Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Experimental Groups
- Control: rats were injected intraperitoneally with saline;
- Control + Hericium erinaceus: rats were injected intraperitoneally with saline, and Hericium erinaceus (200 mg/kg) was administered daily orally by gavage;
- AD group: rats were injected intraperitoneally with AlCl3 as already described;
- AD group + Hericium erinaceus: rats were injected intraperitoneally with AlCl3 as already described, and Hericium erinaceus (200 mg/kg) was administered daily orally by gavage.
2.4. Behavioral Assessment
2.4.1. Morris Water Maze (MWM)
2.4.2. Elevated Plus Maze (EPM)
2.4.3. Novel Object Recognition (NOR)
2.5. Histological Analysis
2.6. Western Blot Analysis
2.7. Biochemical Analysis
2.7.1. Measurement of SOD Activity
2.7.2. Measurement of CAT Activity
2.7.3. Determination of GSH Levels
2.7.4. Measurement of Nitrite Levels
2.7.5. Measurement of Lipid Peroxidation
2.7.6. Measurement of Reactive Oxygen Species
2.8. Cytokines Measurement
2.9. Statistical Evaluation
3. Results
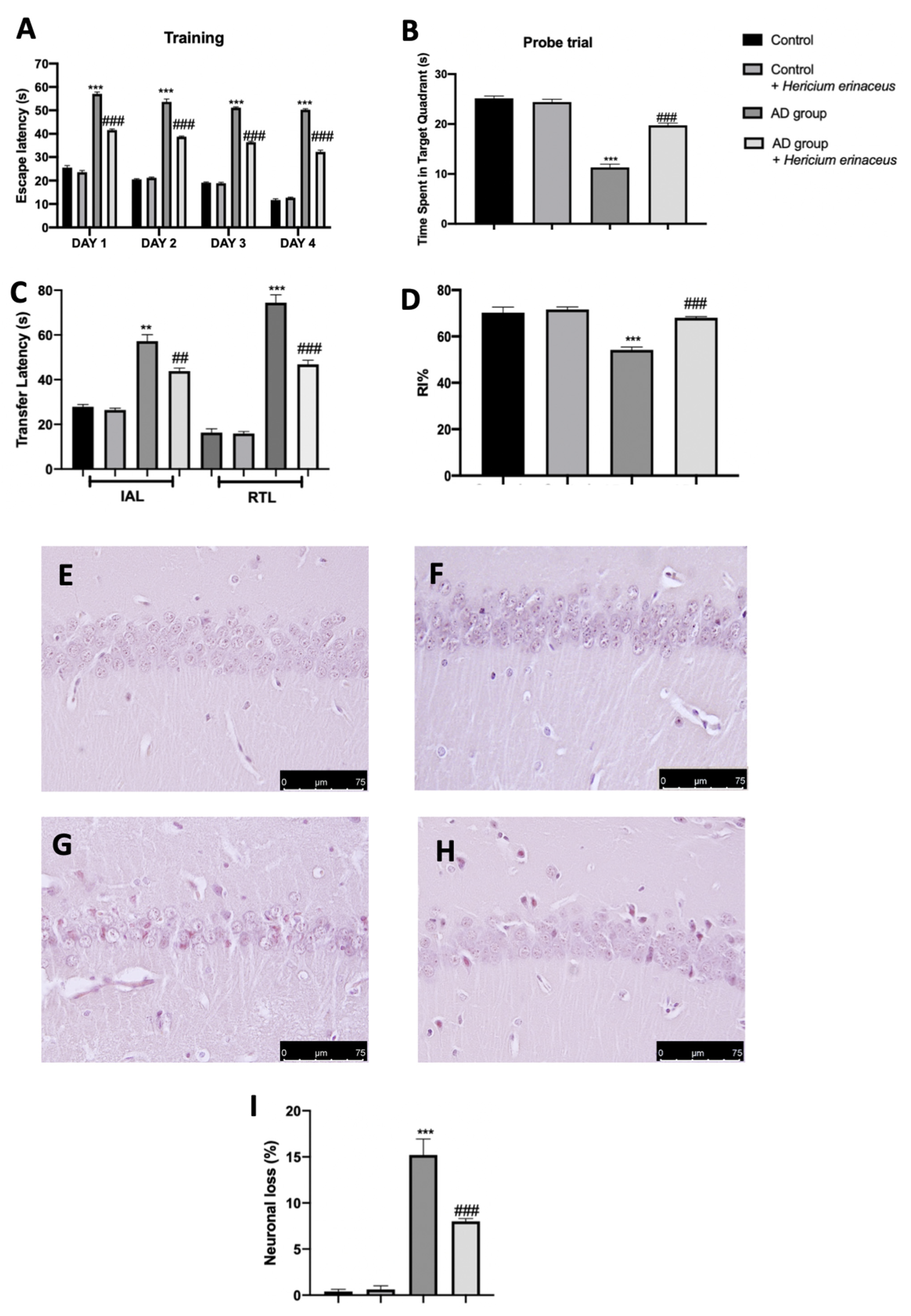
3.1. Effects of Hericium erinaceus Treatment on Behavioral and Histological Alterations
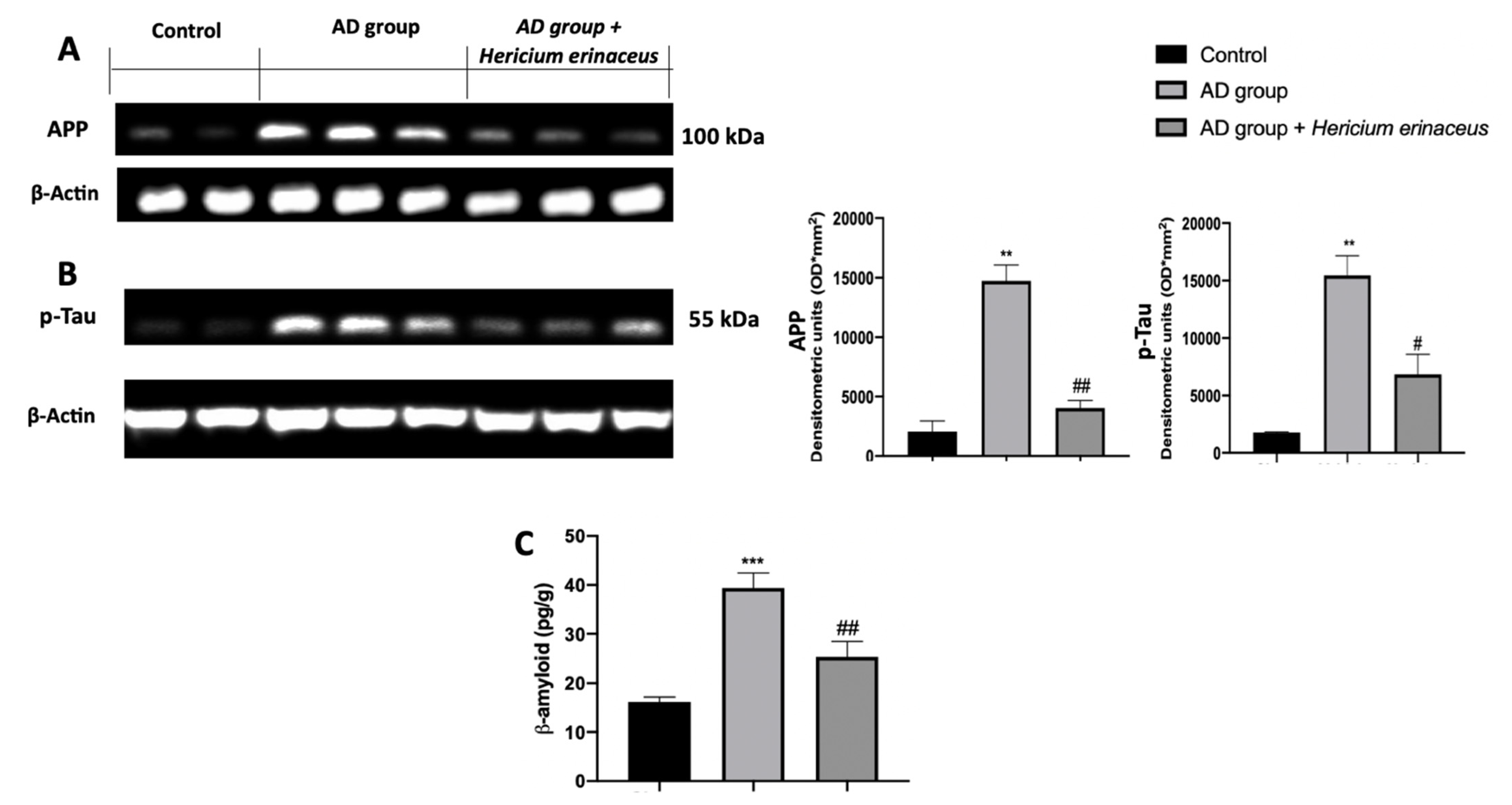
3.2. Effects of Hericium erinaceus Treatment on APP and p-Tau Over-Expression and β-Amyloid Accumulation
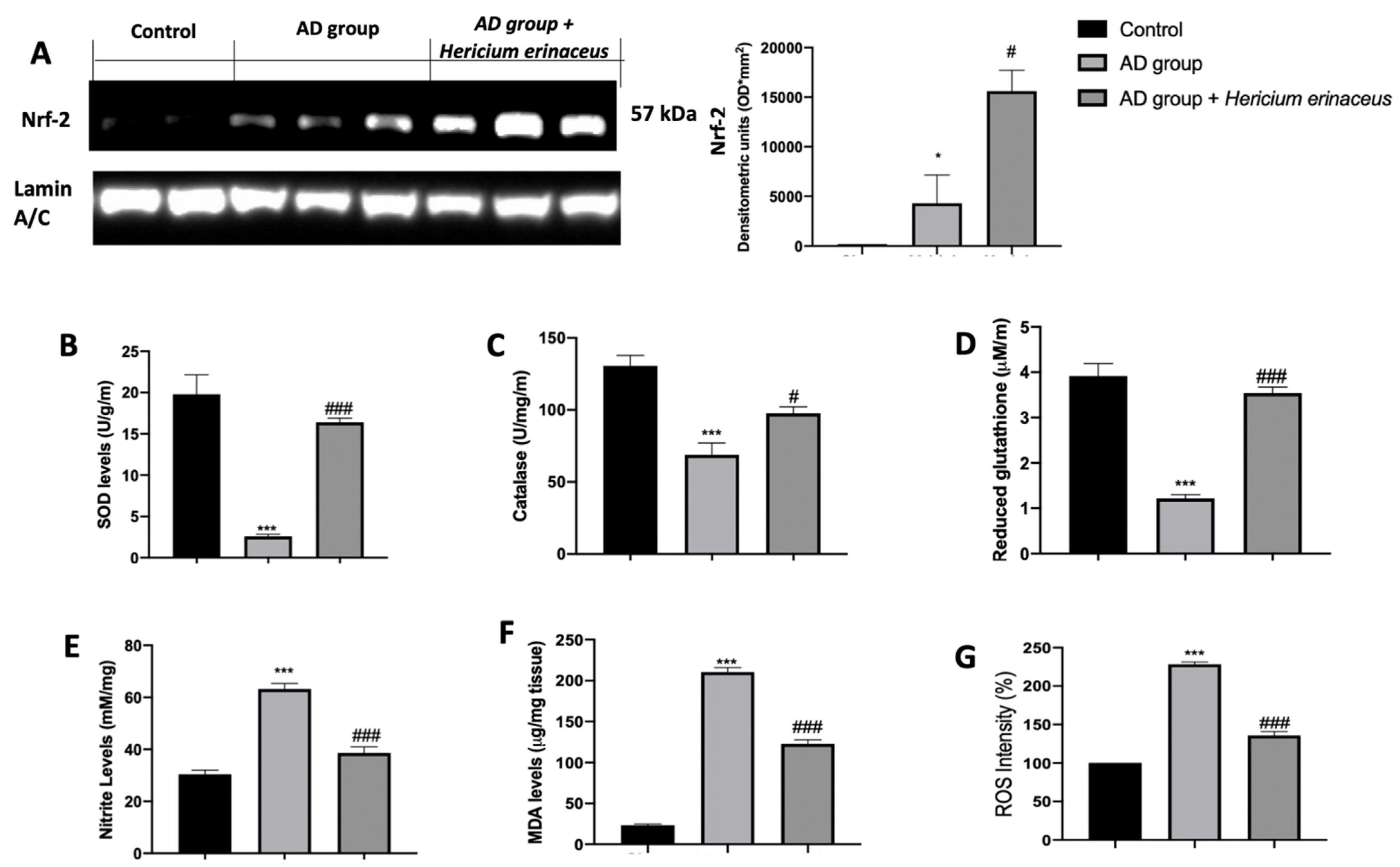
3.3. Effects of Hericium erinaceus Treatment on Oxidative Hippocampal Modifications
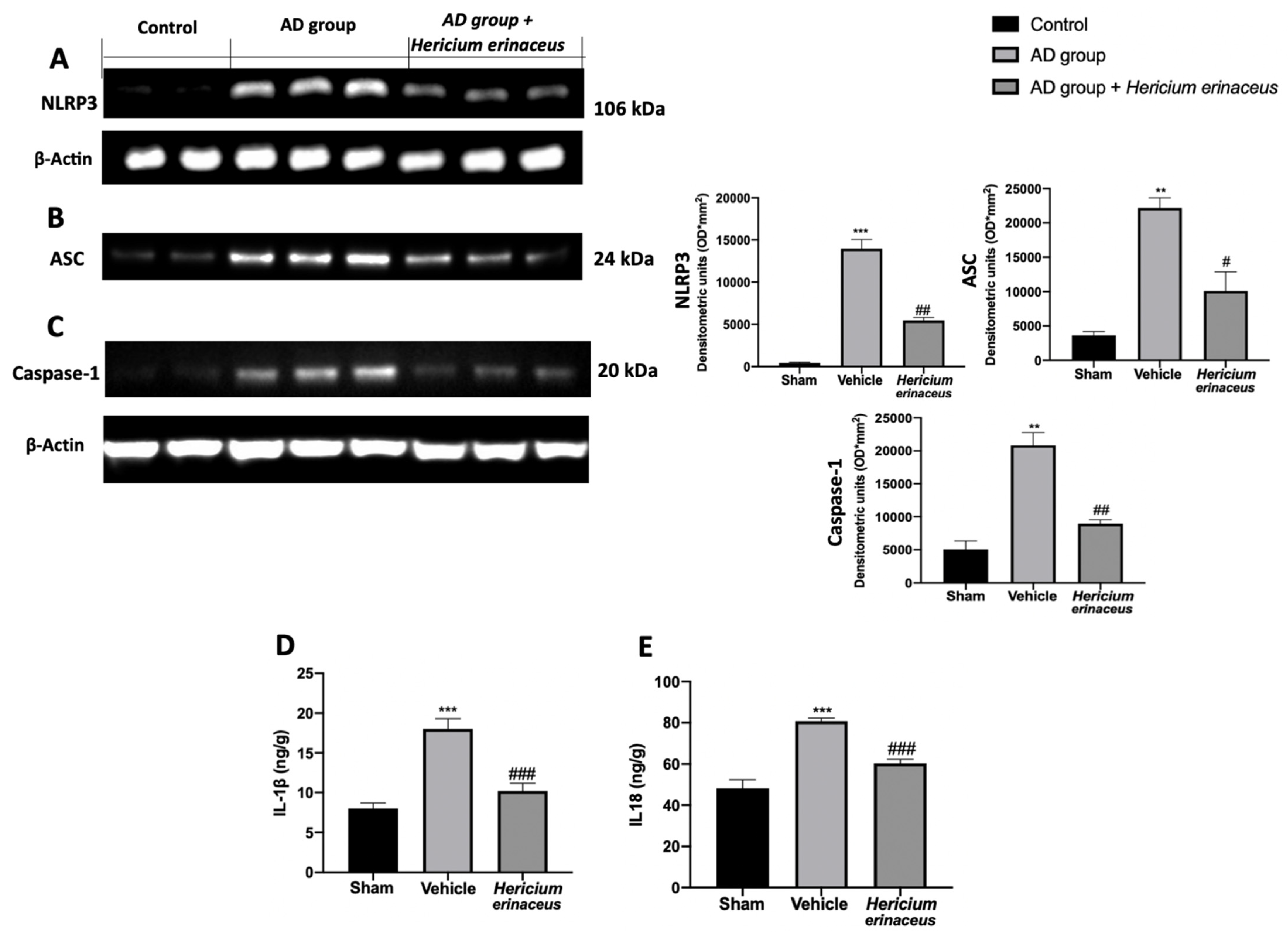
3.4. Effects of Hericium erinaceus Treatment on NLRP3 Inflammasome Activation
