantly by EBL Seed Priming
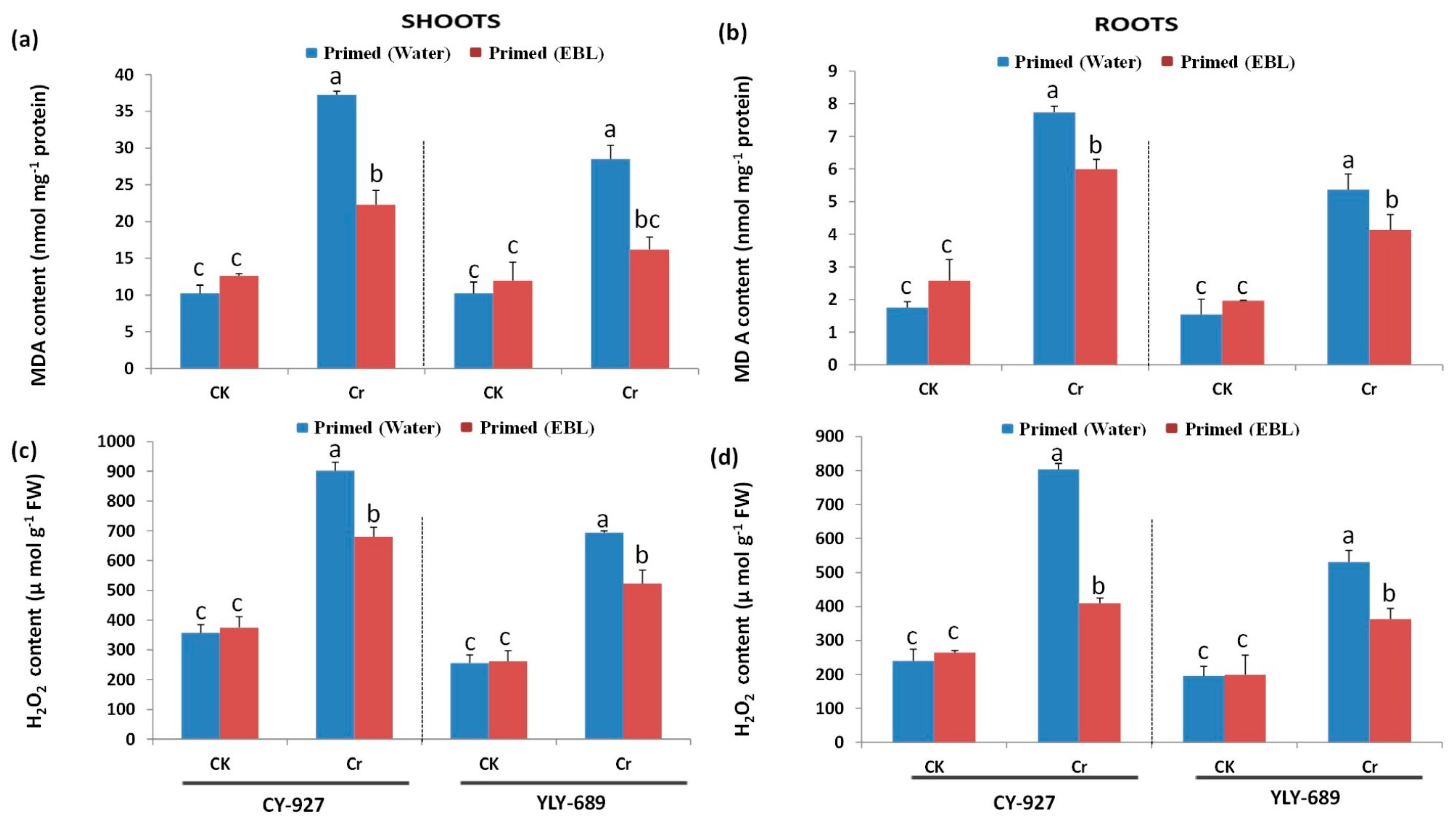
3.4. Significant Reduction of MDA Contents and H2O2 Production by Seed Priming with EBL
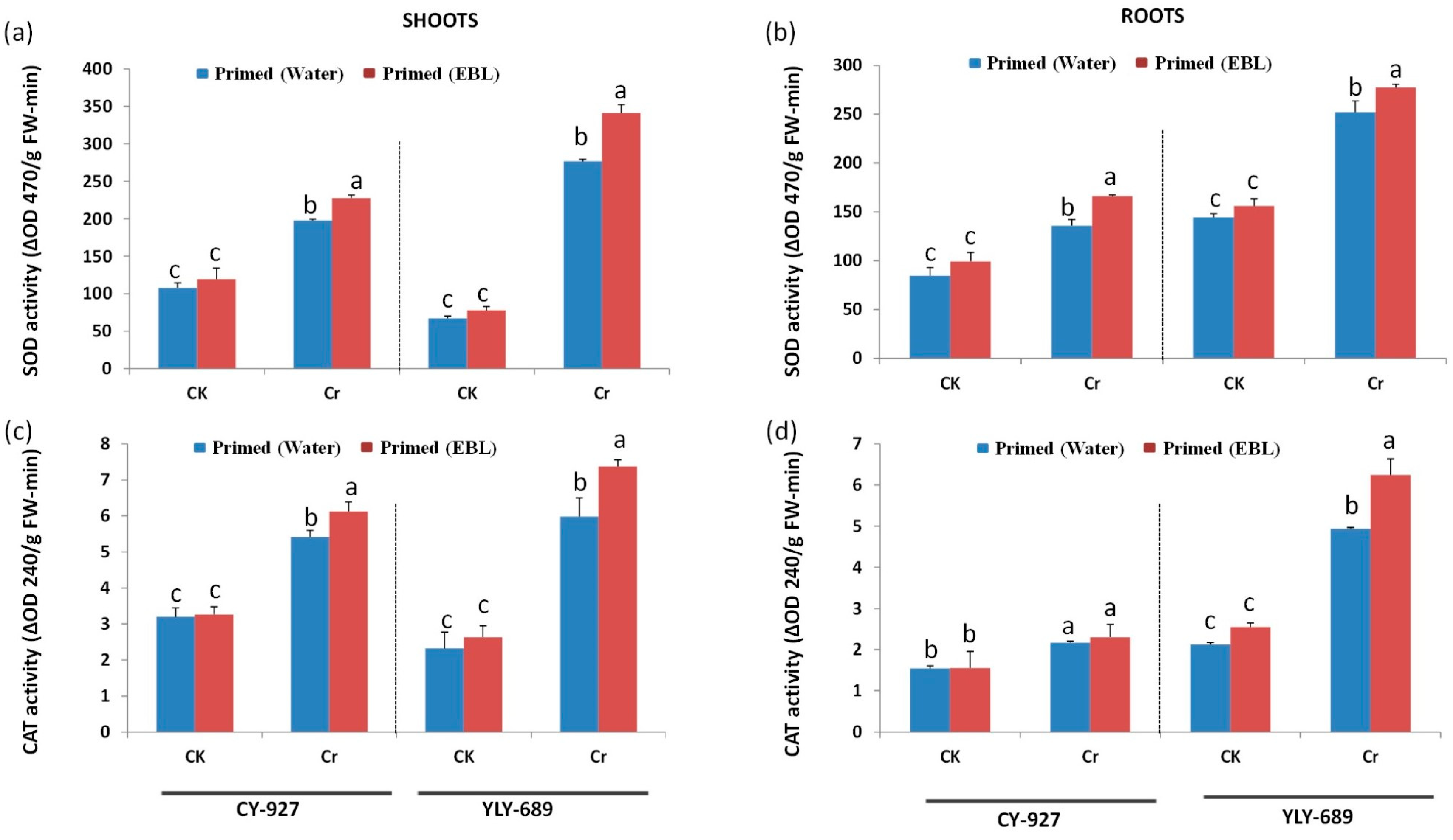
3.5. Regulation of Antioxidant Activities via Seed Priming with EBL
3.6. Determination of Ultrastructure Analysis
3.7. Determination of Gene Expression Analysis
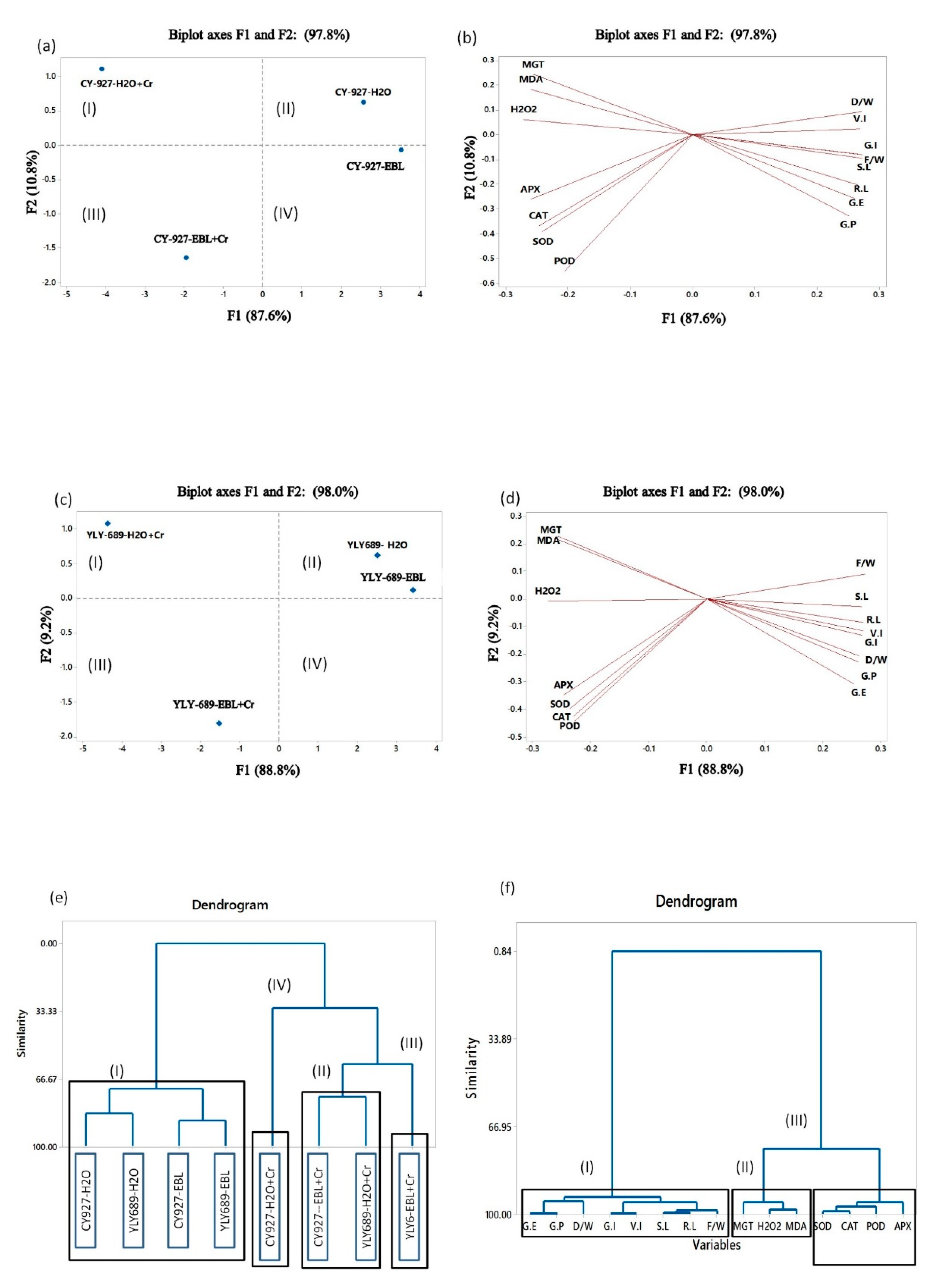
3.8. Determination of Interaction among Growth and Physiological Parameters through Principal Component Analysis, Clustering, and Correlation Analysis
4. Discussion
4.1. EBL Improve Physio-Biochemical Effects Caused by Cr Toxicity in Rice Plants
4.2. EBL Prevented Degradation of Chlorophyll Pigments under Cr Stress
4.3. Seed Priming with EBL Reduced MDA Contents as Well as H2O2 Production
4.4. EBL Enhanced Antioxidant Activity to Mitigate Cr Toxicity
4.5. Rice Cultivar YLY-689 Was More Resistant to Cr Stress Than CY-927
4.6. Ultrastructural Changing Induced by Cr in Rice Plants
4.7. Gene Expression Level
4.8. Clustering and Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cr | Chromium |
| CK | Control |
| Chla | Chlorophyll a |
| Chlb | Chlorophyll b |
| Chl a+b | Chlorophyll a+b |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| POD | Peroxidase |
| G.E | Germination energy |
| G.P | Germination percentage |
| G.I | Germination index |
| V.I | Vigor index |
| MGT | Mean germination time |
| S.L | Shoot length |
| R.L | Root length |
| F/W | Fresh weight |
| D/W | Dry weight |
References
- Ali, S.; Bai, P.; Zeng, F.; Cai, S.; Shamsi, I.H.; Qiu, B.; Wu, F.; Zhang, G. The ecotoxicological and interactive effects of chromium and aluminum on growth, oxidative damage and antioxidant enzymes on two barley genotypes differing in Al tolerance. Environ. Exp. Bot. 2011, 70, 185–191. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Effects of heavy metals on soil, plants, human health and aquatic life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Arain, B.A.; Amin, F.; Surhio, M.A. Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am. J. Plant Sci. 2013, 2013. [Google Scholar]
