Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort
2.2. Cytokine Profiling
2.3. Measurement of Antioxidant Enzymatic Activities
2.4. Measurement of Telomere Length
2.5. Statistical Analysis
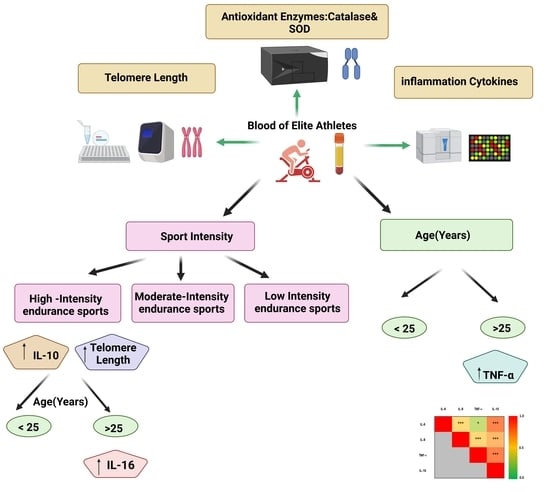
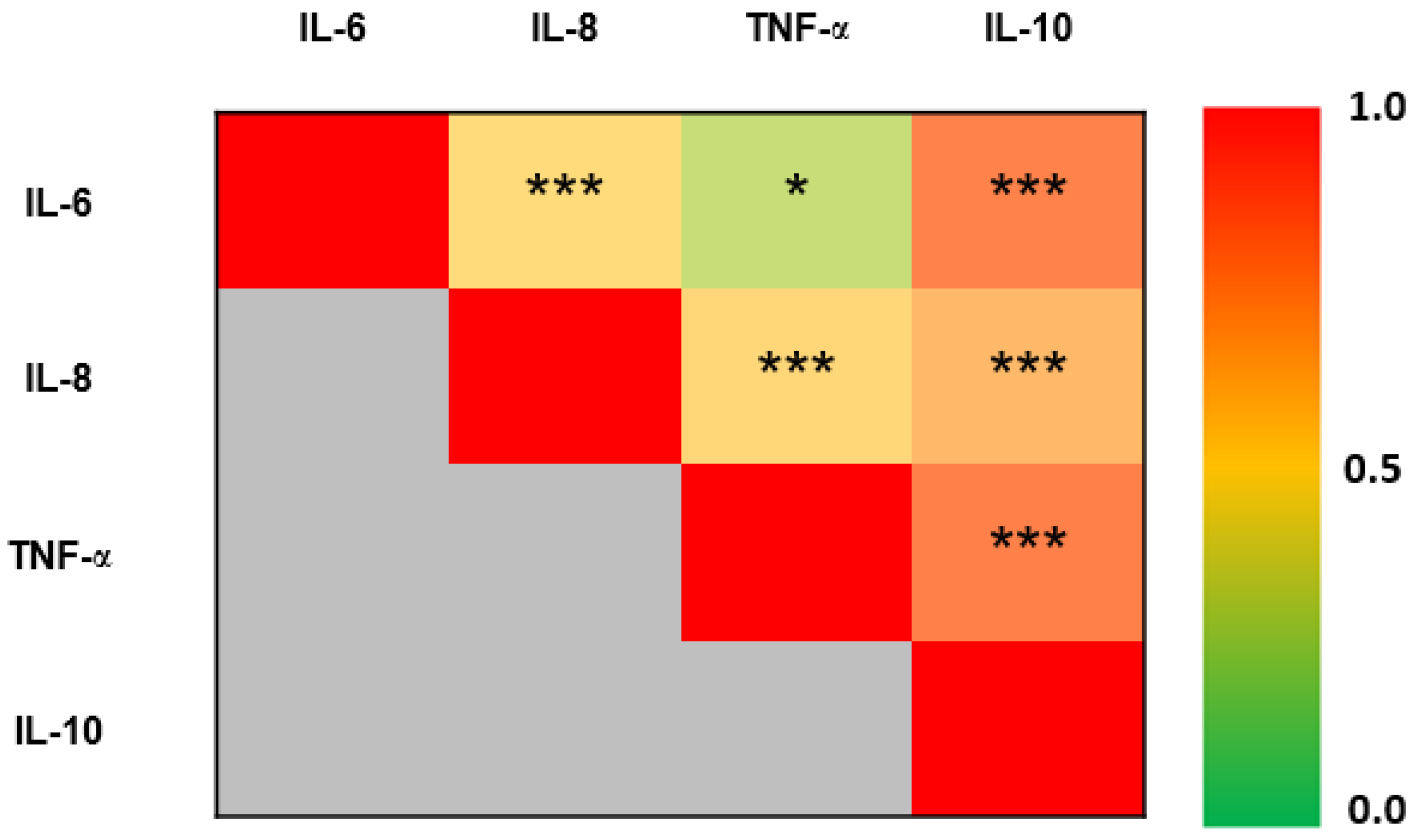
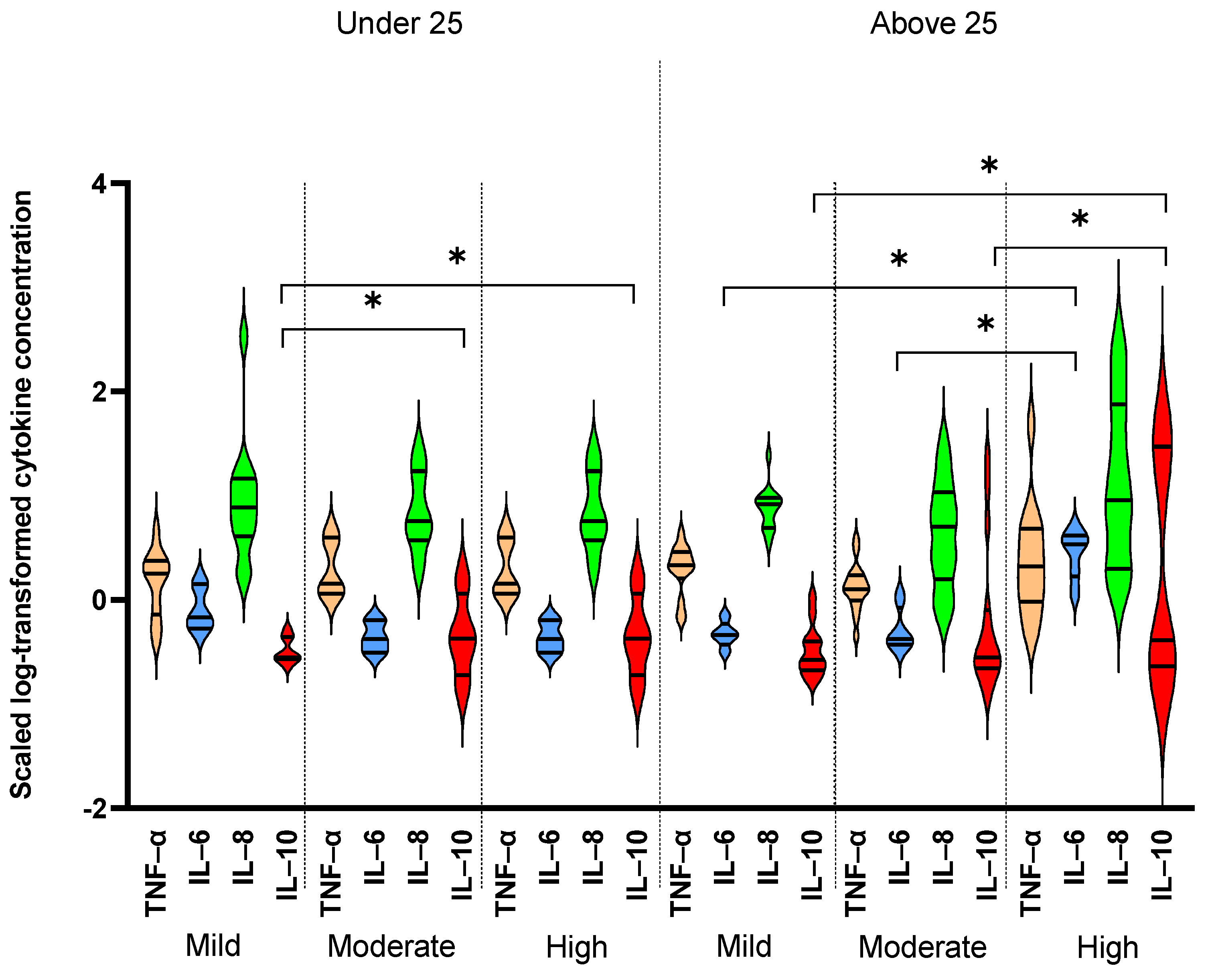
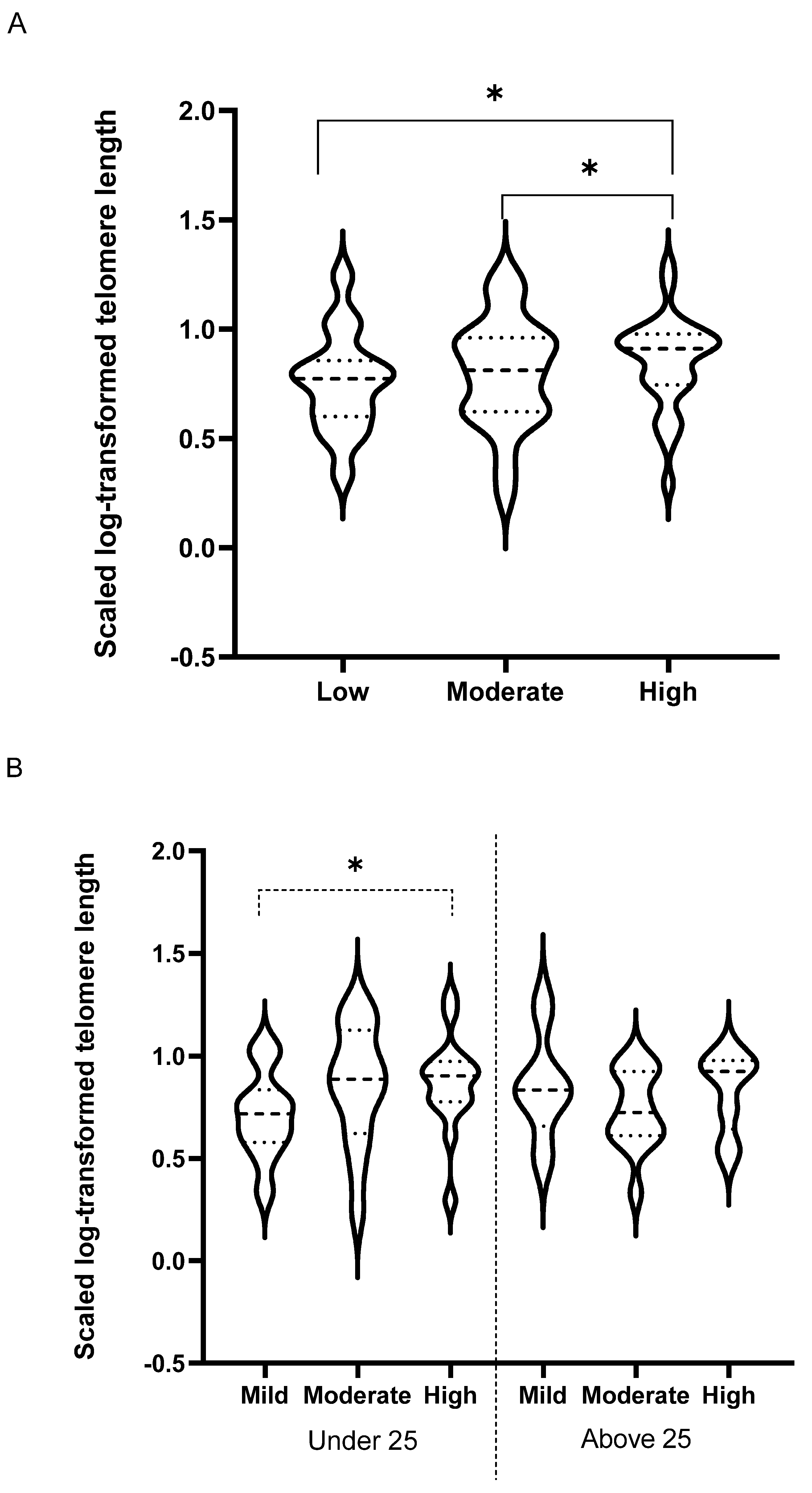
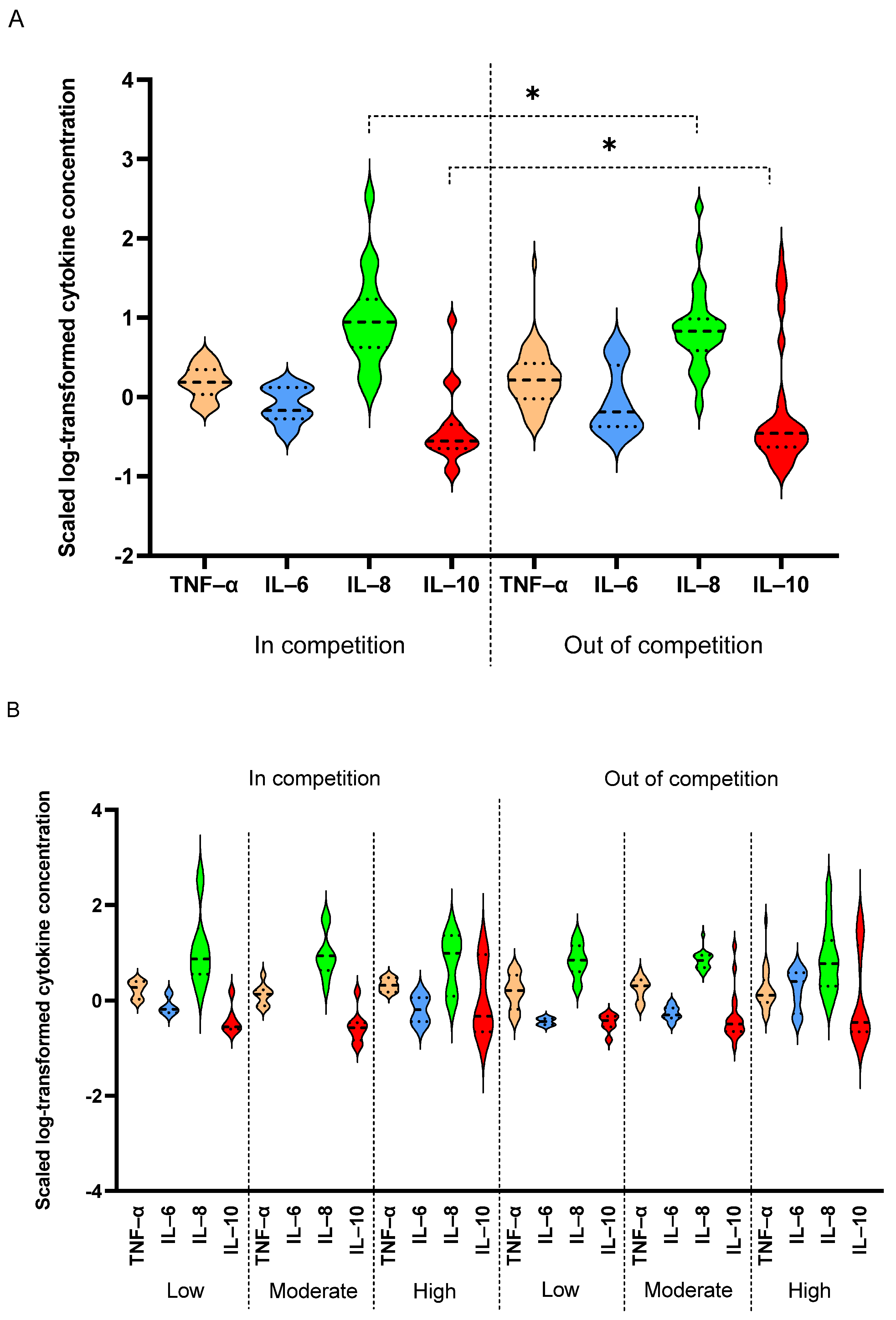
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rea, I.M.; Gibson, D.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, A.L.; Silva, L.C.; Fernandes, J.R.; Benard, G. Preventing or reversing immunosenescence: Can exercise be an immunotherapy? Immunotherapy 2013, 5, 879–893. [Google Scholar] [CrossRef] [Green Version]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Søreide, K. Molecular and biological hallmarks of ageing. BJS 2016, 103, e29–e46. [Google Scholar] [CrossRef] [Green Version]
- Viña, A.G.J.; Viña, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardó, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; et al. Mechanism of Free Radical Production in Exhaustive Exercise in Humans and Rats; Role of Xanthine Oxidase and Protection by Allopurinol. IUBMB Life 2000, 49, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Jackson, J.R.; Hao, Y.; Leonard, S.S.; Alway, S.E. Inhibition of xanthine oxidase reduces oxidative stress and improves skeletal muscle function in response to electrically stimulated isometric contractions in aged mice. Free Radic. Biol. Med. 2011, 51, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Wadley, A.; Chen, Y.-W.; Lip, G.Y.; Fisher, J.P.; Aldred, S. Low volume–high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J. Sports Sci. 2015, 34, 1–9. [Google Scholar] [CrossRef]
