Plant Extracts as Alternative Additives for Sperm Preservation
Abstract
1. Introduction
2. Semen Preservation and Its Effects on Sperm Quality
3. Herbal Pharmacognosy and Its Use in Andrology
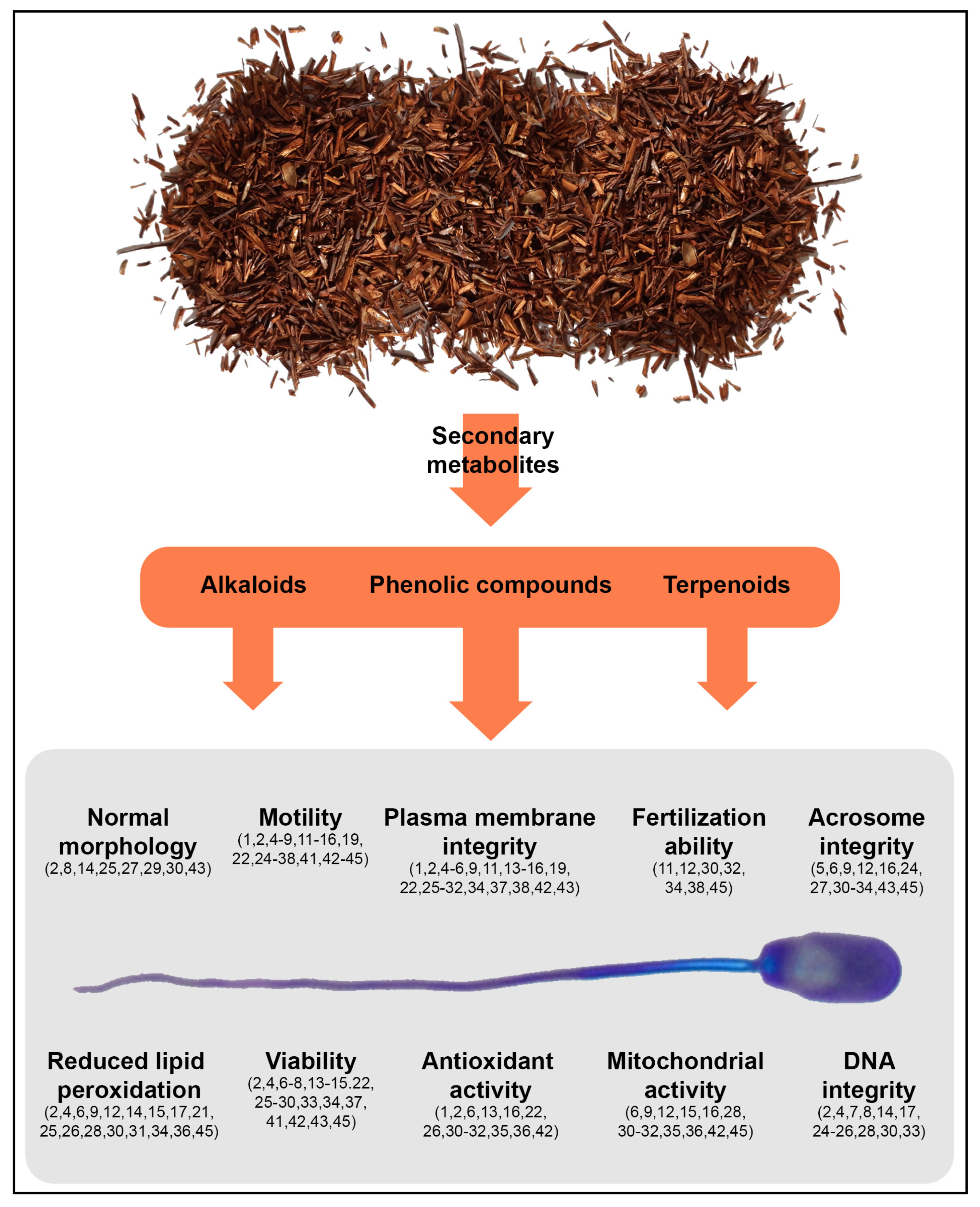
3.1. Plant Families and Species Used as Preservatives for Sperm Preservation
3.1.1. Family Adoxaceae
Sambucus nigra (elder)
3.1.2. Family Apiaceae
Foeniculum vulgare (fennel)
3.1.3. Family Aquifoliaceae
Ilex paraguariensis (yerba mate)
3.1.4. Family Arecaceae
Phoenix dactylifera (date palm)
3.1.5. Family Asphodelaceae
Aloe vera (aloe)
3.1.6. Family Asteraceae
Achillea millefolium (yarrow)
Echinacea purpurea (echinacea)
3.1.7. Family Cactaceae
Opuntia ficus-indica (prickly pear)
3.1.8. Family Capparaceae
Capparis spinosa (caper bush)
3.1.9. Family Crassulaceae
Rhodiola rosea (golden root)
3.1.10. Family Cucurbitaceae
Gynostemma pentaphyllum (jiaogulan)
3.1.11. Family Ebenaceae
Diospyros kaki (kaki)
3.1.12. Family Fabaceae
Albizia harveyi (silk tree)
Aspalathus linearis (rooibos)
Ceratonia siliqua (carob)
Cyclopia intermedia (honeybush)
Entada abyssinica (Splinter bean)
3.1.13. Family Lamiaceae
Melissa officinalis (lemon balm)
Origanum vulgare (oregano)
Salvia miltiorrhiza (red sage)
Salvia officinalis (sage)
Salvia rosmarinus (rosmarinus)
Thymus capitatus (Spanish oregano)
Thymus munbyanus (thyme)
3.1.14. Family Lythraceae
Punica granatum (pomegranate)
3.1.15. Family Moringaceae
Moringa oleifera (moringa)
3.1.16. Family Myrtaceae
Melaleuca alternifolia (tea tree)
Myrrhinium atropurpureum (palo de fierro)
Psidium guajava (common guava)
Syzygium aromaticum (clove)
Syzygium cumini (Jambul)
Ugni molinae (Chilean guava)
3.1.17. Family Poaceae
Cymbopogon citratus (lemon grass)
Lolium perenne (rye grass)
3.1.18. Family Portulacaceae
Portulaca oleracea (pursley)
3.1.19. Family Ranunculaceae
Nigella sativa (black caraway)
3.1.20. Family Santalaceae
Viscum album (Mistletoe)
3.1.21. Family Sapotaceae
Argania spinosa (argan)
3.1.22. Family Schisandraceae
Schisandra chinensis (omija)
3.1.23. Family Simaroubaceae
Eurycoma longifolia (tongkat ali)
3.1.24. Family Theaceae
Camellia sinensis (tea plant)
3.1.25. Family Urticaceae
Urtica dioica (common nettle)
3.1.26. Family Verbenaceae
Lippia origanoides (salva-de-Marajó)
3.1.27. Family Zingiberaceae
Zingiber officinale (ginger)
3.1.28. Family Zygophyllaceae
Tribulus terrestris (calthrop)
| Plant Species (Family) | Animal Species [References] | Extraction Method | Plant Part | Toxicity | Antimicrobial Activity |
|---|---|---|---|---|---|
| Achillea millefolium (Asteraceae) | Chicken [44] | Water | Aerial parts | No | NE |
| Albizia harveyi (Fabaceae) | Cattle [52] | Methanol | Leaves | No | NE |
| Aloe vera (Asphodelaceae) | Peccary [43] | Pulp | Leaves | No | NE |
| Argania spinosa (Sapotaceae) | Sheep [86] | Oil | Seeds | DD | NE |
| Aspalathus linearis (Fabaceae) | Pig [53] | Water | Leaves, stems | DD | NE |
| Camellia sinensis (Theaceae) | Dog, mouse, pig, sheep [89,90,91,92,93,94] | Water | Leaves, powder | DD | NE |
| Capparis spinosa (Capparaceae) | Human [48] | Ethanol | Leaves | No | NE |