3.5. Effects of Hericium erinaceus Treatment on Pro-Inflammatory Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziegler-Graham, K.; Brookmeyer, R.; Johnson, E.; Arrighi, H.M. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimers Dement. 2008, 4, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.R.; Wang, M.X. APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer’s disease. J. Inorg. Biochem. 2009, 103, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Karlawish, J.H.; Caine, E.D. Current state of research on decision-making competence of cognitively impaired elderly persons. Am. J. Geriatr. Psychiatry 2002, 10, 151–165. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Zilka, N.; Filipcik, P.; Koson, P.; Fialova, L.; Skrabana, R.; Zilkova, M.; Rolkova, G.; Kontsekova, E.; Novak, M. Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006, 580, 3582–3588. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Lavados, M.; Guillon, M.; Mujica, C.; Bosch, R.; Farias, G.; Fuentes, P. Anomalously phosphorylated tau and Abeta fragments in the CSF correlates with cognitive impairment in MCI subjects. Neurobiol. Aging 2006, 27, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, L.; Eriksdotter Jonhagen, M.; Kilander, L.; Soininen, H.; Hallikainen, M.; Waldemar, G.; Nygaard, H.; Andreasen, N.; Winblad, B.; Wimo, A. Determinants of costs of care for patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 449–459. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium Erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 13, 23. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xiong, S.; Xie, C.; Davies, P.; Markesbery, W.R. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J. Alzheimers Dis. 2004, 6, 659–671; discussion 673–681. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H. Pinpointing plaques with PIB. Nat. Med. 2006, 12, 753–754; discussion 754. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med. J. 2016, 57, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Fusco, R.; Ginestra, G.; D’Amico, R.; Bisignano, C.; Mandalari, G.; Cuzzocrea, S.; Di Paola, R. Involvement of TLR4 and PPAR-alpha Receptors in Host Response and NLRP3 Inflammasome Activation, Against Pulmonary Infection With Pseudomonas Aeruginosa. Shock 2019, 51, 221–227. [Google Scholar] [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing 2018, 15, 8. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Biundo, F.; Campolo, M.; Di Paola, R.; Cuzzocrea, S. Inhibition of inflammasome activation improves lung acute injury induced by carrageenan in a mouse model of pleurisy. FASEB J. 2017, 31, 3497–3511. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N.; Dahlen, S.E.; Drazen, J.M.; Hay, D.W.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharm. Rev. 2006, 58, 463–487. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- Liang, B.; Guo, Z.; Xie, F.; Zhao, A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013, 13, 253. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmed, H.I.; Abu-Elfotuh, K. Modeling stages mimic Alzheimer’s disease induced by different doses of aluminum in rats: Focus on progression of the disease in response to time. of 2016, 11, 2. [Google Scholar]
- D’Amico, R.; Trovato Salinaro, A.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Lo Dico, G.; Cuzzocrea, S.; Di Paola, R.; et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants 2021, 10, 898. [Google Scholar] [CrossRef]
- Mahmoodzadeh, T.; Kashani, M.H.K.; Ramshini, H.; Moslem, A.; Mohammad-Zadeh, M. Effect of Camellia sinensis on Spatial Memory in a Rat Model of Alzheimer’s Disease. J. Biomed. 2016, 1, e5340. [Google Scholar] [CrossRef]
- Mutlu, O.; Akar, F.; Celikyurt, I.K.; Tanyeri, P.; Ulak, G.; Erden, F. 7-NI and ODQ Disturbs Memory in the Elevated Plus Maze, Morris Water Maze, and Radial Arm Maze Tests in Mice. Drug Targets Insights 2015, 9, 1–8. [Google Scholar] [CrossRef]