- Tiwari, K.; Dwivedi, S.; Singh, N.; Rai, U.; Tripathi, R. Chromium (VI) induced phytotoxicity and oxidative stress in pea (Pisum sativum L.): biochemical changes and translocation of essential nutrients. J. Environ. Biol. 2009, 30, 389–394. [Google Scholar] [PubMed]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Arain, B.A.; Amin, F.; Surhio, M.A. Analysis of growth response and tolerance index of Glycine max (L.) Merr. under hexavalent chromium stress. Adv. Life Sci. 2014, 1, 231–241. [Google Scholar]
- Singh, S.; Srivastava, P.K.; Kumar, D.; Tripathi, D.K.; Chauhan, D.K.; Prasad, S.M. Morpho-anatomical and biochemical adapting strategies of maize (Zea mays L.) seedlings against lead and chromium stresses. Biocatal. Agric. Biotechnol. 2015, 4, 286–295. [Google Scholar] [CrossRef]
- Karthik, C.; Kadirvelu, K.; Bruno, B.; Maharajan, K.; Rajkumar, M.; Manoj, S.R.; Arulselvi, P.I. Cellulosimicrobium funkei strain AR6 alleviate Cr (VI) toxicity in Lycopersicon esculentum by regulating the expression of growth responsible, stress tolerant and metal transporter genes. Rhizosphere 2021, 18, 100351. [Google Scholar] [CrossRef]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide alleviates the injurious effects of Cr (VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of Ascorbate–glutathione and Glyoxalase cycles. J. Plant. Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicol. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.-J.; Feng, Y.-X.; Li, Y.-H.; Lin, Y.-J.; Yu, X.-Z. Unraveling genes promoting ROS metabolism in subcellular organelles of Oryza sativa in response to trivalent and hexavalent chromium. Sci. Total Environ. 2020, 744, 140951. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyer, A.; Dietz, K.-J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Sirhindi, G. Brassinosteroids: biosynthesis and role in growth, development, and thermotolerance responses. In Molecular Stress Physiology of Plants; Springer: Berlin, Germany, 2013; pp. 309–329. [Google Scholar]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, J.; Zhang, S.; Gao, C.; Song, W. Identification of chilling-tolerance in maize inbred lines at germination and seedling growth stages. J. Zhejiang Univer. Agric. Life Sci. 2006, 32, 41–45. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents; Portland Press: London, UK, 1983. [Google Scholar]
- Shentu, J.; He, Z.; Yang, X.-E.; Li, T. Accumulation properties of cadmium in a selected vegetable-rotation system of southeastern China. J. Agric. Food Chem. 2008, 56, 6382–6388. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Kwasniewski, M.; Chwialkowska, K.; Kwasniewska, J.; Kusak, J.; Siwinski, K.; Szarejko, I. Accumulation of peroxidase-related reactive oxygen species in trichoblasts correlates with root hair initiation in barley. J. Plant Physiol. 2013, 170, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Chance, B.; Maehly, A. 136, Assay of Catalases and Peroxidases; Elsevier: Amsteradam, The Netherlands, 1955. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Sah, S.K.; Kaur, G.; Kaur, A. Rapid and reliable method of high-quality RNA extraction from diverse plants. Am. J. Plant Sci. 2014, 5, 3129. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shakirova, F.; Allagulova, C.; Maslennikova, D.; Fedorova, K.; Yuldashev, R.; Lubyanova, A.; Bezrukova, M.; Avalbaev, A. Involvement of dehydrins in 24-epibrassinolide-induced protection of wheat plants against drought stress. Plant Physiol. Biochem. 2016, 108, 539–548. [Google Scholar] [CrossRef]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological changes induced by chromium stress in plants: an overview. Protoplasma 2012, 249, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Surti, M.; Ashraf, S.A.; Adnan, M. Physiological and Molecular Responses to Heavy Metal Stresses in Plants. Harsh Environ. Plant Resil. Mol. Funct. Asp. 2021, 171. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Physiology and Biochemistry of Seeds in Relation to Germination: Volume 2: Viability, Dormancy, and Environmental Control; Springer: Berlin, Germany, 2012. [Google Scholar]
