Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatment
2.3. In Vitro Cytotoxicity
2.4. Intracellular ROS Generation
2.5. Lipid Peroxidation Assay
2.6. Comparison of Cytotoxicity between Pure Hydrolysates and Their Bioavailable Fraction
2.7. Cytoprotective Effect of Hydrolysates Exposed Together with T-2 Toxin
2.8. Determination of Fungal Metabolites in Fish Hydrolysates through Liquid Chromatography Quadrupole Time of Fliht Mass Spectrometry
2.9. Statistical Analysis
3. Results
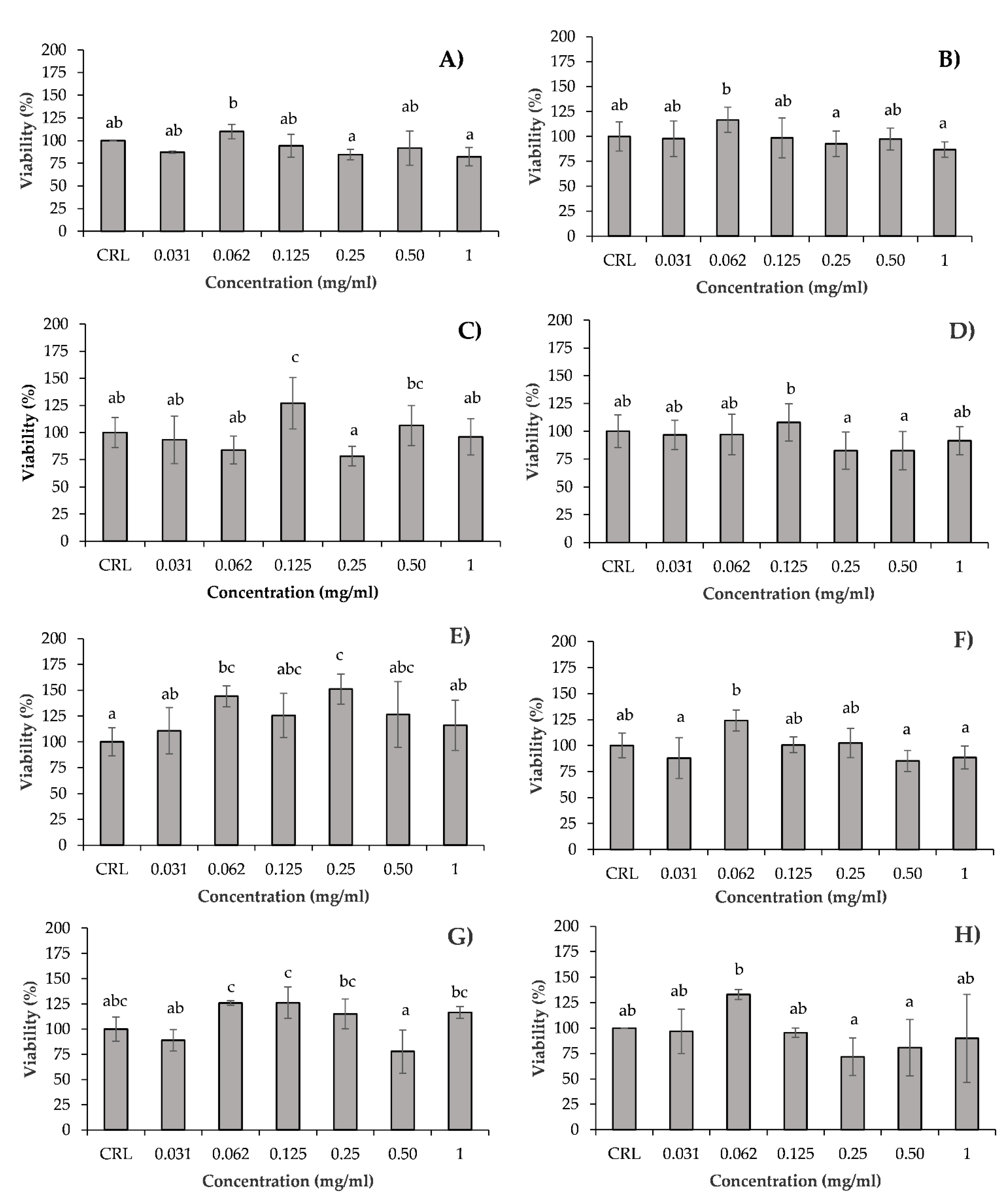
3.1. In Vitro Cytotoxicity
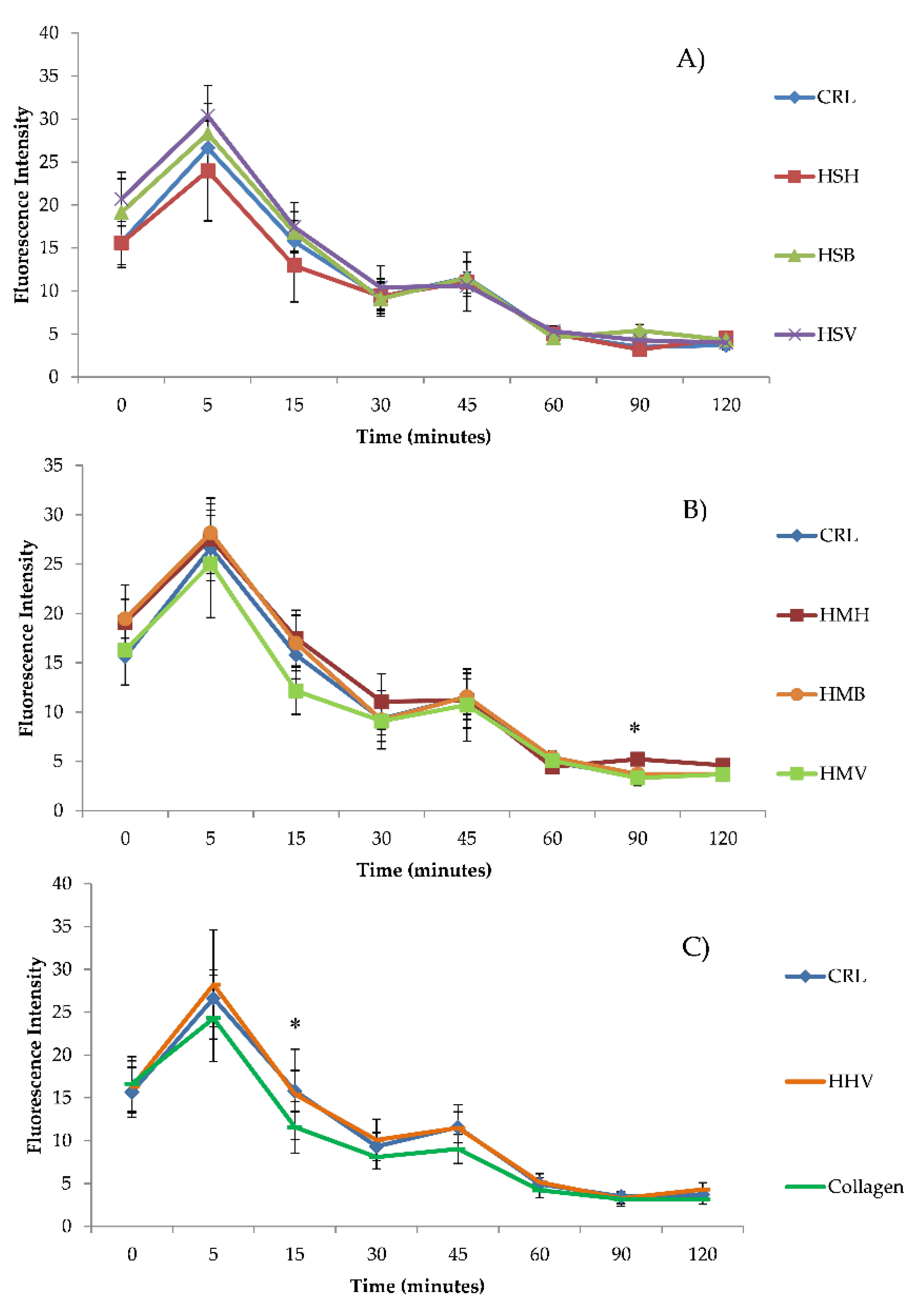
3.2. Intracellular ROS Generation
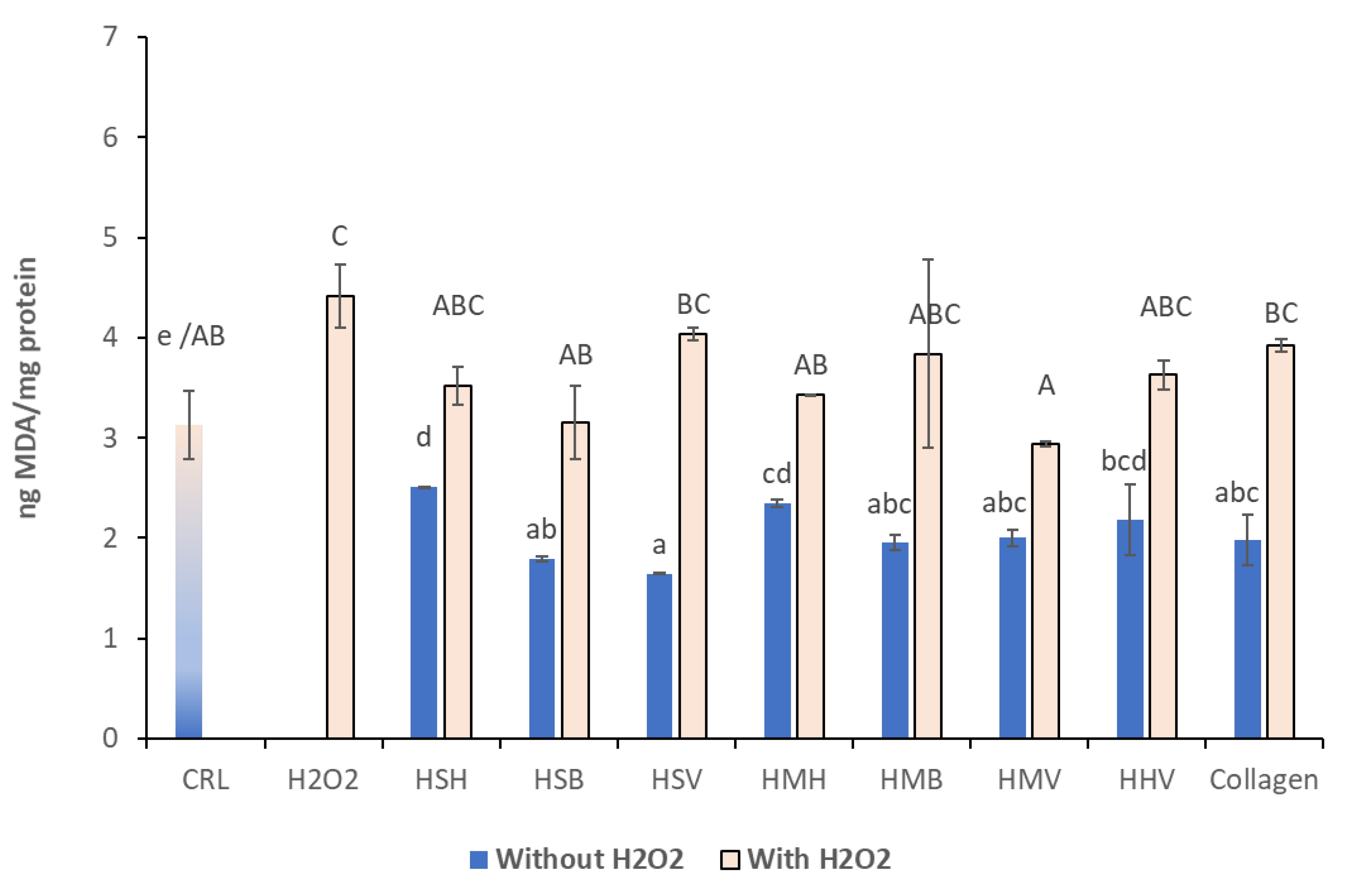
3.3. Lipid Peroxidation Assay
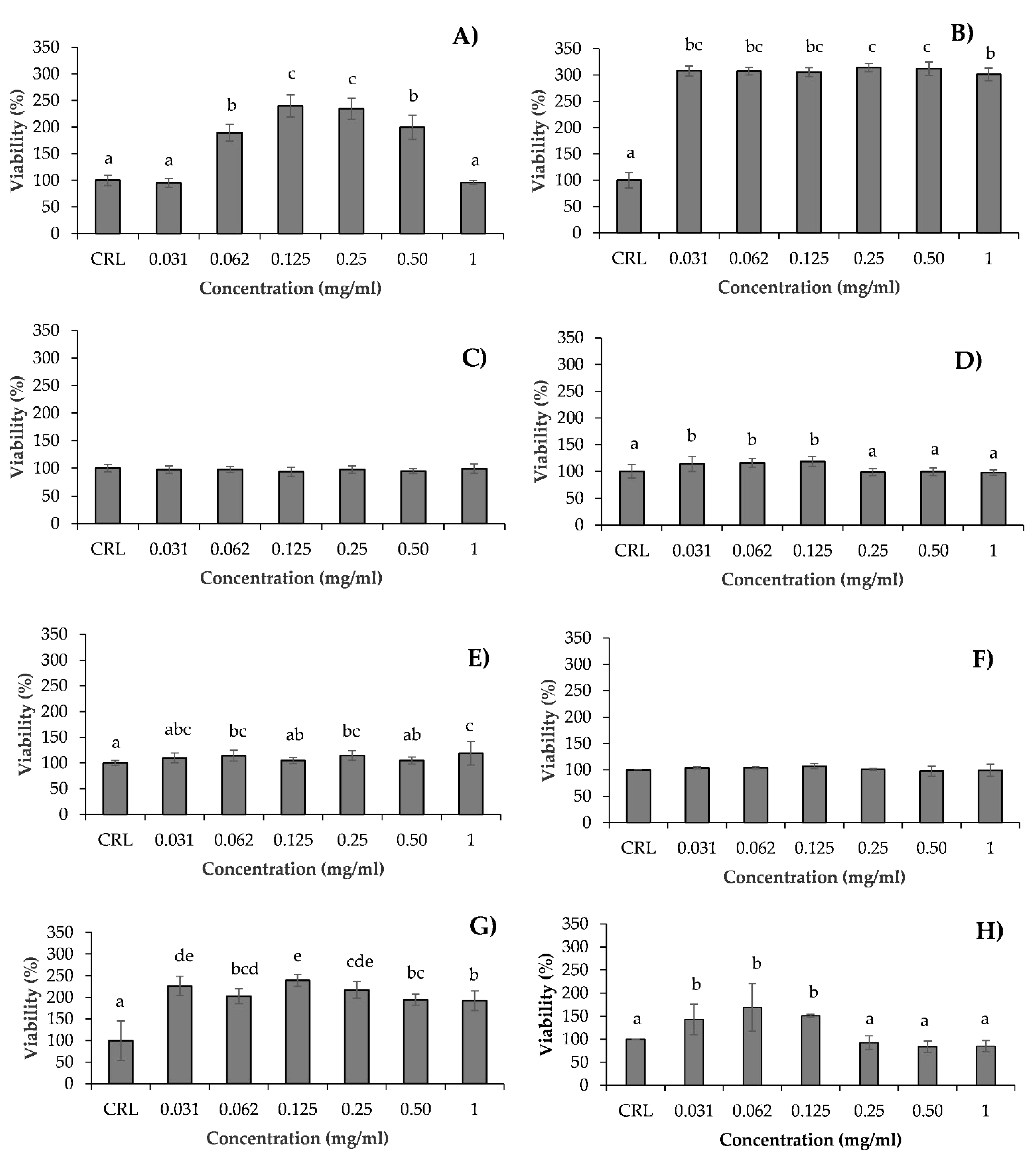
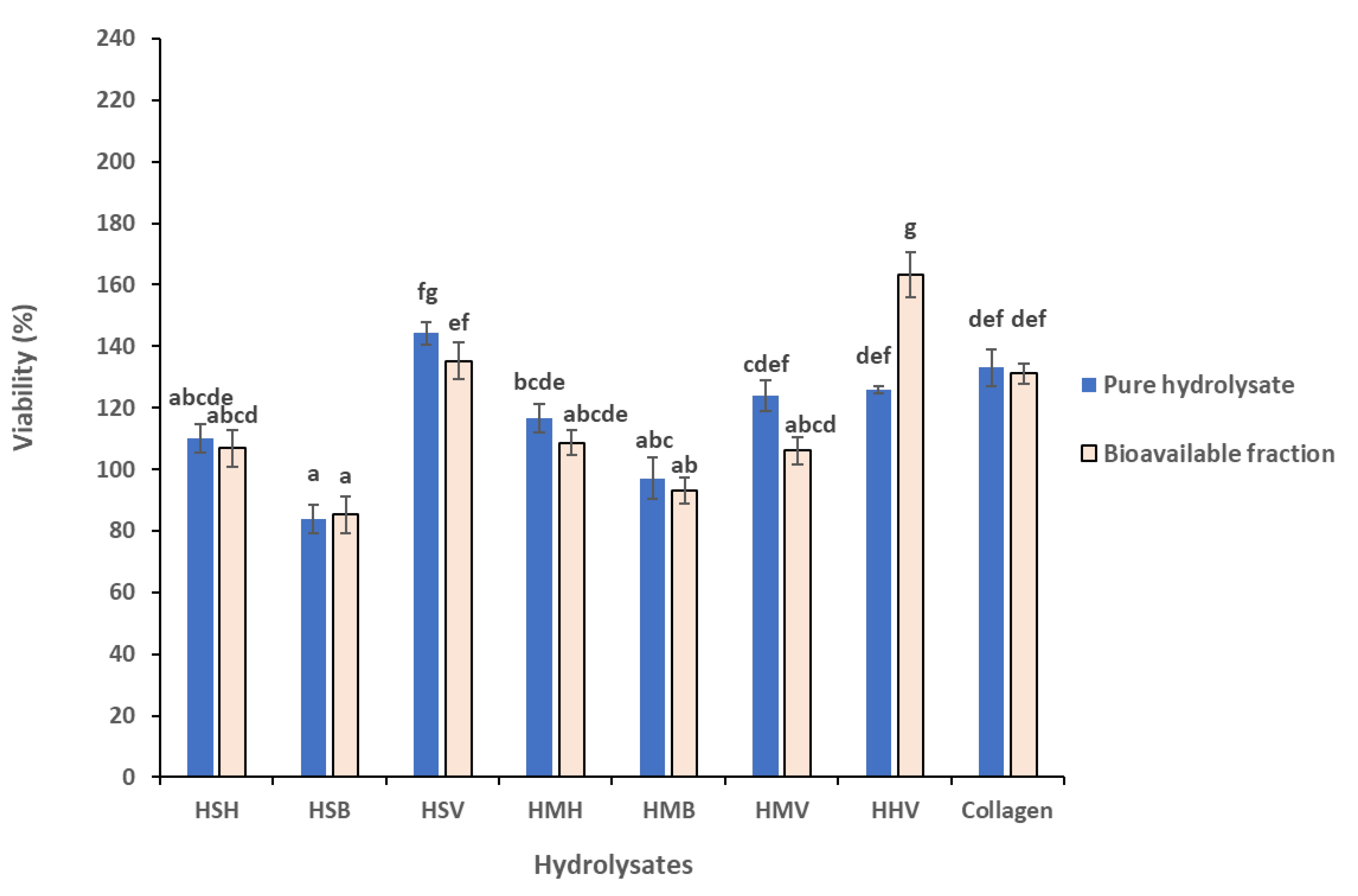
3.4. Comparison of Cytotoxicity between pure Hydrolysates and Their Bioavailable Fraction
3.5. Cytoprotective Effect of Hydrolysates against T-2 Mycotoxin
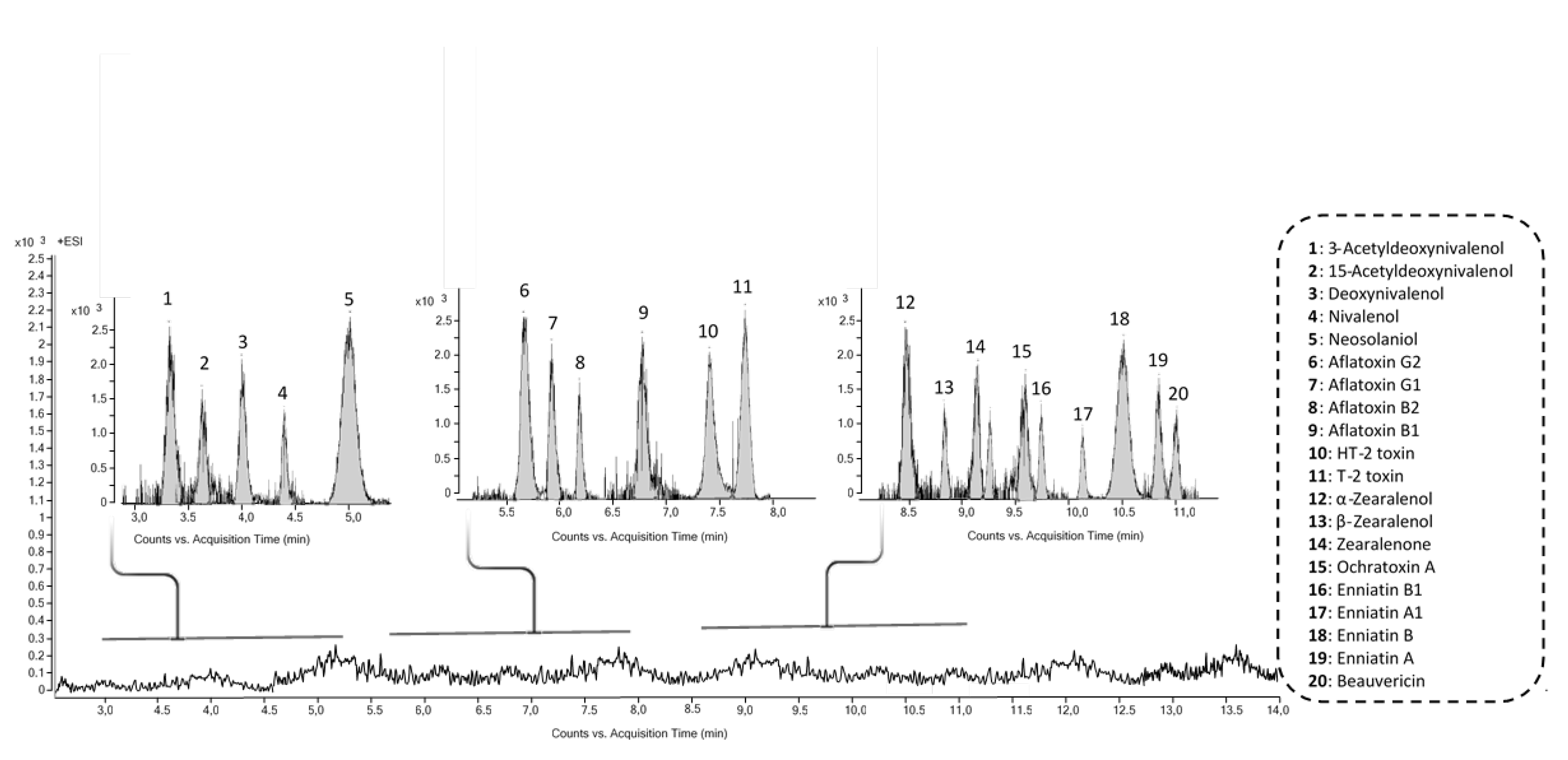
3.6. Identification of Fungal Metabolites in Fish Hydrolysates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT. Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [PubMed]
- Nagai, F.; Ushiyama, K.; Kano, I. DNA cleavage by metabolites of butylated hydroxytoluene. Arch. Toxicol. 1993, 67, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, R.; Dwyer-Nield, L.D.; Malkinson, A.M.; Thompson, J.A. Lung toxicity and tumor promotion by hydroxylated derivatives of 2,6-di-tert-butyl-4-methylphenol (BHT) and 2-tert-butyl-4-methyl-6-iso-propylphenol: Correlation with quinone methide reactivity. Chem. Res. Toxicol. 2002, 15, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Granato, D.; Nunes, D.S.; Barba, F.J. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovacević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and bioactive properties of peptides derived from marine side streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef]
- Ballatore, M.B.; del Rosario Bettiol, M.; Vanden Braber, N.L.; Aminahuel, C.A.; Rossi, Y.E.; Petroselli, G.; Erra-Balsells, R.; Cavaglieri, L.R.; Montenegro, M.A. Antioxidant and cytoprotective effect of peptides produced by hydrolysis of whey protein concentrate with trypsin. Food Chem. 2020, 319, 126472. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Q.; Chen, X.; Tian, Y.; Liu, Z.; Wang, S. Bioactive peptides derived from crimson snapper and in vivo anti-aging effects on fat diet-induced high fat Drosophila melanogaster. Food Funct. 2020, 11, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Axarlis, K.; Daskalaki, M.G.; Michailidou, S.; Androulaki, N.; Tsoureki, A.; Mouchtaropoulou, E.; Kolliniati, O.; Lapi, I.; Dermitzaki, E.; Venihaki, M.; et al. Diet supplementation with fish-derived extracts suppresses diabetes and modulates intestinal microbiome in a murine model of diet-induced obesity. Mar. Drugs 2021, 19, 268. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, M.G.; Axarlis, K.; Aspevik, T.; Orfanakis, M.; Kolliniati, O.; Lapi, I.; Tzardi, M.; Dermitzaki, E.; Venihaki, M.; Kousoulaki, K.; et al. Fish side stream-derived protein hydrolysates suppress DSS-induced colitis by modulating intestinal inflammation in mice. Mar. Drugs 2021, 19, 312. [Google Scholar] [CrossRef]
- Aspevik, T.; Oterhals, Å.; Rønning, S.B.; Altintzoglou, T.; Wubshet, S.G.; Gildberg, A.; Afseth, N.K.; Whitaker, R.D.; Lindberg, D. Valorization of proteins from co-and by-products from the fish and meat industry. Chem. Chem. Technol. Waste Valorization 2017, 375, 123–150. [Google Scholar]
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shan, T.; Ma, Y.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Chi, C.-F.; Cao, Z.-H.; Wang, B.; Hu, F.-Y.; Li, Z.-R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Lee, E.J.; Hur, J.; Ham, S.A.; Jo, Y.; Lee, S.; Choi, M.-J.; Seo, H.G. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 2017, 104, 281–286. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.-H.; Li, M.-Z.; Li, C.-J.; Chen, H.-Q.; Jiang, Y.; Tang, T.; Qi, W.-Y.; Xu, H.-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, Y.-J. Static hydrothermal processing and fractionation for production of a collagen peptide with anti-oxidative and anti-aging properties. Process Biochem. 2019, 83, 176–182. [Google Scholar] [CrossRef]
- Jin, J.-E.; Ahn, C.-B.; Je, J.-Y. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed ark shell (Scapharca subcrenata). Process Biochem. 2018, 72, 170–176. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Chi, C.-F.; Zhao, Y.-Q.; Wang, B. Preparation, physicochemical and antioxidant properties of acid-and pepsin-soluble collagens from the swim bladders of miiuy croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.