| Ceratonia siliqua (Fabaceae) | Human [54] | Water | Not specified | DD | NE |
| Cyclopia intermedia (Fabaceae) | Pig [55] | Water | Leaves, stems | No | NE |
| Cymbopogon citratus (Poaceae) | Pig [76] | Oil | Leaves | Toxic | No |
| Diospyros kaki (Ebenaceae) | Cattle [51] | Pulp | Pulp (fruit) | No | NE |
| Echinacea purpurea (Asteraceae) | Sheep [45] | Water | Leaves | No | NE |
| Entada abyssinica (Fabaceae) | Sheep [56] | Methanol | Bark | DD | NE |
| Eurycoma longifolia (Simaroubaceae) | Cattle [88] | Water | Not specified | DD | NE |
| Foeniculum vulgare (Apiaceae) | Pig, sheep [37,38] | Water | Leaves, seeds | No | NE |
| Gynostemma pentaphyllum (Cucurbitaceae) | Pig [50] | Several steps | Not specified | DD | NE |
| Ilex paraguariensis (Aquifoliaceae) | Pig [39] | Water | Leaves | No | NE |
| Lippia origanoides (Verbenaceae) | Buffalo [95] | Oil | Not specified | No | NE |
| Lolium perenne (Poaceae) | Buffalo [81] | Several steps | Leaves | No | NE |
| Melaleuca alternifolia (Myrtaceae) | Pig [64,75] | Oil | Not specified | DD | Yes |
| Melissa officinalis (Lamiaceae) | Pig [39] | Water | Leaves | No | NE |
| Moringa oleifera (Moringaceae) | Sheep, shrimp [72,73,74] | Ethanol, methanol | Leaves, seeds | No | Yes |
| Myrrhinium atropurpureum (Myrtaceae) | Pig [76] | Oil | Leaves | DD | No |
| Nigella sativa (Ranunculaceae) | Goat, sheep [83,84] | Oil | Seeds | DD | NE |
| Opuntia ficus-indica (Cactaceae) | Sheep [46,47] | Acetone, oil | Cladodes, seeds | DD | NE |
| Origanum vulgare (Lamiaceae) | Cattle, human, rabbit [57,58,59] | Ethanol, oil | Whole plant, leaves | DD | NE |
| Phoenix dactylifera (Arecaceae) | Buffalo, cattle, horse [40,41,42] | Semen extender | Pollen | DD | NE |
| Portulaca oleracea (Portulacaceae) | Goat [82] | Ethanol, methanol, water | Whole plant | DD | NE |
| Psidium guajava (Myrtaceae) | Goat [77] | Several steps | Leaves | DD | NE |
| Punica granatum (Lythraceae) | Buffalo, cattle, sheep [69,70,71] | Juice | Fruit | DD | NE |
| Rhodiola rosea (Crassulaceae) | Pig [49] | Water | Caudex | No | NE |
| Salvia miltiorrhiza (Lamiaceae) | Pig [60] | Several steps | Not specified | No | NE |
| Salvia officinalis (Lamiaceae) | Pig [61] | Water | Leaves | No | NE |
| Salvia rosmarinus (Lamiaceae) | Chicken, goat, pig, sheep [62,63,64,65,66,67] | Water, oil | Leaves, stems | DD | Yes |
| Sambucus nigra (Adoxaceae) | Cattle [36] | Ethanol | Fruit | DD | NE |
| Schisandra chinensis (Schisandraceae) | Cattle [87] | Ethanol | Fruit | No | Yes |
| Syzygium aromaticum (Myrtaceae) | Sheep [78] | Ethanol | Buds | DD | NE |
| Syzygium cumini (Myrtaceae) | Cattle [79] | Juice | Fruit | No | NE |
| Thymus capitatus (Lamiaceae) | Pig [63] | Oil | Not specified | Toxic | NE |
| Thymus munbyanus (Lamiaceae) | Human [68] | Oil | Leaves, flowers | Toxic | NE |
| Tribulus terrestris (Zygophyllaceae) | Human [96] | Water | Not specified | No | NE |
| Ugni molinae (Myrtaceae) | Pig [80] | Water | Fruit | DD | NE |
| Urtica dioica (Urticaceae) | Cattle [42] | Water | Not specified | No | NE |
| Viscum album (Santalaceae) | Rabbit [85] | Water | Not specified | DD | NE |