- Shinwari, K.I.; Jan, M.; Shah, G.; Khattak, S.R.; Urehman, S.; Daud, M.; Naeem, R.; Jamil, M. Seed priming with salicylic acid induces tolerance against chromium (VI) toxicity in rice (Oryza sativa L.). Pak. J. Bot. 2015, 47, 161–170. [Google Scholar]
- Sharma, I.; Pati, P.K.; Bhardwaj, R. Effect of 28-homobrassinolide on antioxidant defence system in Raphanus sativus L. under chromium toxicity. Ecotoxicology 2011, 20, 862–874. [Google Scholar] [CrossRef]
- Ali, A.A.; Abdel-Fattah, R.I. Osmolytes-antioxidant behaviour in Phaseolus vulgaris and Hordeum vulgare with brassinosteroid under salt stress. J. Agron. 2006. [Google Scholar]
- Hayat, S.; Hasan, S.A.; Hayat, Q.; Ahmad, A. Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma 2010, 239, 3–14. [Google Scholar] [CrossRef]
- Vajpayee, P.; Tripathi, R.; Rai, U.; Ali, M.; Singh, S. Chromium (VI) accumulation reduces chlorophyll biosynthesis, nitrate reductase activity and protein content in Nymphaea alba L. Chemosphere 2000, 41, 1075–1082. [Google Scholar] [CrossRef]
- Yu, X.-Z.; Lin, Y.-J.; Zhang, Q. Metallothioneins enhance chromium detoxification through scavenging ROS and stimulating metal chelation in Oryza sativa. Chemosphere 2019, 220, 300–313. [Google Scholar] [CrossRef]
- Wakeel, A.; Ali, I.; Wu, M.; Kkan, A.R.; Jan, M.; Ali, A.; Liu, Y.; Ge, S.; Wu, J.; Gan, Y. Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 166–179. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Hameed, A.; Hafeez, F.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L. Role of zinc–lysine on growth and chromium uptake in rice plants under Cr stress. Plant Growth Regul. 2018, 37, 1413–1422. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: an overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Wang, Y.; Li, J.; Bian, Z. Effects of brassinosteroids on photosynthetic performance and nitrogen metabolism in pepper seedlings under chilling stress. Agronomy 2019, 9, 839. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hasan, S.; Hayat, S.; Hayat, Q.; Yadav, S.; Fariduddin, Q.; Ahmad, A. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ. Exp. Bot. 2008, 62, 153–159. [Google Scholar] [CrossRef]