-C.; Xiao, J.; Ong, M.G.-L.; Pang, M.-J.; Wong, S.-J.; Teh, L.-K.; Chai, T.-T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Maqsood, S. Effect of pretreatment on lipid oxidation and fishy odour development in protein hydrolysates from the muscle of Indian mackerel. Food Chem. 2012, 135, 2474–2482. [Google Scholar] [CrossRef]
- Sheriff, S.A.; Sundaram, B.; Ramamoorthy, B.; Ponnusamy, P. Synthesis and in vitro antioxidant functions of protein hydrolysate from backbones of Rastrelliger kanagurta by proteolytic enzymes. Saudi J. Biol. Sci. 2014, 21, 19–26. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 1496–1501. [Google Scholar] [CrossRef]
- Wang, B.; Xie, N.; Li, B. Charge properties of peptides derived from casein affect their bioavailability and cytoprotection against H2O2-induced oxidative stress. J. Dairy Sci. 2016, 99, 2468–2479. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Bai, F.; Fang, Y.; Wang, J.; Gao, R. The anti-skin-aging effect of oral administration of gelatin from the swim bladder of Amur sturgeon (Acipenser schrenckii). Food Funct. 2019, 10, 3890–3897. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. Optimization of the red tilapia (Oreochromis spp.) viscera hydrolysis for obtaining iron-binding peptides and evaluation of in vitro iron bioavailability. Foods 2020, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Chen, H.; Jin, W.; Yang, Z.; Cao, Y.; Miao, J. Three newly isolated calcium-chelating peptides from tilapia bone collagen hydrolysate enhance calcium absorption activity in intestinal Caco-2 Cells. J. Agric. Food Chem. 2020, 68, 2091–2098. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Bakken, H.E. Preventive effect of Nile tilapia hydrolysate against oxidative damage of HepG2 cells and DNA mediated by H2O2 and AAPH. J. Food Sci. Technol. 2015, 52, 6194–6205. [Google Scholar] [CrossRef]
- Turco, L.; Catone, T.; Caloni, F.; Consiglio, E.D.; Testai, E.; Stammati, A. Caco-2/TC7 cell line characterization for intestinal absorption: How reliable is this in vitro model for the prediction of the oral dose fraction absorbed in human? Toxicol. Vitr. 2011, 25, 13–20. [Google Scholar] [CrossRef]