- Chavali, V.D.; Agarwal, M.; Vyas, V.K.; Saxena, B. Neuroprotective Effects of Ethyl Pyruvate against Aluminum Chloride-Induced Alzheimer’s Disease in Rats via Inhibiting Toll-Like Receptor 4. J. Mol. Neurosci. 2020, 70, 836–850. [Google Scholar] [CrossRef]
- Mudò, G.; Frinchi, M.; Nuzzo, D.; Scaduto, P.; Plescia, F.; Massenti, M.F.; Di Carlo, M.; Cannizzaro, C.; Cassata, G.; Cicero, L. Anti-inflammatory and cognitive effects of interferon-β1a (IFNβ1a) in a rat model of Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Neuroprotective Effect of Artesunate in Experimental Model of Traumatic Brain Injury. Front. Neurol. 2018, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, W.; Xu, F.; Cao, Z.; Jia, F.; Li, Y. Iron Dyshomeostasis Participated in Rat Hippocampus Toxicity Caused by Aluminum Chloride. Biol. Trace Elem. Res. 2020, 197, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; D’Amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment With a Flavonoid-Rich Fraction of Bergamot Juice Improved Lipopolysaccharide-Induced Periodontitis in Rats. Front. Pharm. 2018, 9, 1563. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Cordaro, M.; Crupi, R.; Siracusa, R.; Campolo, M.; Bruschetta, G.; Fusco, R.; Pugliatti, P.; Esposito, E.; Cuzzocrea, S. Protective Effects of Ultramicronized Palmitoylethanolamide (PEA-um) in Myocardial Ischaemia and Reperfusion Injury in VIVO. Shock 2016, 46, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.G.; Frinchi, M.; Nuzzo, D.; Jinnah, H.A.; Mudo, G.; Condorelli, D.F.; Caciagli, F.; Ciccarelli, R.; Di Iorio, P.; Mule, F.; et al. Altered gastrointestinal motility in an animal model of Lesch-Nyhan disease. Auton. Neurosci. 2018, 210, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox((R)) Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wu, W.; Xu, T.; Yin, Y.; Zhang, J.; Huang, D.; Li, W. Chronic glucocorticoid exposure activates BK-NLRP1 signal involving in hippocampal neuron damage. J. Neuroinflamm. 2017, 14, 139. [Google Scholar] [CrossRef]