- Somssich, M.; Vandenbussche, F.; Ivakov, A.; Funke, N.; Ruprecht, C.; Vissenberg, K.; Van Der Straeten, D.; Persson, S.; Suslov, D. Brassinosteroids Influence Arabidopsis Hypocotyl Graviresponses Through Changes in Mannans and Cellulose; University Ghent: Ghent, Belguim, 2019. [Google Scholar]
- Chen, L.; Hu, W.-f.; Long, C.; Wang, D. Exogenous plant growth regulator alleviate the adverse effects of U and Cd stress in sunflower (Helianthus annuus L.) and improve the efficacy of U and Cd remediation. Chemosphere 2021, 262, 127809. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.; Soares, C.; Oliveira, F.; Martins, M.; Branco-Neves, S.; Barbosa, B.; Ataíde, I.; Teixeira, J.; Azenha, M.; Azevedo, R.A. Foliar application of 24-epibrassinolide improves Solanum nigrum L. tolerance to high levels of Zn without affecting its remediation potential. Chemosphere 2020, 244, 125579. [Google Scholar] [CrossRef]
- Soares, T.F.S.N.; dos Santos Dias, D.C.F.; Oliveira, A.M.S.; Ribeiro, D.M.; dos Santos Dias, L.A. Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar] [CrossRef]
- Horton, A.; Fairhurst, S.; Bus, J.S. Lipid peroxidation and mechanisms of toxicity. Crit. Rev. Toxicol. 1987, 18, 27–79. [Google Scholar] [CrossRef]
- Khan, M.; Samrana, S.; Zhang, Y.; Malik, Z.; Khan, M.D.; Zhu, S. Reduced glutathione protects subcellular compartments from Pb-induced ROS injury in leaves and roots of upland cotton (Gossypium hirsutum L.). Front. Plant Sci. 2020, 11, 412. [Google Scholar] [CrossRef] [Green Version]
- Samrana, S.; Ali, A.; Muhammad, U.; Azizullah, A.; Ali, H.; Khan, M.; Naz, S.; Khan, M.D.; Zhu, S.; Chen, J. Physiological, ultrastructural, biochemical, and molecular responses of glandless cotton to hexavalent chromium (Cr6+) exposure. Environ. Pollut. 2020, 266, 115394. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol Plant. 2020. [Google Scholar] [CrossRef]