| Zingiber officinale (Zingiberaceae) | Sheep, shrimp [45,72] | Water, ethanol | Rhizome | No | Yes |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Browne, R.K.; Silla, A.J.; Upton, R.; Della-Togna, G.; Marcec-Greaves, R.; Shishova, N.V.; Uteshev, V.K.; Proaño, B.; Pérez, O.D.; Mansour, N.; et al. Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology 2019, 133, 187–200. [Google Scholar] [CrossRef]
- Valipour, A.; Osowski, S.; Rey, J.; Ochsendorf, F.; Weberschock, T. Semen cryopreservation in adolescent and adult men undergoing fertility compromising cancer treatment: A systematic review. Andrologia 2019, 51, e13392. [Google Scholar] [CrossRef]
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009, 10, 49–62. [Google Scholar] [CrossRef]
- Benson, J.D.; Woods, E.J.; Walters, E.M.; Critser, J.K. The cryobiology of spermatozoa. Theriogenology 2012, 78, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016, 169, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Karimi, I.; Ahmadi, S.; Mohammed, L.J. The necessity of antioxidant inclusion in caprine and ovine semen extenders: A systematic review complemented with computational insight. Reprod. Domest. Anim. 2020, 55, 1027–1043. [Google Scholar] [CrossRef]
- Bresciani, C.; Cabassi, C.S.; Morini, G.; Taddei, S.; Bettini, R.; Bigliardi, E.; Di Ianni, F.; Sabbioni, A.; Parmigiani, E. Boar semen bacterial contamination in Italy and antibiotic efficacy in a modified extender. Ital. J. Anim. Sci. 2014, 13, 83–87. [Google Scholar] [CrossRef]
- Koziorowska-Gilun, M.; Koziorowski, M.; Fraser, L.; Strzezek, J. Antioxidant defence system of boar cauda epididymidal spermatozoa and reproductive tract fluids. Reprod. Domest. Anim. 2011, 46, 527–533. [Google Scholar] [CrossRef]
- Tosic, J.; Walton, A. Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem. J. 1950, 47, 199–212. [Google Scholar] [CrossRef]
- Aitken, J.B.; Naumovski, N.; Curry, B.; Grupen, C.G.; Gibb, Z.; Aitken, R.J. Characterization of an L-amino acid oxidase in equine spermatozoa. Biol. Reprod. 2015, 92, 125. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Althouse, G.C.; Lu, K.G. Bacteriospermia in extended porcine semen. Theriogenology 2005, 63, 573–584. [Google Scholar] [CrossRef]
- Gączarzewicz, D.; Udała, J.; Piasecka, M.; Błaszczyk, B.; Stankiewicz, T. Bacterial contamination of boar semen and its relationship to sperm quality preserved in commercial extender containing gentamicin sulfate. Pol. J. Vet. Sci. 2016, 19, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Harrison, R.A.P.; Petrunkina, A.M. Cryobiological determinants of frozen semen quality, with special reference to stallion. Anim. Reprod. Sci. 2008, 107, 276–292. [Google Scholar] [CrossRef]
- Ros-Santaella, J.L.; Domínguez-Rebolledo, A.E.; Garde, J.J. Sperm flagellum volume determines freezability in red deer spermatozoa. PLoS ONE 2014, 9, e112382. [Google Scholar] [CrossRef]
- Billard, R.; Cosson, J.; Linhart, O. Changes in the flagellum morphology of intact and frozen/thawed Siberian sturgeon Acipenser baerii (Brandt) sperm during motility. Aquac. Res. 2000, 31, 283–287. [Google Scholar] [CrossRef]