- Mufson, E.J.; Chen, E.Y.; Cochran, E.J.; Beckett, L.A.; Bennett, D.A.; Kordower, J.H. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp. Neurol. 1999, 158, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R.; Schmitt, F.A.; Kryscio, R.J.; Davis, D.G.; Smith, C.D.; Wekstein, D.R. Neuropathologic substrate of mild cognitive impairment. Arch. Neurol. 2006, 63, 38–46. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L.; Crain, B.J.; Pletnikova, O.; Rudow, G.; Iacono, D.; Riudavets, M.A.; Driscoll, I.; et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA). J. Alzheimers Dis. 2009, 18, 665–675. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Stancu, I.C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brone, B.; Huaux, F.; Octave, J.N.; et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Mao, P.; Calkins, M.J.; Reddy, A.P.; Shirendeb, U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: Implications for synaptic damage and cognitive decline. J. Alzheimers Dis. 2010, 20 (Suppl S2), S499–S512. [Google Scholar] [CrossRef]
- Zhao, J.; O’Connor, T.; Vassar, R. The contribution of activated astrocytes to Abeta production: Implications for Alzheimer’s disease pathogenesis. J. Neuroinflamm. 2011, 8, 150. [Google Scholar] [CrossRef]
- Scoville, W.B. The limbic lobe in man. J. Neurosurg. 1954, 11, 64–66. [Google Scholar] [CrossRef]
- Amaral, D.G.; Witter, M.P. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 1989, 31, 571–591. [Google Scholar] [CrossRef]
- Nakashiba, T.; Young, J.Z.; McHugh, T.J.; Buhl, D.L.; Tonegawa, S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 2008, 319, 1260–1264. [Google Scholar] [CrossRef]