- Mullins, A.L.; Van Rosendal, S.P.; Briskey, D.R.; Fassett, R.G.; Wilson, G.R.; Coombes, J.S. Variability in oxidative stress biomarkers following a maximal exercise test. Biomarkers 2013, 18, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Flynn, M.G.; Campbell, W.W.; Craig, B.A.; Robinson, J.; Timmerman, K.L.; Mcfarlin, B.K.; Coen, P.; Talbert, E. The Influence of Exercise Training on Inflammatory Cytokines and C-Reactive Protein. Med. Sci. Sports Exerc. 2007, 39, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.C.; Simtchouk, S.; Durrer, C.; Jung, M.E.; Mui, A.L.; Little, J.P. Short-term exercise training reduces anti-inflammatory action of interleukin-10 in adults with obesity. Cytokine 2018, 111, 460–469. [Google Scholar] [CrossRef]
- Sohail, M.U.; Al-Mansoori, L.; Al-Jaber, H.; Georgakopoulos, C.; Donati, F.; Botrè, F.; Sellami, M.; Elrayess, M.A. Assessment of Serum Cytokines and Oxidative Stress Markers in Elite Athletes Reveals Unique Profiles Associated with Different Sport Disciplines. Front. Physiol. 2020, 11, 600888. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Balan, E.; Decottignies, A.; Deldicque, L. Physical Activity and Nutrition: Two Promising Strategies for Telomere Maintenance? Nutrition 2018, 10, 1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.; Zuo, L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [Green Version]
- Semeraro, M.D.; Smith, C.; Kaiser, M.; Levinger, I.; Duque, G.; Gruber, H.-J.; Herrmann, M. Physical activity, a modulator of aging through effects on telomere biology. Aging 2020, 12, 13803–13823. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, R. The immune system in sport: Getting the balance right. Br. J. Sports Med. 2000, 34, 161. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: Classification of sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef] [Green Version]