- Watson, P. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Aparicio, I.M.; Martin Muñoz, P.; Salido, G.M.; Peña, F.J.; Tapia, J.A. The autophagy-related protein LC3 is processed in stallion spermatozoa during short-and long-term storage and the related stressful conditions. Animal 2016, 10, 1182–1191. [Google Scholar] [CrossRef]
- Leisegang, K. Herbal Pharmacognosy: An introduction. In Herbal Medicine in Andrology, 1st ed.; Henkel, R., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 3; pp. 17–26. [Google Scholar]
- Gonzales, G.F.; Gasco, M.; Vasquez-Velasquez, C.; Fano-Sizgorich, D.; Alarcón-Yaquetto, D.E. Herbal medicine used to treat andrological problems: Americas. In Herbal Medicine in Andrology, 1st ed.; Henkel, R., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 5.1; pp. 47–66. [Google Scholar]
- Silberstein, T.; Har-Vardi, I.; Harlev, A.; Friger, M.; Hamou, B.; Barac, T.; Levitas, E.; Saphier, O. Antioxidants and Polyphenols: Concentrations and Relation to Male Infertility and Treatment Success. Oxid. Med. Cell. Longev. 2016, 2016, 9140925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, Y.; Du, M.; Liu, W.; Ren, Y.; Zhang, C.; Zhang, J. The effect of dietary grape pomace supplementation on epididymal sperm quality and testicular antioxidant ability in ram lambs. Theriogenology 2017, 97, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ros-Santaella, J.L.; Kotrba, R.; Pintus, E. High-energy diet enhances spermatogenic function and increases sperm midpiece length in fallow deer (Dama dama) yearlings. R. Soc. Open Sci. 2019, 6, 181972. [Google Scholar] [CrossRef]
- Pini, T.; Makloski, R.; Maruniak, K.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Mitigating the effects of oxidative sperm DNA damage. Antioxidants 2020, 9, 589. [Google Scholar] [CrossRef]
- Glimn-Lacy, J.; Kaufman, P.B. Botany Illustrated. Introduction to Plants, Major Groups, Flowering Plant Families, 1st ed.; Springer: New York, NY, USA, 2006; 278p. [Google Scholar]
- Heywood, V.H.; Brummitt, R.K.; Culham, A.; Seberg, O. Flowering Plant Families of the World, 1st ed.; Firefly Books: Richmond Hill, ON, Canada, 2007; 424p. [Google Scholar]
- Kubitzki, K.; Rohwer, G.; Bittrich, V. The Families and Genera of Vascular Plants. Flowering Plants. Dicotyledons. Magnoliid, Hamamelid and Caryophyllid Families, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume II, 663p. [Google Scholar]
- Bayton, R.; Maughan, S. Plant Families: A Guide for Gardeners and Botanists, 1st ed.; The University of Chicago Press: Chicago, IL, USA, 2017; 224p. [Google Scholar]
- Zomlefer, W.B. Guide to Flowering Plant Families, 1st ed.; University of North Carolina Press: Chapel Hill, NC, USA, 1994; 430p. [Google Scholar]
- Alves, I.A.B.S.; Miranda, H.M.; Soares, L.A.L.; Randau, K.P. Simaroubaceae family: Botany, chemical composition and biological activities. Rev. Bras. Farmacogn. 2014, 24, 481–501. [Google Scholar] [CrossRef]
- Clayton, J.W.; Fernando, E.S.; Soltis, P.S.; Soltis, D.E. Molecular phylogeny of the tree-of-heaven family (Simaroubaceae) based on chloroplast and nuclear markers. Int. J. Plant Sci. 2007, 168, 1325–1339. [Google Scholar] [CrossRef]