- Sethi, P.; Jyoti, A.; Singh, R.; Hussain, E.; Sharma, D. Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. Neurotoxicology 2008, 29, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Moumen, R.; Ait-Oukhatar, N.; Bureau, F.; Fleury, C.; Bougle, D.; Arhan, P.; Neuville, D.; Viader, F. Aluminium increases xanthine oxidase activity and disturbs antioxidant status in the rat. J. Trace Elem. Med. Biol. 2001, 15, 89–93. [Google Scholar] [CrossRef]
- Bondy, S.C.; Guo-Ross, S.X.; Truong, A.T. Promotion of transition metal-induced reactive oxygen species formation by beta-amyloid. Brain Res. 1998, 799, 91–96. [Google Scholar] [CrossRef]
- Becaria, A.; Bondy, S.C.; Campbell, A. Aluminum and copper interact in the promotion of oxidative but not inflammatory events: Implications for Alzheimer’s disease. J. Alzheimers Dis. 2003, 5, 31–38. [Google Scholar] [CrossRef]
- Niu, P.Y.; Niu, Q.; Zhang, Q.L.; Wang, L.P.; He, S.E.; Wu, T.C.; Conti, P.; Di Gioacchino, M.; Boscolo, P. Aluminum impairs rat neural cell mitochondria in vitro. Int. J. Immunopathol. Pharm. 2005, 18, 683–689. [Google Scholar] [CrossRef]
- Kumar, V.; Bal, A.; Gill, K.D. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Res. 2008, 1232, 94–103. [Google Scholar] [CrossRef]
- Kumar, V.; Bal, A.; Gill, K.D. Susceptibility of mitochondrial superoxide dismutase to aluminium induced oxidative damage. Toxicology 2009, 255, 117–123. [Google Scholar] [CrossRef]
- Starke, R.M.; Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr. Neurovasc. Res. 2013, 10, 247–255. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Limon-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Campolo, M.; Latteri, S.; Carughi, A.; Mandalari, G.; Cuzzocrea, S. The Antioxidant Activity of Pistachios Reduces Cardiac Tissue Injury of Acute Ischemia/Reperfusion (I/R) in Diabetic Streptozotocin (STZ)-Induced Hyperglycaemic Rats. Front. Pharm. 2018, 9, 51. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef]