- Paolisso, G.; Rizzo, M.R.; Mazziotti, G.; Tagliamonte, M.R.; Gambardella, A.; Rotondi, M.; Carella, C.; Giugliano, D.; Varricchio, M.; D’Onofrio, F. Advancing age and insulin resistance: Role of plasma tumor necrosis factor-α. Am. J. Physiol. Metab. 1998, 275, E294–E299. [Google Scholar] [CrossRef]
- Hager, K.; Machein, U.; Krieger, S.; Platt, D.; Seefried, G.; Bauer, J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol. Aging 1994, 15, 771–772. [Google Scholar] [CrossRef]
- Lupertz, R.; Chovolou, Y.; Kampkotter, A.; Watjen, W.; Kahl, R. Catalase overexpression impairs TNF-alpha induced NF-kappaB activation and sensitizes MCF-7 cells against TNF-alpha. J. Cell Biochem. 2008, 103, 1497–1511. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Exercise and interleukin-6. Curr. Opin. Hematol. 2001, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.A.; Callister, R.; Taylor, R.D.; Sibbritt, D.W.; MacDonald-Wicks, L.K.; Garg, M.L. Antioxidant Restriction and Oxidative Stress in Short-Duration Exhaustive Exercise. Med. Sci. Sports Exerc. 2005, 37, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Vincent, H.K.; Morgan, J.W.; Vincent, K.R. Obesity Exacerbates Oxidative Stress Levels after Acute Exercise. Med. Sci. Sports Exerc. 2004, 36, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Rivier, A.; Pène, J.; Chanez, P.; Anselme, F.; Caillaud, C.; Prefaut, C.; Godard, P.; Bousquet, J. Release of Cytokines by Blood Monocytes During Strenuous Exercise. Int. J. Sports Med. 1994, 15, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, R.G. Are we becoming less disposable? EMBO Rep. 2004, 5, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, G.; Sagui, E.; Fabien, Z.; Martin-Krumm, C.; Canini, F.; Trousselard, M. Anxiety and Psycho-Physiological Stress Response to Competitive Sport Exercise. Front. Psychol. 2018, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J.S. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.; Thomas, A.W.; Webb, R.; Hughes, M.G. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: The effect of exercise intensity and volume. Appl. Physiol. Nutr. Metab. 2016, 41, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Konrad, M.; Henson, D.A.; Kennerly, K.; Shanely, R.A.; Wallner-Liebmann, S.J. Variance in the acute in-flammatory response to prolonged cycling is linked to exercise intensity. J. Interferon Cytokine Res. 2012, 32, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.P.R.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. Effect of Exercise Intensity on the Cytokine Response to an Acute Bout of Running. Med. Sci. Sports Exerc. 2011, 43, 2297–2306. [Google Scholar] [CrossRef]
- Rapoport, B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010, 6, e1000960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef]
- Glund, S.; Deshmukh, A.; Long, Y.C.; Moller, T.; Koistinen, H.A.; Caidahl, K.; Zierath, J.; Krook, A. Interleukin-6 Directly Increases Glucose Metabolism in Resting Human Skeletal Muscle. Diabetes 2007, 56, 1630–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, D.A.; Rocha-Vieira, E.; Soares, B.A.; Nonato, L.F.; Fonseca, S.R.; Martins, J.B.; Mendonça, V.A.; Lacerda, A.C.; Massensini, A.R.; Poortamns, J.R.; et al. High intensity interval training modulates hippocampal oxidative stress, BDNF and inflammatory mediators in rats. Physiol. Behav. 2018, 184, 6–11. [Google Scholar] [CrossRef]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef]
- Bönig, H.; Packeisen, J.; Röhne, B.; Hempel, L.; Hannen, M.; Klein-Vehne, A.; Burdach, S.; Körholz, D. Interaction Between Interleukin 10 and Interleukin 6 in Human B-Cell Differentiation. Immunol. Investig. 1998, 27, 267–280. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Borghini, A.; Giardini, G.; Tonacci, A.; Mastorci, F.; Mercuri, A.; Sposta, S.M.; Moretti, S.; Andreassi, M.G.; Pratali, L. Chronic and acute effects of endurance training on telomere length. Mutagenesis 2015, 30, 711–716. [Google Scholar] [CrossRef]
