(−)-Epicatechin-Enriched Extract from Camellia sinensis Improves Regulation of Muscle Mass and Function: Results from a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquid Chromatography Analysis and Radical-Scavenging Assay
2.3. Subjects and Study Design
2.4. Analysis of Muscle-Related Factors
2.5. Statistical Analyses
3. Results
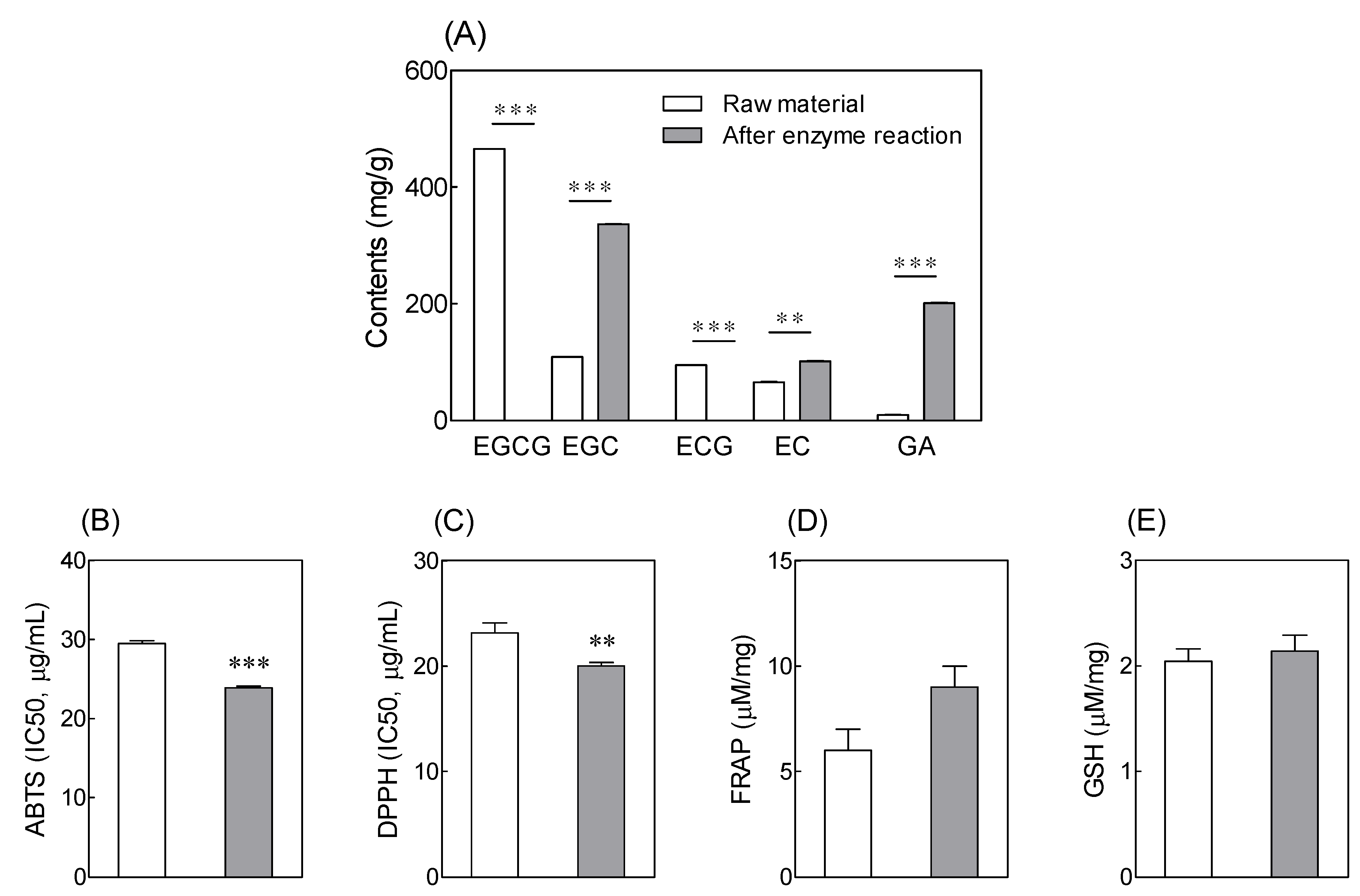
3.1. Catechin Content and Antioxidant Activity of Tannase-Treated Green Tea Extract
3.2. Effects of Tannase-Treated Green Tea Extract on Isokinetic Muscular Strength
3.3. Effects of Tannase-Treated Green Tea Extract on Grip Strength
3.4. Effects of Tannase-Treated Green Tea Extract on Body Muscle Mass
3.5. Effects of Tannase-Treated Green Tea Extract on Levels of Follistatin and Myostatin
3.6. Safety Parameters and Adverse Events of Administration of Tannase-Treated Green Tea Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate Supplementation Accelerates Recovery of Muscle Damage and Soreness and Inflammatory Markers after a Weightlifting Training Session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colon, C.J.P.; Molina-Vicenty, I.L.; Frontera-Rodriguez, M.; Garcia-Ferre, A.; Rivera, B.P.; Cintron-Velez, G.; Frontera-Rodriguez, S. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J. Biomed. 2018, 3, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Taaffe, D.R.; Marcus, R. Musculoskeletal health and the older adult. J. Rehabilit. Res. Dev. 2000, 37, 245–254. [Google Scholar]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Fang, Q.; Xu, T.; Wu, C.; Xu, L.; Wang, L.; Yang, X.; Yu, S.; Zhang, Q.; Ding, F.; et al. Mechanistic Role of Reactive Oxygen Species and Therapeutic Potential of Antioxidants in Denervation- or Fasting-Induced Skeletal Muscle Atrophy. Front. Physiol. 2018, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Smuder, A.J.; Judge, A.R. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Broome, S.C.; Woodhead, J.S.T.; Merry, T.L. Mitochondria-Targeted Antioxidants and Skeletal Muscle Function. Antioxidants 2018, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Vastag, D. Flavonoids and phenolic acids as potential natural antioxidants. In Antioxidants; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Pabón-Baquero, L.C.; Otálvaro-Álvarez, Á.M.; Fernández, M.R.R.; Chaparro-González, M.P. Plant extracts as antioxidant additives for food industry. In Antioxidants in Foods and Its Applications; Shalaby, E., Mostafa Azzam, G., Eds.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-379-7. [Google Scholar]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Suzuki, T.; Saito, K.; Yoshida, H.; Kojima, N.; Kim, M.; Sudo, M.; Yamashiro, Y.; Tokimitsu, I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 458–465. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. Biomed. Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.B.; Lee, H.S.; Hong, J.S.; Kim, D.H.; Moon, J.M.; Park, Y. Effects of tannase-converted green tea extract on skeletal muscle development. BMC Complement. Med. Ther. 2020, 20, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.B.; Lee, H.S.; Kim, D.H.; Moon, J.M.; Park, Y. Tannase-Converted Green Tea Extract with High (-)-Epicatechin Inhibits Skeletal Muscle Mass in Aged Mice. Evid. Based Complement. Altern. Med. 2020, 2020, 4319398. [Google Scholar] [CrossRef] [Green Version]