- Zucco, F.; Batto, A.-F.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G.; et al. An inter-laboratory study to evaluate the effects of medium composition on the differentiation and barrier function of Caco-2 cell lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Decastelli, L.; Lai, J.; Gramaglia, M.; Monaco, A.; Nachtmann, C.; Oldano, F.; Ruffier, M.; Sezian, A.; Bandirola, C. Aflatoxins occurrence in milk and feed in Northern Italy during 2004–2005. Food Control 2007, 18, 1263–1266. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 324–365. [Google Scholar]
- Hashem, M. Isolation of mycotoxin-producing fungi from fishes growing in aquacultures. Res. J. Microbiol. 2011, 6, 862–872. [Google Scholar] [CrossRef]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Dänicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins (Basel) 2013, 5, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Aspevik, T.; Thoresen, L.; Steinsholm, S.; Carlehög, M.; Kousoulaki, K. Sensory and chemical properties of protein hydrolysates based on mackerel (Scomber scombrus) and salmon (Salmo salar) side stream materials. J. Aquat. Food Prod. Technol. 2021, 30, 176–187. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Festila, L.E.; Fernández, M. Comparison of basal cytotoxicity of seven carbamates in CHO-K1 cells. Toxicol. Environ. Chem. 2006, 88, 345–354. [Google Scholar] [CrossRef]
- Ruiz-Leal, M.; George, S. An in vitro procedure for evaluation of early stage oxidative stress in an established fish cell line applied to investigation of PHAH and pesticide toxicity. Twelfth Int. Symp. Pollut. Responses Mar. Org. 2004, 58, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells. Toxicol Vitr. 2009, 23, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Ruiz, M.-J. T-2 toxin and its metabolites: Characterization, cytotoxic mechanisms and adaptive cellular response in human hepatocarcinoma (HepG2) cells. Food Chem. Toxicol. 2020, 145, 111654. [Google Scholar] [CrossRef] [PubMed]
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Poojary, M.; Barba, F.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. Evaluating the potential of cell disruption technologies for green selective extraction of antioxidant compounds from Stevia rebaudiana Bertoni leaves. J. Food Eng. 2015, 149, 222–228. [Google Scholar] [CrossRef]