- Ludlow, A.T.; Roth, S.M. Physical Activity and Telomere Biology: Exploring the Link with Aging-Related Disease Prevention. J. Aging Res. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniesa, C.A.; Verde, Z.; Diaz-Ureña, G.; Santiago, C.; Gutiérrez, F.; Díaz, E.; Gómez-Gallego, F.; Pareja-Galeano, H.; Soares-Miranda, L.; Lucia, A. Telomere Length in Elite Athletes. Int. J. Sports Physiol. Perform. 2017, 12, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Loenneke, J.P. Leukocyte telomere length and mortality among U.S. adults: Effect modification by physical activity behaviour. J. Sports Sci. 2018, 36, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A. Physical activity and telomere length in U.S. men and women: An NHANES investigation. Prev. Med. 2017, 100, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Prescott, J.; Kraft, P.; Han, J.; Giovannucci, E.; Hankinson, S.E.; de Vivo, I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am. J. Epidemiol. 2012, 175, 414–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saghebjoo, M.; Sadeghi-Tabas, S.; Saffari, I.; Ghane, A.; Dimauro, I. Sex Differences in antiaging response to short- and long-term high-intensity interval exercise in rat cardiac muscle: Telomerase activity, total antioxidant/oxidant status. Chin. J. Physiol. 2019, 62, 261–266. [Google Scholar] [CrossRef]
- Caire-Juvera, G.; Navarro-Ibarra, M.J.; Hernández, J. Diet, physical activity and telomere length in adults. Nutr. Hosp. 2019, 36, 1403–1417. [Google Scholar] [CrossRef] [Green Version]