- Baik, J.H.; Suh, H.J.; Cho, S.Y.; Park, Y.; Choi, H.S. Differential activities of fungi-derived tannases on biotransformation and substrate inhibition in green tea extract. J. Biosci. Bioeng. 2014, 118, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, T.; Xu, J.; Li, J.; Chen, F.; Xiang, Z.; Huang, Y.; Zhang, D.; Hu, L.; Zhang, B. Interactions between β-cyclodextrin and tea catechins, and potential anti-osteoclastogenesis activity of the (−)-epigallocatechin-3-gallate–β-cyclodextrin complex. RSC Adv. 2019, 9, 28006–28018. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Paineiras-Domingos, L.L.; da Cunha Sa-Caputo, D.; Reis, A.S.; Francisca Santos, A.; Sousa-Goncalves, C.R.; Dos Anjos, E.M.; Dos Santos Pereira, M.J.; Sartorio, A.; Bernardo-Filho, M. Assessment Through the Short Physical Performance Battery of the Functionality in Individuals with Metabolic Syndrome Exposed to Whole-Body Vibration Exercises. Dose Response 2018, 16, 1559325818794530. [Google Scholar] [CrossRef] [Green Version]
- Lakshman, K.M.; Bhasin, S.; Corcoran, C.; Collins-Racie, L.A.; Tchistiakova, L.; Forlow, S.B.; St Ledger, K.; Burczynski, M.E.; Dorner, A.J.; Lavallie, E.R. Measurement of myostatin concentrations in human serum: Circulating concentrations in young and older men and effects of testosterone administration. Mol. Cell. Endocrinol. 2009, 302, 26–32. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.A.; Meza, R.; Lieber, R.L. Skeletal muscle fibroblasts in health and disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Brioche, T.; Lemoine-Morel, S. Oxidative Stress, Sarcopenia, Antioxidant Strategies and Exercise: Molecular Aspects. Curr. Pharm. Des. 2016, 22, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senanayake, S.N. Green tea extract: Chemistry, antioxidant properties and food applications–A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Macedo, J.A.; Battestin, V.; Ribeiro, M.; Macedo, G.A. Increasing the antioxidant power of tea extracts by biotransformation of polyphenols. Food Chem. 2011, 126, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Koch, W.; Kukula-Koch, W.; Czop, M.; Helon, P.; Gumbarewicz, E. The Role of Extracting Solvents in the Recovery of Polyphenols from Green Tea and Its Antiradical Activity Supported by Principal Component Analysis. Molecules 2020, 25, 2173. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Krakowska-Sieprawska, A.; Rafinska, K.; Walczak-Skierska, J.; Buszewski, B. The Influence of Plant Material Enzymatic Hydrolysis and Extraction Conditions on the Polyphenolic Profiles and Antioxidant Activity of Extracts: A Green and Efficient Approach. Molecules 2020, 25, 2074. [Google Scholar] [CrossRef]