- Der, J.P.; Nickrent, D.L. A molecular phylogeny of Santalaceae (Santalales). Syst. Bot. 2008, 33, 107–116. [Google Scholar] [CrossRef]
- Wallnöfer, B. The biology and systematics of Ebenaceae: A review. Ann. Nat. Mus. Wien. Ser. B Bot. Zool. 2001, 103, 485–512. [Google Scholar]
- Abdramanov, A.; Massanyi, P.; Sarsembayeva, N.; Usenbayev, A.; Alimov, J.; Tvrdá, E. The in vitro effect of elderberry (Sambucus nigra) extract on the activity and oxidative profile of bovine spermatozoa. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1319–1322. [Google Scholar] [CrossRef]
- Malo, C.; Gil, L.; Cano, R.; González, N.; Luño, V. Fennel (Foeniculum vulgare) provides antioxidant protection for boar semen cryopreservation. Andrologia 2012, 44, 710–715. [Google Scholar] [CrossRef]
- Najafi, A.; Daghigh Kia, H.; Mehdipour, M.; Shamsollahi, M.; Miller, D.J. Does fennel extract ameliorate oxidative stress frozen-thawed ram sperm? Cryobiology 2019, 87, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Luño, V.; Gil, L.; Olaciregui, M.; Jerez, R.A.; de Blas, I.; Hozbor, F. Antioxidant effect of lemon balm (Melissa officinalis) and mate tea (Ilex paraguensys) on quality, lipid peroxidation and DNA oxidation of cryopreserved boar epididymal spermatozoa. Andrologia 2015, 47, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- El-Sheshtawy, R.I.; El-Nattat, W.S.; Shalaby, S.I.A.; Shahba, M.I.; Al-Sedawy, I.E. Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG). Asian Pac. J. Reprod. 2016, 5, 252–255. [Google Scholar] [CrossRef]
- El-Sisy, G.A.; El-Badry, D.A.; El-Sheshtawy, R.I.; El-Nattat, W.S. Effects of Phoenix dactylifera pollen grains extract supplementation on post-thaw quality of arabian stallion semen. Bulg. J. Vet. Med. 2018, 21, 40–49. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Abdulkareem, T.A. Some post-cryopreserved semen characteristics of Holstein Bulls as influenced by adding aquoeus extract of Urtica dioica and date palm pollen powder to Tris extender. Plant Arch. 2020, 20, 461–467. [Google Scholar]
- Souza, A.L.P.; Lima, G.L.; Peixoto, G.C.X.; Silva, A.M.; Oliveira, M.F.; Silva, A.R. Use of Aloe vera–based extender for chilling and freezing collared peccary (Pecari tajacu) semen. Theriogenology 2016, 85, 1432–1438. [Google Scholar] [CrossRef]
- Najafi, D.; Taheri, R.A.; Najafi, A.; Rouhollahi, A.A.; Alvarez-Rodriguez, M. Effect of Achillea millefolium-loaded nanophytosome in the post-thawing sperm quality and oxidative status of rooster semen. Cryobiology 2018, 82, 37–42. [Google Scholar] [CrossRef]
- Merati, Z.; Farshad, A. Ginger and echinacea extracts improve the quality and fertility potential of frozen-thawed ram epididymal spermatozoa. Cryobiology 2020, 92, 138–145. [Google Scholar] [CrossRef]
- Allai, L.; Druart, X.; Louanjli, N.; Contell, J.; Nasser, B.; El Amiri, B. Improvements of ram semen quality using cactus seed oil during liquid preservation in Tris egg yolk and skim milk based extenders. Small Rumin. Res. 2017, 151, 16–21. [Google Scholar] [CrossRef]