- Abrahin, O.; Cortinhas-Alves, E.A.; Vieira, R.P.; Guerreiro, J.F. Elite athletes have longer telomeres than sedentary subjects: A meta-analysis. Exp. Gerontol. 2019, 119, 138–145. [Google Scholar] [CrossRef]
- Østhus, I.B.Ø.; Sgura, A.; Berardinelli, F.; Alsnes, I.V.; Brønstad, E.; Rehn, T.; Støbakk, P.K.; Hatle, H.; Wisløff, U.; Nauman, J. Telomere Length and Long-Term Endurance Exercise: Does Exercise Training Affect Biological Age? A Pilot Study. PLoS ONE 2012, 7, e52769. [Google Scholar] [CrossRef]
- Simoes, H.G.; Rosa, T.S.; Sousa, C.V.; Aguiar, S.D.S.; Motta-Santos, D.; Degens, H.; Korhonen, M.T.; Campbell, C.S.G. Does Longer Leukocyte Telomere Length and Higher Physical Fitness Protect Master Athletes from Consequences of Coronavirus (SARS-CoV-2) Infection? Front. Sports Act Living 2020, 2, 87. [Google Scholar] [CrossRef]
- Aguiar, S.S.; Sousa, C.V.; Santos, P.A.; Barbosa, L.P.; Maciel, L.A.; Coelho-Júnior, H.J.; Motta-Santos, D.; Rosa, T.S.; Degens, H.; Simões, H.G. Master athletes have longer telomeres than age-matched non-athletes. A systematic review, meta-analysis and discussion of possible mechanisms. Exp. Gerontol. 2021, 146, 111212. [Google Scholar] [CrossRef]
- Mastaloudis, A.; Leonard, S.; Traber, M. Oxidative stress in athletes during extreme endurance exercise. Free. Radic. Biol. Med. 2001, 31, 911–922. [Google Scholar] [CrossRef]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2019, 40, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.-A.; Lee, J.-H.; Song, W.; Jun, T.-W. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech. Ageing Dev. 2008, 129, 254–260. [Google Scholar] [CrossRef]