- Hammed, A.M.; Jaswir, I.; Amid, A.; Alam, Z.; Asiyanbi-H, T.T.; Ramli, N. Enzymatic hydrolysis of plants and algae for extraction of bioactive compounds. Food Res. Int. 2013, 29, 352–370. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; De los Santos, S.; Gonzalez-Basurto, S.; Canto, P.; Mendoza-Lorenzo, P.; Palma-Flores, C.; Ceballos-Reyes, G.; Villarreal, F.; Zentella-Dehesa, A.; Coral-Vazquez, R. (-)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic delta-sarcoglycan null mouse striated muscle. FEBS J. 2014, 281, 5567–5580. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Salmean, G.; Ciaraldi, T.P.; Nogueira, L.; Barboza, J.; Taub, P.R.; Hogan, M.C.; Henry, R.R.; Meaney, E.; Villarreal, F.; Ceballos, G.; et al. Effects of (-)-epicatechin on molecular modulators of skeletal muscle growth and differentiation. J. Nutr. Biochem. 2014, 25, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, N.A.; Blahnik, Z.J.; Prahadeeswaran, S.; McKinley-Barnard, S.K.; Holden, S.L.; Waldhelm, A. (-)-Epicatechin Supplementation Inhibits Aerobic Adaptations to Cycling Exercise in Humans. Front. Nutr. 2018, 5, 132. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Wang, X.; Zhang, L.; Parnell, L.D.; Admed, B.; LeRoith, T.; Ansah, T.A.; Zhang, L.; Li, J.; Ordovas, J.M.; et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. 2019, 33, 965–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haramizu, S.; Ota, N.; Hase, T.; Murase, T. Catechins suppress muscle inflammation and hasten performance recovery after exercise. Med. Sci. Sport. Exerc. 2013, 45, 1694–1702. [Google Scholar] [CrossRef]
- Ota, N.; Soga, S.; Shimotoyodome, A. Daily consumption of tea catechins improves aerobic capacity in healthy male adults: A randomized double-blind, placebo-controlled, crossover trial. Biosci. Biotechnol. Biochem. 2016, 80, 2412–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, N.; Soga, S.; Haramizu, S.; Yokoi, Y.; Hase, T.; Murase, T. Tea catechins prevent contractile dysfunction in unloaded murine soleus muscle: A pilot study. Nutrition 2011, 27, 955–959. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Booth, F.W.; Gordon, S.E. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am. J. Physiol. 1999, 277, R601–R606. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Cadavid, N.F.; Taylor, W.E.; Yarasheski, K.; Sinha-Hikim, I.; Ma, K.; Ezzat, S.; Shen, R.; Lalani, R.; Asa, S.; Mamita, M.; et al. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc. Natl. Acad. Sci. USA 1998, 95, 14938–14943. [Google Scholar] [CrossRef] [Green Version]