- Allai, L.; Druart, X.; Öztürk, M.; BenMoula, A.; Nasser, B.; El Amiri, B. Protective effects of Opuntia ficus-indica extract on ram sperm quality, lipid peroxidation and DNA fragmentation during liquid storage. Anim. Reprod. Sci. 2016, 175, 1–9. [Google Scholar] [CrossRef]
- Khojasteh Rad, M.; Ghani, A.; Ghani, E. In vitro effects of Capparis spinosa L. extract on human sperm function, DNA fragmentation, and oxidative stress. J. Ethnopharmacol. 2021, 269, 113702. [Google Scholar] [CrossRef]
- Zhao, H.W.; Li, Q.W.; Ning, G.Z.; Han, Z.S.; Jiang, Z.L.; Duan, Y.F. Rhodiola sacra aqueous extract (RSAE) improves biochemical and sperm characteristics in cryopreserved boar semen. Theriogenology 2009, 71, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Li, Q.W.; Zhang, T.; Jiang, Z.L. Effect of Gynostemma Pentaphyllum Polysaccharide on boar spermatozoa quality following freezing-thawing. Cryobiology 2009, 59, 244–249. [Google Scholar] [CrossRef]
- El-Sheshtawy, R.I.; El-Nattat, W.S. Effect of Diospyros kaki enriched extender on cattle bull sperm parameters and conception rate. Asian Pac. J. Reprod. 2017, 6, 128–132. [Google Scholar] [CrossRef]
- Sobeh, M.; Hassan, S.A.; El Raey, M.A.; Khalil, W.A.; Hassan, M.A.E.; Wink, M. Polyphenolics from Albizia harveyi exhibit antioxidant activities and counteract oxidative damage and ultra-structural changes of cryopreserved bull semen. Molecules 2017, 22, 1993. [Google Scholar] [CrossRef]
- Ros-Santaella, J.L.; Pintus, E. Rooibos (Aspalathus linearis) extract enhances boar sperm velocity up to 96 hours of semen storage. PLoS ONE 2017, 12, e0183682. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, A.; Aghaz, F.; Golestan Jahromi, M.; Bakhtiari, M.; Khazaei, M. Does supplementation of sperm freezing/thawing media with Ceratonia siliqua improve detrimental effect of cryopreservation on sperm parameters and chromatin quality in normozoospermic specimens? Cell Tissue Bank. 2019, 20, 403–409. [Google Scholar] [CrossRef]
- Ros-Santaella, J.L.; Kadlec, M.; Pintus, E. Pharmacological Activity of Honeybush (Cyclopia intermedia) in Boar Spermatozoa during Semen Storage and under Oxidative Stress. Animals 2020, 10, 463. [Google Scholar] [CrossRef]
- Sobeh, M.; Hassan, S.A.; Hassan, M.A.E.; Khalil, W.A.; Abdelfattah, M.A.O.; Wink, M.; Yasri, A. A Polyphenol-Rich Extract From Entada abyssinica Reduces Oxidative Damage in Cryopreserved Ram Semen. Front. Vet. Sci. 2020, 7, 604477. [Google Scholar] [CrossRef]
- Daghigh Kia, H.; Farhadi, R.; Ashrafi, I.; Mehdipour, M. Anti-oxidative effects of ethanol extract of Origanum vulgare on kinetics, microscopic and oxidative parameters of cryopreserved holstein bull spermatozoa. Iran. J. Appl. Anim. Sci. 2016, 6, 783–789. [Google Scholar]
- Mbaye, M.M.; El Khalfi, B.; Ouzamode, S.; Saadani, B.; Louanjli, N.; Soukri, A. Effect of Origanum vulgare Essential Oil Supplementation on the Advanced Parameters of Mobility and on the Integrity of Human Sperm DNA. Int. J. Reprod. Med. 2020, 2020, 1230274. [Google Scholar] [CrossRef] [PubMed]