- Varamenti, E.; Tod, D.; Pullinger, S.A. Redox Homeostasis and Inflammation Responses to Training in Adolescent Athletes: A Systematic Review and Meta-analysis. Sports Med. Open 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, R.; Chandrashekara, S.; VasanthaKumar, K. Cytokine response to strenuous exercise in athletes and non-athletes—An adaptive response. Cytokine 2007, 40, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Cooper, D.M.; Nemet, D. The GH-IGF-I Response to Typical Field Sports Practices in Adolescent Athletes: A Summary. Pediatr. Exerc. Sci. 2014, 26, 428–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Low Intensity (LI) | Moderate Intensity (MI) | High Intensity (HI) | |
|---|---|---|---|
| In Competition | 1 Cricket (M), 1 Equestrian (M), 1 Golf (M), 1 Powerboating (M), 1 Sport climbing (M) | 4 Football (4M) | 2 Cycling—track endurance (2M), 4 Cycling—cross (2M, 2F), 5 Triathlon (4M, 1F) |
| Out of Competition | 1 Athletics—throws (M), 2 Bobsleigh (1M, 1F), 2 Gymnastics (2F), 1 Luge (M), 2 Sport climbing (1M, 1F), 1 Volleyball (F), 4 Wrestling (4M) | 27 Football (26M) | 12 Athletics—long distance (11M, 1F), 4 Cycling—track endurance (2M, 2F), 2 Cycling—road (2M), 2 Triathlon (2M) |
| Cohort | Under 25 | Above 25 | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | All (n = 80) | Combined (n = 45) | Low (n = 12) | Moderate (n = 16) | High (n = 17) | Combined (n = 35) | Low (n = 6) | Moderate (n = 14) | High (n = 15) | Age (Combined) | Age (Intensity) | Intensity (Age) | Age × Intensity |
| Age (years) | 26.4 (7.6) | 21.0 (2.2) | 20.67 (2.02) | 22 (1.51) | 20.35 (2.52) | 33.5 (6) | 33.8 (9.5) | 33.2 (2.2) | 33.7 (7.3) | *** | *** | NS | NS |
| IL-6 (pg/mL) | 0.7 [0.4–1.4] | 0.7 [0.4–1.1] | 0.7 [0.5–0.7] | 0.9 [0.7–0.9] | 0.4 [0.4–0.9] | 0.7 [0.4–3.4] | 0.4 [0.3–0.4] | 0.5 [0.4–0.6] | 3.4 [1.7–4.2] | NS | NS | ** | *** |
| IL-8 (pg/mL) | 6.9 [3.9–11.7] | 6.4 [3.3–10.4] | 7.7 [4.1–14.6] | 6.7 [5.1–8.9] | 5 [1.6–11] | 8.1 [4.7–16.2] | 5.8 [3.9–17.6] | 8.3 [4.9–9.5] | 9.1 [2–75.6] | NS | NS | NS | * |