- Travelli, C.; Aprile, S.; Rahimian, R.; Grolla, A.A.; Rogati, F.; Bertolotti, M.; Malagnino, F.; Di Paola, R.; Impellizzeri, D.; Fusco, R.; et al. Identification of Novel Triazole-Based Nicotinamide Phosphoribosyltransferase (NAMPT) Inhibitors Endowed with Antiproliferative and Antiinflammatory Activity. J. Med. Chem. 2017, 60, 1768–1792. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’ Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Gugliandolo, E.; Campolo, M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. Effect of a new formulation of micronized and ultramicronized N-palmitoylethanolamine in a tibia fracture mouse model of complex regional pain syndrome. PLoS ONE 2017, 12, e0178553. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants 2021, 10, 1664. https://doi.org/10.3390/antiox10111664
Cordaro M, Salinaro AT, Siracusa R, D’Amico R, Impellizzeri D, Scuto M, Ontario ML, Cuzzocrea S, Di Paola R, Fusco R, et al. Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants. 2021; 10(11):1664. https://doi.org/10.3390/antiox10111664
Chicago/Turabian StyleCordaro, Marika, Angela Trovato Salinaro, Rosalba Siracusa, Ramona D’Amico, Daniela Impellizzeri, Maria Scuto, Maria Laura Ontario, Salvatore Cuzzocrea, Rosanna Di Paola, Roberta Fusco, and et al. 2021. "Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease" Antioxidants 10, no. 11: 1664. https://doi.org/10.3390/antiox10111664
APA StyleCordaro, M., Salinaro, A. T., Siracusa, R., D’Amico, R., Impellizzeri, D., Scuto, M., Ontario, M. L., Cuzzocrea, S., Di Paola, R., Fusco, R., & Calabrese, V. (2021). Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants, 10(11), 1664. https://doi.org/10.3390/antiox10111664