- Ďuračka, M.; Galovičová, L.; Slávik, M.; Árvay, J.; Tvrdá, E. The in vitro effect of the Origanum vulgare extract on semen. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1089–1092. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, Z.L.; Liu, H.; Li, Q.W. Effect of Salvia miltiorrhiza polysaccharides on boar spermatozoa during freezing-thawing. Anim. Reprod. Sci. 2015, 159, 25–30. [Google Scholar] [CrossRef]
- Monton, A.; Gil, L.; Malo, C.; Olaciregui, M.; Gonzalez, N.; de Blas, I. Sage (Salvia officinalis) and fennel (Foeniculum vulgare) improve cryopreserved boar epididymal semen quality study. Cryo Lett. 2015, 36, 83–90. [Google Scholar]
- Malo, C.; Gil, L.; Gonzalez, N.; Martínez, F.; Cano, R.; de Blas, I.; Espinosa, E. Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: Comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 2010, 61, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Ventrella, D.; Barone, F.; Filippini, G.; Benvenuti, S.; Pisi, A.; Scozzoli, M.; Bacci, M.L. Thymbra capitata (L.) cav. and Rosmarinus officinalis (L.) Essential oils: In vitro effects and toxicity on swine spermatozoa. Molecules 2017, 22, 2162. [Google Scholar] [CrossRef]
- Elmi, A.; Prosperi, A.; Zannoni, A.; Bertocchi, M.; Scorpio, D.G.; Forni, M.; Foni, E.; Bacci, M.L.; Ventrella, D. Antimicrobial capabilities of non-spermicidal concentrations of tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis) essential oils on the liquid phase of refrigerated swine seminal doses. Res. Vet. Sci. 2019, 127, 76–81. [Google Scholar] [CrossRef]
- Zanganeh, Z.; Zhandi, M.; Zare-Shahneh, A.; Najafi, A.; Mahdi Nabi, M.; Mohammadi-Sangcheshmeh, A. Does rosemary aqueous extract improve buck semen cryopreservation? Small Rumin. Res. 2013, 114, 120–125. [Google Scholar] [CrossRef]
- Motlagh, M.K.; Sharafi, M.; Zhandi, M.; Mohammadi-Sangcheshmeh, A.; Shakeri, M.; Soleimani, M.; Zeinoaldini, S. Antioxidant effect of rosemary (Rosmarinus officinalis L.) extract in soybean lecithin-based semen extender following freeze-thawing process of ram sperm. Cryobiology 2014, 69, 217–222. [Google Scholar] [CrossRef]
- Touazi, L.; Aberkane, B.; Bellik, Y.; Moula, N.; Iguer-Ouada, M. Effect of the essential oil of Rosmarinus officinalis (L.) on rooster sperm motility during 4 °C short-term storage. Vet. World 2018, 11, 590–597. [Google Scholar] [CrossRef]
- Chikhoune, A.; Stouvenel, L.; Iguer-Ouada, M.; Hazzit, M.; Schmitt, A.; Lorès, P.; Wolf, J.P.; Aissat, K.; Auger, J.; Vaiman, D.; et al. In-vitro effects of Thymus munbyanus essential oil and thymol on human sperm motility and function. Reprod. Biomed. Online 2015, 31, 411–420. [Google Scholar] [CrossRef]
- Javed, M.; Tunio, M.T.; Abdul Rauf, H.; Bhutta, M.F.; Naz, S.; Iqbal, S. Addition of pomegranate juice (Punica granatum) in tris-based extender improves post-thaw quality, motion dynamics and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull spermatozoa. Andrologia 2019, 51, e13322. [Google Scholar] [CrossRef] [PubMed]
- El-Sheshtawy, R.I.; El-Sisy, G.A.; El-Nattat, W.S. Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation. Asian Pac. J. Reprod. 2016, 5, 335–339. [Google Scholar] [CrossRef]