- Rattan, A.; Kapoor, D.; Kapoor, N.; Bhardwaj, R.; Sharma, A. Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. Plant Growth Regul. 2020, 39, 1465–1475. [Google Scholar] [CrossRef]
- Liaqat, S.; Umar, S.; Saffeullah, P.; Iqbal, N.; Siddiqi, T.O.; Khan, M.I.R. Protective Effect of 24-Epibrassinolide on Barley Plants Growing Under Combined Stress of Salinity and Potassium Deficiency. Plant Growth Regul. 2020, 39, 1543–1558. [Google Scholar] [CrossRef]
- Surgun-Acar, Y.; Zemheri-Navruz, F. Exogenous application of 24-epibrassinolide improves manganese tolerance in Arabidopsis thaliana L. via the modulation of antioxidant system. Plant Growth Regul. 2021, 1–12. [Google Scholar]
- Kabir, A. Biochemical and molecular changes in rice seedlings (Oryza sativa L.) to cope with chromium stress. Plant Biol. 2016, 18, 710–719. [Google Scholar] [CrossRef]
- Ma, J.; Lv, C.; Xu, M.; Chen, G.; Lv, C.; Gao, Z. Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ. Sci. Pollut. Res. 2016, 23, 1768–1778. [Google Scholar] [CrossRef]
- Qiu, B.; Zeng, F.; Cai, S.; Wu, X.; Haider, S.I.; Wu, F.; Zhang, G. Alleviation of chromium toxicity in rice seedlings by applying exogenous glutathione. J. Plant Physiol. 2013, 170, 772–779. [Google Scholar] [CrossRef]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef]
- Li, L.; Long, M.; Islam, F.; Farooq, M.A.; Wang, J.; Mwamba, T.M.; Shou, J.; Zhou, W. Synergistic effects of chromium and copper on photosynthetic inhibition, subcellular distribution, and related gene expression in Brassica napus cultivars. Environ. Sci. Pollut. Res. 2019, 26, 11827–11845. [Google Scholar] [CrossRef]
- dos Santos, R.W.; Schmidt, É.C.; Martins, R.d.P.; Latini, A.; Maraschin, M.; Horta, P.A.; Bouzon, Z.L. Effects of cadmium on growth, photosynthetic pigments, photosynthetic performance, biochemical parameters and structure of chloroplasts in the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales). Am. J. Plant Sci. 2012, 3, 1077–1084. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saif, S.; Khan, M.S. Assessment of toxic impact of metals on proline, antioxidant enzymes, and biological characteristics of Pseudomonas aeruginosa inoculated Cicer arietinum grown in chromium and nickel-stressed sandy clay loam soils. Environ. Monit. Assess. 