- Zimmers, T.A.; Davies, M.V.; Koniaris, L.G.; Haynes, P.; Esquela, A.F.; Tomkinson, K.N.; McPherron, A.C.; Wolfman, N.M.; Lee, S.J. Induction of cachexia in mice by systemically administered myostatin. Science 2002, 296, 1486–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.A.; LeBrasseur, N.K. Myostatin and sarcopenia: Opportunities and challenges-a mini-review. Gerontology 2014, 60, 289–293. [Google Scholar] [CrossRef]
- Aoki, Y.; Ozawa, T.; Takemasa, T.; Numata, O. Black Tea High-Molecular-Weight Polyphenol-Rich Fraction Promotes Hypertrophy during Functional Overload in Mice. Molecules 2017, 22, 548. [Google Scholar] [CrossRef] [Green Version]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar] [PubMed]
| Control Group (n = 34) | Treatment Group (n = 33) | Total (n = 67) | p-Value | |
|---|---|---|---|---|
| Age (years) | 63.5 ± 3.7 | 64.8 ± 3.7 | 64.1 ± 3.7 | 0.177 |
| Sex | ||||
| Male (n, %) | 5 (14.7) | 5 (15.2) | 10 (14.9) | 0.959 |
| Female (n, %) | 29 (85.3) | 28 (84.8) | 57 (85.1) | |
| Height (cm) | 157.0 ± 5.2 | 157.7 ± 6.4 | 157.3 ± 5.8 | 0.639 |
| Weight (kg) | 58.2 ± 9.3 | 61.2 ± 8.0 | 59.7 ± 8.8 | 0.158 |
| BMI (kg/m2) | 23.5 ± 2.9 | 24.6 ± 2.5 | 24.0 ± 2.8 | 0.121 |
| SBP (mmHg) | 125.9 ± 12.2 | 124.5 ± 14.0 | 120.9 ± 13.5 | 0.664 |
| DBP (mmHg) | 75.5 ± 7.8 | 77.2 ± 9.7 | 74.3 ± 10.3 | 0.665 |
| Pulse (times/min) | 73.5 ± 9.3 | 76.2 ± 9.7 | 72.7 ± 10.1 | 0.263 |
| Alcohol (n, %) | 8 (23.5%) | 5 (15.2%) | 13 (19.4%) | 0.493 |
| Smoking (n, %) | 0 (0.0%) | 1 (3.0%) | 1 (1.5%) | 0.306 |
| Control Group (n = 34) | Treatment Group (n = 33) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Diff | p-Value | Baseline | 12 Weeks | Diff | p-Value | ||||
| Peak Torque (N·m) | Left | Flexor | 33.6 ± 12.6 | 34.4 ± 12.3 | 0.83 ± 11.0 | 0.662 | 31.0 ± 12.1 | 34.1 ± 17.5 | 3.11 ± 18.3 | 0.336 | 0.541 |
| Extensor | 72.9 ± 18.6 | 73.8 ± 18.8 | 0.84 ± 12.4 | 0.695 | 73.6 ± 21.2 | 72.9 ± 22.8 | −0.72 ± 12.8 | 0.748 | 0.614 | ||
| Right | Flexor | 35.8 ± 12.7 | 33.9 ± 14.2 | −1.85 ± 9.3 | 0.255 | 30.1 ± 12.9 | 36.3 ± 18.4 | 6.18 ± 15.3 | 0.027 | 0.013 | |
| Extensor | 74.7 ± 16.7 | 73.5 ± 15.5 | −1.24 ± 9.8 | 0.467 | 74.7 ± 20.8 | 75.7 ± 19.6 | 1.02 ± 13.1 | 0.657 | 0.425 | ||
| Peak TQ/BW (%) | Left | Flexor | 57.7 ± 19.5 | 59.9 ± 20.3 | 2.26 ± 18.2 | 0.475 | 50.7 ± 18.4 | 55.1 ± 24.4 | 4.36 ± 28.1 | 0.379 | 0.718 |
| Extensor | 125.8 ± 27.0 | 128.1 ± 29.4 | 2.25 ± 21.3 | 0.542 | 118.8 ± 28.6 | 118.1 ± 30.3 | −0.63 ± 24.6 | 0.883 | 0.610 | ||
| Right | Flexor | 62.1 ± 21.6 | 58.9 ± 23.4 | −3.13 ± 16.7 | 0.283 | 49.2 ± 19.8 | 58.9 ± 27.7 | 9.71 ± 25.8 | 0.038 | 0.019 | |
| Extensor | 128.8 ± 22.5 | 127.4 ± 22.8 | −1.44 ± 16.1 | 0.606 | 120.1 ± 26.4 | 123.3 ± 24.3 | 3.22 ± 18.7 | 0.330 | 0.278 | ||
| Control Group (n = 34) | Treatment Group (n = 33) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Diff | p-Value | Baseline | 12 Weeks | Diff | p-Value | ||||
| Hand grip | Left | 27.7 ± 6.3 | 26.9 ± 5.8 | −0.81 ± 2.9 | 0.112 | 26.6 ± 6.11 | 26.2 ± 5.6 | −0.44 ± 2.9 | 0.388 | 0.602 | |
| Right | 29.7 ± 6.1 | 28.1 ± 5.6 | −1.57 ± 3.0 | 0.004 | 28.2 ± 4.8 | 28.1 ± 5.2 | −0.15 ± 2.5 | 0.726 | 0.037 | ||