- Meiliana, A.; Wijaya, A. Hormesis in Health and Disease: Molecular Mechanisms. Indones. Biomed. J. 2020, 12, 288–303. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a multifaceted hormetic agent, mediates an intricate crosstalk between mitochondrial turnover, autophagy, and apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Karbarz, M.; Mytych, J.; Solek, P.; Stawarczyk, K.; Tabecka-Lonczynska, A.; Koziorowski, M.; Luczaj, L. Cereal grass juice in wound healing: Hormesis and cell-survival in normal fibroblasts, in contrast to toxic events in cancer cells. J. Physiol. Pharmacol. 2019, 70, 595–604. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Xiao, H.; Decker, E.A.; Yongsawatdigul, J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015, 167, 7–15. [Google Scholar] [CrossRef]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. In-vitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef]
- Zhong, S.; Ma, C.; Lin, Y.C.; Luo, Y. Antioxidant properties of peptide fractions from silver carp (Hypophthalmichthys molitrix) processing by-product protein hydrolysates evaluated by electron spin resonance spectrometry. Food Chem. 2011, 126, 1636–1642. [Google Scholar] [CrossRef]
- Hu, X.-M.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Antioxidant peptides from the protein hydrolysate of monkfish (Lophius litulon) muscle: Purification, identification, and cytoprotective function on HepG2 cells damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, X.; Xu, B.; Yuan, F.; Gong, J.; Yang, Z. Collagen peptides from swim bladders of giant croaker (Nibea japonica) and their protective effects against H2O2-induced oxidative damage toward human umbilical vein endothelial cells. Mar. Drugs 2020, 18, 430. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, S.; Tang, Y. Marine collagen peptides promote cell proliferation of NIH-3T3 fibroblasts via NF-κB signaling pathway. Molecules 2019, 24, 4201. [Google Scholar] [CrossRef]
- Muinde, K.B.; Donglu, F.; Zhao, L.; Qiuhui, H. Agaricus bisporus peptide fractions confer cytoprotective ability against hydrogen peroxide-induced oxidative stress in HepG2 and Caco-2 cells. J. Food Meas. Charact. 2020, 14, 2503–2519. [Google Scholar]