2018, 190, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Mahmood, T.; Mahmood, Z.; Shazadi, K.; Mujeeb-Kazi, A.; Rasheed, A. Genotypic Variation and Genotype× Environment Interaction for Yield-Related Traits in Synthetic Hexaploid Wheats under a Range of Optimal and Heat-Stressed Environments. Crop Sci. 2018, 58, 295–303. [Google Scholar] [CrossRef]
- Ali, Q.; Perveen, R.; El-Esawi, M.A.; Ali, S.; Hussain, S.M.; Amber, M.; Iqbal, N.; Rizwan, M.; Alyemeni, M.N.; El-Serehy, H.A. Low doses of Cuscuta reflexa extract act as natural biostimulants to improve the germination vigor, growth, and grain yield of wheat grown under water stress: photosynthetic pigments, antioxidative defense mechanisms, and nutrient acquisition. Biomolecules 2020, 10, 1212. [Google Scholar] [CrossRef]



| Varieties Name | Treatment | G.E | G.P | G.I | MGT | V.I |
|---|---|---|---|---|---|---|
| CY-927 | H2O | 90.00 ± 2.00 b | 94.67 ± 1.15 b | 20.13 ± 1.17 b | 2.91 ± 0.17 b | 2.04 ± 0.11 b |
| EBL | 96.00 ± 2.00 a | 99.33 ± 1.15 a | 28.01 ± 1.47 a | 2.16 ± 0.11 c | 2.87 ± 0.13 a | |
| H2O+Cr | 38.67 ± 3.06 d | 46.67 ± 3.06 d | 7.15 ± 0.74 d | 4.06 ± 0.07 a | 0.31 ± 0.07 d | |
| EBL+Cr | 72.67 ± 3.06 c | 82.67 ± 3.06 c | 12.90 ± 0.35 c | 3.21 ± 0.14 b | 0.77 ± 0.03 c | |
| YLY-689 | H2O | 93.33 ± 2.31 a | 99.33 ± 1.15 a | 27.29 ± 0.76 b | 2.44 ± 0.13 c | 2.63 ± 0.09 b |
| EBL | 98.00 ± 2.00 a | 100.00 ± 0.00 a | 32.98 ± 0.59 a | 2.07 ± 0.08 d | 3.24 ± 0.05 a | |
| H2O+Cr | 54.00 ± 2.00 c | 66.00 ± 2.00 c | 12.26 ± 0.88 d | 3.66 ± 0.19 a | 0.90 ± 0.07 d | |
| EBL+Cr | 86.67 ± 1.15 b | 89.33 ± 1.15 b | 21.50 ± 0.30 c | 2.74 ± 0.14 b | 2.00 ± 0.03 c |
| Varieties Name | Treatment | S.L | R.L | F/W | D/W |
|---|---|---|---|---|---|
| CY-927 | H2O | 15.51 ± 0.02 b | 13.04 ± 0.02 b | 0.90 ± 0.01 b | 0.10 ± 0.001 b |
| EBL | 17.19 ± 0.04 a | 15.03 ± 0.16 a | 0.96 ± 0.01 a | 0.10 ± 0.001 a | |
| H2O+Cr | 8.30 ± 0.03 d | 7.56 ± 0.03 d | 0.51 ± 0.01 d | 0.05 ± 0.001 d | |