| TNF-α (pg/mL) | 1.6 [1.0–2.5] | 1.4 [0.9–2] | 1.8 [0.7–2.4] | 1.4 [0.9–2.1] | 1.3 [1–1.7] | 2.1 [1.1–3.3] | 1.4 [1.2–4] | 2.1 [1.7–2.9] | 2.1 [1–4.9] | ** | * | NS | NS |
| IL-10 (pg/mL) | 0.3 [0.2–0.5] | 0.3 [0.2–0.5] | 0.3 [0.3–0.4] | 0.3 [0.2–0.7] | 0.3 [0.2–0.8] | 0.4 [0.2–1.2] | 0.4 [0.2–1.3] | 0.3 [0.2–0.4] | 0.4 [0.2–29.7] | NS | NS | * | NS |
| TL length (kb) | 7.2 (4.0) | 7.5 (4.4) | 5 (3.1) | 8.6 (5) | 8.3 (4.3) | 6.7 (3.3) | 6.6 (5.7) | 6 (2.5) | 7.3 (2.7) | NS | NS | * | NS |
| SOD (U/mL) | 2.5 (0.1) | 2.5 (0.07) | 2.5 (0.1) | 2.6 (0.1) | 2.5 (0.1) | 2.5 (0.05) | 2.6 (0.03) | 2.5 (0.05) | 2.5 (0.05) | NS | NS | NS | NS |
| Catalase (U/mL) | 0.2 (0.1) | 0.16 (0.12) | 0.2 (0.2) | 0.1 (0.1) | 0.2 (0.1) | 0.18 (0.14) | 0.2 (0.2) | 0.2 (0.08) | 0.2 (0.2) | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellami, M.; Al-muraikhy, S.; Al-Jaber, H.; Al-Amri, H.; Al-Mansoori, L.; Mazloum, N.A.; Donati, F.; Botre, F.; Elrayess, M.A. Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes. Antioxidants 2021, 10, 1035. https://doi.org/10.3390/antiox10071035
Sellami M, Al-muraikhy S, Al-Jaber H, Al-Amri H, Al-Mansoori L, Mazloum NA, Donati F, Botre F, Elrayess MA. Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes. Antioxidants. 2021; 10(7):1035. https://doi.org/10.3390/antiox10071035
Chicago/Turabian StyleSellami, Maha, Shamma Al-muraikhy, Hend Al-Jaber, Hadaia Al-Amri, Layla Al-Mansoori, Nayef A. Mazloum, Francesco Donati, Francesco Botre, and Mohamed A. Elrayess. 2021. "Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes" Antioxidants 10, no. 7: 1035. https://doi.org/10.3390/antiox10071035
APA StyleSellami, M., Al-muraikhy, S., Al-Jaber, H., Al-Amri, H., Al-Mansoori, L., Mazloum, N. A., Donati, F., Botre, F., & Elrayess, M. A. (2021). Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes. Antioxidants, 10(7), 1035. https://doi.org/10.3390/antiox10071035