- Benzie, I.F.F.; Choi, S.-W. Chapter One—Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 1–53. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, X.; Tian, Y.; Wu, D.; Du, M.; Wang, S. Protection against oxidative stress and anti-aging effect in Drosophila of royal jelly-collagen peptide. Food Chem. Toxicol. 2020, 135, 110881. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Lin, S.-J.; Yang, Z.-S.; Jin, H.-X. Preparation of antioxidant peptide by microwave-assisted hydrolysis of collagen and its protective effect against H2O2-induced damage of RAW264. 7 Cells. Mar. Drugs 2019, 17, 642. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Qian, Z.-J.; Ryu, B.; Park, J.W.; Kim, S.-K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Zhao, Y.-Q.; Zhao, G.-X.; Chi, C.-F.; Wang, B. Antioxidant peptides from collagen hydrolysate of redlip croaker (Pseudosciaena polyactis) scales: Preparation, characterization, and cytoprotective effects on H2O2-damaged HepG2 cells. Mar. Drugs 2020, 18, 156. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Cytoprotective effect of antioxidant pentapeptides from the protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy) against H2O2-mediated human umbilical vein endothelial cell (HUVEC) injury. Int. J. Mol. Sci. 2019, 20, 5425. [Google Scholar] [CrossRef]
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox–Transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef]
- Matejova, I.; Svobodova, Z.; Vakula, J.; Mares, J.; Modra, H. Impact of mycotoxins on aquaculture fish species: A review. J. World Aquac. Soc. 2017, 48, 186–200. [Google Scholar] [CrossRef]
- Mahmoud, A.F.; Escrivá, L.; Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H. Determination of trichothecenes in chicken liver using gas chromatography coupled with triple-quadrupole mass spectrometry. LWT 2018, 93, 237–242. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Izzo, L.; Gaspari, A.; Graziani, G.; Mañes, J.; Ritieni, A. Simultaneous determination of AFB1 and AFM1 in milk samples by ultra high performance liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Beverages 2018, 4, 43. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.; Rivas-Caceres, R.R.; Salem, A.Z. Mycotoxin toxicity and residue in animal products: Prevalence, consumer exposure and reduction strategies–A review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef]
- Tolosa, J.; Barba, F.J.; Pallarés, N.; Ferrer, E. Mycotoxin Identification and In Silico Toxicity Assessment Prediction in Atlantic Salmon. Mar. Drugs 2020, 18, 629. [Google Scholar] [CrossRef]