| EBL+Cr | 11.59 ± 0.02 c | 10.96 ± 0.02 c | 0.68 ± 0.01 c | 0.06 ± 0.001 c | |
| YLY-689 | H2O | 15.16 ± 0.08 b | 13.31 ± 0.02 b | 0.91 ± 0.01 b | 0.10 ± 0.003 a |
| EBL | 18.39 ± 0.06 a | 15.28 ± 0.04 a | 0.96 ± 0.01 a | 0.10 ± 0.001 a | |
| H2O+Cr | 9.34 ± 0.04 d | 8.43 ± 0.02 d | 0.59 ± 0.01 d | 0.07 ± 0.002 c | |
| EBL+Cr | 12.22 ± 0.04 c | 11.22 ± 0.03 c | 0.68 ± 0.01 c | 0.09 ± 0.001 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basit, F.; Chen, M.; Ahmed, T.; Shahid, M.; Noman, M.; Liu, J.; An, J.; Hashem, A.; Fahad Al-Arjani, A.-B.; Alqarawi, A.A.; et al. Seed Priming with Brassinosteroids Alleviates Chromium Stress in Rice Cultivars via Improving ROS Metabolism and Antioxidant Defense Response at Biochemical and Molecular Levels. Antioxidants 2021, 10, 1089. https://doi.org/10.3390/antiox10071089
Basit F, Chen M, Ahmed T, Shahid M, Noman M, Liu J, An J, Hashem A, Fahad Al-Arjani A-B, Alqarawi AA, et al. Seed Priming with Brassinosteroids Alleviates Chromium Stress in Rice Cultivars via Improving ROS Metabolism and Antioxidant Defense Response at Biochemical and Molecular Levels. Antioxidants. 2021; 10(7):1089. https://doi.org/10.3390/antiox10071089
Chicago/Turabian StyleBasit, Farwa, Min Chen, Temoor Ahmed, Muhammad Shahid, Muhammad Noman, Jiaxin Liu, Jianyu An, Abeer Hashem, Al-Bandari Fahad Al-Arjani, Abdulaziz A. Alqarawi, and et al. 2021. "Seed Priming with Brassinosteroids Alleviates Chromium Stress in Rice Cultivars via Improving ROS Metabolism and Antioxidant Defense Response at Biochemical and Molecular Levels" Antioxidants 10, no. 7: 1089. https://doi.org/10.3390/antiox10071089
APA StyleBasit, F., Chen, M., Ahmed, T., Shahid, M., Noman, M., Liu, J., An, J., Hashem, A., Fahad Al-Arjani, A.-B., Alqarawi, A. A., Alsayed, M. F. S., Fathi Abd_Allah, E., Hu, J., & Guan, Y. (2021). Seed Priming with Brassinosteroids Alleviates Chromium Stress in Rice Cultivars via Improving ROS Metabolism and Antioxidant Defense Response at Biochemical and Molecular Levels. Antioxidants, 10(7), 1089. https://doi.org/10.3390/antiox10071089