| Control Group (n = 34) | Treatment Group (n = 33) | Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Diff | p-Value | Baseline | 12 weeks | Diff | p-Value | ||||
| Body muscle mass (g) | Arm | Left | 1863.0 ± 462.2 | 1783.3 ± 453.6 | −79.7 ± 148.0 | 0.004 | 1888.6 ± 487.5 | 1798.1 ± 453.8 | −90.4 ± 357.7 | 0.156 | 0.874 |
| Right | 1975.0 ± 432.4 | 1891.9 ± 464.2 | −83.0 ± 175.7 | 0.009 | 1998.8 ± 441.8 | 1929.5 ± 453.1 | −69.2 ± 378.4 | 0.301 | 0.850 | ||
| Trunk | 18,159.7 ± 2972.4 | 18,961.9 ± 3381.5 | 802.3 ± 1228.3 | 0.001 | 18,486.8 ± 3766.2 | 19,845.1 ± 3143.4 | 1358.3 ± 2801.1 | 0.009 | 0.301 | ||
| Leg | Left | 5304.7 ± 1077.6 | 5312.3 ± 1111.6 | 7.6 ± 227.2 | 0.847 | 5205.1 ± 1290.1 | 5406.6 ± 974.0 | 201.4 ± 916.6 | 0.216 | 0.246 | |
| Right | 5431.3 ± 1100.5 | 5396.1 ± 1119.0 | −35.2 ± 277.6 | 0.465 | 5283.2 ± 1191.9 | 5421.1 ± 920.2 | 137.9 ± 899.3 | 0.385 | 0.297 | ||
| Total | 35,660.1 ± 6099.3 | 36,052.9 ± 6350.5 | 392.8 ± 1188.2 | 0.063 | 35,789.5 ± 7243.6 | 37,147.5 ± 5707.7 | 1358.0 ± 5272.2 | 0.149 | 0.312 | ||
| Control Group (n = 34) | Treatment Group (n = 33) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Diff | p-Value | Baseline | 12 Weeks | Diff | p-Value | ||
| Follistatin (pg/mL) | 2109.7 ± 539.6 | 2225.6 ± 739.5 | 115.9 ± 558.3 | 0.235 | 1980.2 ± 720.1 | 2020.2 ± 633.0 | 40.1 ± 517.0 | 0.659 | 0.566 |
| Myostatin (ng/mL) | 1.08 ± 0.61 | 1.10 ± 0.94 | 0.02 ± 0.47 | 0.677 | 1.23 ± 1.27 | 1.00 ± 0.66 | −0.22 ± 0.62 | 0.007 | 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Lee, S.-H.; Park, Y.; Lee, H.-S.; Hong, J.S.; Lim, C.Y.; Kim, D.H.; Park, S.-S.; Suh, H.J.; Hong, K.-B. (−)-Epicatechin-Enriched Extract from Camellia sinensis Improves Regulation of Muscle Mass and Function: Results from a Randomized Controlled Trial. Antioxidants 2021, 10, 1026. https://doi.org/10.3390/antiox10071026
Seo H, Lee S-H, Park Y, Lee H-S, Hong JS, Lim CY, Kim DH, Park S-S, Suh HJ, Hong K-B. (−)-Epicatechin-Enriched Extract from Camellia sinensis Improves Regulation of Muscle Mass and Function: Results from a Randomized Controlled Trial. Antioxidants. 2021; 10(7):1026. https://doi.org/10.3390/antiox10071026
Chicago/Turabian StyleSeo, Hyeyeong, Seok-Hee Lee, Yooheon Park, Hee-Seok Lee, Jeong Sup Hong, Cho Young Lim, Dong Hyeon Kim, Sung-Soo Park, Hyung Joo Suh, and Ki-Bae Hong. 2021. "(−)-Epicatechin-Enriched Extract from Camellia sinensis Improves Regulation of Muscle Mass and Function: Results from a Randomized Controlled Trial" Antioxidants 10, no. 7: 1026. https://doi.org/10.3390/antiox10071026
APA StyleSeo, H., Lee, S.-H., Park, Y., Lee, H.-S., Hong, J. S., Lim, C. Y., Kim, D. H., Park, S.-S., Suh, H. J., & Hong, K.-B. (2021). (−)-Epicatechin-Enriched Extract from Camellia sinensis Improves Regulation of Muscle Mass and Function: Results from a Randomized Controlled Trial. Antioxidants, 10(7), 1026. https://doi.org/10.3390/antiox10071026