- Tolosa, J.; Font, G.; Mañes, J.; Ferrer, E. Natural occurrence of emerging Fusarium mycotoxins in feed and fish from aquaculture. J. Agric. Food Chem. 2014, 62. [Google Scholar] [CrossRef]
- Tolosa, J.; Barba, F.J.; Font, G.; Ferrer, E. Mycotoxin incidence in some fish products: QuEChERS methodology and liquid chromatography linear ion trap tandem mass spectrometry approach. Molecules 2019, 24, 527. [Google Scholar] [CrossRef]
- Nogueira, W.V.; de Oliveira, F.K.; Marimón Sibaja, K.V.; de Oliveira Garcia, S.; Kupski, L.; de Souza, M.M.; Tesser, M.B.; Garda-Buffon, J. Occurrence and bioacessibility of mycotoxins in fish feed. Food Addit. Contam. Part B 2020, 13, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Meucci, V.; Armani, A.; Tinacci, L.; Guardone, L.; Battaglia, F.; Intorre, L. Natural occurrence of ochratoxin A (OTA) in edible and not edible tissue of farmed gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) sold on the Italian market. Food Control 2020, 120, 107537. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Heilos, D.; Richter, L.; Süssmuth, R.D.; Heffeter, P.; Sulyok, M.; Kenner, L.; Berger, W.; Dornetshuber-Fleiss, R. Mouse tissue distribution and persistence of the food-born fusariotoxins Enniatin B and Beauvericin. Toxicol. Lett. 2016, 247, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Krug, I.; Behrens, M.; Esselen, M.; Humpf, H.-U. Transport of enniatin B and enniatin B1 across the blood-brain barrier and hints for neurotoxic effects in cerebral cells. PLoS ONE 2018, 13, e0197406. [Google Scholar] [CrossRef]


| Salmon | Mackerel | Herring | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | HSH | HSB | HSV | HMH | HMB | HMV | HHV | ||
| Salmon | HSH | HSH < CTR (0.1 > p > 0.05) | |||||||
| HSB | HSB > CTR (0.1 > p > 0.05) | HSB > HSH (p ≤ 0.05) | |||||||
| HSV | HSV > CTR (0.1 > p > 0.05) | HSV > HSH (p ≤ 0.05) | NS | ||||||
| Mackerel | HMH | HMH > CTR (p ≤ 0.05) | HMH > HSH (p ≤ 0.05) | NS | NS | ||||
| HMB | HMB > CTR (0.1 > p > 0.05) | HMB > HSH (0.1> p > 0.05) | NS | NS | NS | ||||
| HMV | NS | NS | HMV < HSB (p ≤ 0.05) | HMV < HSV (p ≤ 0.05) | HMV < HMH (p ≤ 0.05) | HMV < HMB (p ≤ 0.05) | |||
| Herring | HHV | NS | HHV > HSH (0.1 > p > 0.05) | HHV < HSB (0.1 > p > 0.05) | HHV < HSV (0.1 > p > 0.05) | HHV < HMH (p ≤ 0.05) | HHV < HMB (0.1 > p > 0.05) | HMV < HHV (0.1 > p > 0.05) | |
| Flownder | Collagen | Collagen < CTR (p ≤ 0.05) | NS | Collagen < HSB (p ≤ 0.05) | Collagen < HSV (p ≤ 0.05) | Collagen < HMH (p ≤ 0.05) | Collagen < HMB (p ≤ 0.05) | Collagen < HMV (p ≤ 0.05) | Collagen < HHV (p ≤ 0.05) |
| Salmon | Mackerel | Herring | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTR | H2O2 | HSHH2O2 | HSBH2O2 | HSVH2O2 | HMHH2O2 | HMBH2O2 | HMVH2O2 | HHVH2O2 | ||
| H2O2 | H2O2 > CTR (p ≤ 0.05) | |||||||||
| Salmon | HSHH2O2 | HSH > CTR (p ≤ 0.05) | NS | |||||||
| HSBH2O2 | HSB > CTR (p ≤ 0.05) | NS | NS | |||||||
| HSVH2O2 | HSV > CTR (p ≤ 0.05) | HSV < H2O2 (0.1> p > 0.05) | NS | HSV < HSB (p ≤ 0.05) | ||||||
| Mackerel | HMHH2O2 | HMH > CTR (p ≤ 0.05) | NS | NS | NS | HMH > HSV (p ≤ 0.05) | ||||
| HMBH2O2 | HMB > CTR (p ≤ 0.05) | NS | NS | HMB > HSB (p ≤ 0.05) | HMB > HSV (p ≤ 0.05) | HMB > HMH (0.1> p > 0.05) | ||||
| HMVH2O2 | HMV > CTR (p ≤ 0.05) | NS | HMV < HSH (p ≤ 0.05) | NS | NS | NS | NS | |||
| Herring | HHVH2O2 | HHV > CTR (p ≤ 0.05) | NS | HHV > HSH (p ≤ 0.05) | NS | HHV > HSV (p ≤ 0.05) | NS | NS | HHV > HMV (p ≤ 0.05) | |
| Flownder | CollagenH2O2 | Collaben > CTR (p ≤ 0.05) | Collagen < H2O2 (p ≤ 0.05) | NS | Collagen < HSB (p ≤ 0.05) | NS | Collagen > HMH (0.1> p > 0.05) | Collagen < HMB (p ≤ 0.05) | NS | Collagen < HHV (p ≤ 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taroncher, M.; Rodríguez-Carrasco, Y.; Aspevik, T.; Kousoulaki, K.; Barba, F.J.; Ruiz, M.-J. Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells. Antioxidants 2021, 10, 975. https://doi.org/10.3390/antiox10060975
Taroncher M, Rodríguez-Carrasco Y, Aspevik T, Kousoulaki K, Barba FJ, Ruiz M-J. Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells. Antioxidants. 2021; 10(6):975. https://doi.org/10.3390/antiox10060975
Chicago/Turabian StyleTaroncher, Mercedes, Yelko Rodríguez-Carrasco, Tone Aspevik, Katerina Kousoulaki, Francisco J. Barba, and María-José Ruiz. 2021. "Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells" Antioxidants 10, no. 6: 975. https://doi.org/10.3390/antiox10060975
APA StyleTaroncher, M., Rodríguez-Carrasco, Y., Aspevik, T., Kousoulaki, K., Barba, F. J., & Ruiz, M.-J. (2021). Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells. Antioxidants, 10(6), 975. https://doi.org/10.3390/antiox10060975