- Mehdipour, M.; Kia, H.D.; Nazari, M.; Najafi, A. Effect of lecithin nanoliposome or soybean lecithin supplemented by pomegranate extract on post-thaw flow cytometric, microscopic and oxidative parameters in ram semen. Cryobiology 2017, 78, 34–40. [Google Scholar] [CrossRef]
- Nimrat, S.; Noppakun, S.; Sripuak, K.; Boonthai, T.; Vuthiphandchai, V. Cryopreservation of banana shrimp (Fenneropenaeus merguiensis) spermatophores with supplementation of medicinal plant extracts: Development of a programmable controlled-rate method and a practical method. Aquaculture 2020, 515, 734537. [Google Scholar] [CrossRef]
- Nimrat, S.; Playsin, N.; Jong-on, B.; Boonthai, T.; Vuthiphandchai, V. Chilled storage of banana shrimp (Fenneropenaeus merguiensis) spermatophores with supplementation of moringa (Moringa oleifera Lam.) extract. Aquac. Res. 2020, 51, 3582–3592. [Google Scholar] [CrossRef]
- Carrera-Chávez, J.M.; Jiménez-Aguilar, E.E.; Acosta-Pérez, T.P.; Núñez-Gastélum, J.A.; Quezada-Casasola, A.; Escárcega-Ávila, A.M.; Itza-Ortiz, M.F.; Orozco-Lucero, E. Effect of Moringa oleifera seed extract on antioxidant activity and sperm characteristics in cryopreserved ram semen. J. Appl. Anim. Res. 2020, 48, 114–120. [Google Scholar] [CrossRef]
- Elmi, A.; Ventrella, D.; Barone, F.; Carnevali, G.; Filippini, G.; Pisi, A.; Benvenuti, S.; Scozzoli, M.; Bacci, M.L. In vitro effects of tea tree oil (Melaleuca alternifolia essential oil) and its principal component terpinen-4-ol on swine spermatozoa. Molecules 2019, 24, 1071. [Google Scholar] [CrossRef]
- Cavalleri, R.; Becker, J.S.; Pavan, A.M.; Bianchetti, P.; Goettert, M.I.; Ethur, E.M.; Bustamante-Filho, I.C. Essential oils rich in monoterpenes are unsuitable as additives to boar semen extender. Andrologia 2018, 50, e13074. [Google Scholar] [CrossRef]
- Wurlina, W.; Hariadi, M.; Safitri, E.; Susilowati, S.; Meles, D.K. The effect of crude guava leaf tannins on motility, viability, and intact plasma membrane of stored spermatozoa of Etawa crossbred goats. Vet. World 2020, 13, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Baghshahi, H.; Riasi, A.; Mahdavi, A.H.; Shirazi, A. Antioxidant effects of clove bud (Syzygium aromaticum) extract used with different extenders on ram spermatozoa during cryopreservation. Cryobiology 2014, 69, 482–487. [Google Scholar] [CrossRef]
- Suleman, S.; Kanwal, M.A.; Malik, F.; Ali, R.; Siddique, S.; Kanwal, N.; Ahmad, S.N.; Younis, A.; Hussain, I.; Ahmad, K.R. Jambul (Syzygium cumini) Pulp Extract Enhances Viability, Motility, and In Vitro Fertilizability of Cryopreserved Bovine Semen. Biopreserv. Biobank. 2020, 19. [Google Scholar] [CrossRef]
- Jofré, I.; Cuevas, M.; De Castro, L.S.; De Agostini, L.J.D.; Torres, M.A.; Alvear, M.; Scheuermann, E.; Andrade, A.F.C.; Nichi, M.; Assumpção, M.E.O.; et al. Antioxidant Effect of a Polyphenol-Rich Murtilla (Ugni molinae Turcz.) extract and its effect on the regulation of metabolism in refrigerated boar sperm. Oxid. Med. Cell. Longev. 2019, 2019, 2917513. [Google Scholar] [CrossRef]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Husna, A.U.; Azam, A.; Ullah, N.; Walker, V.K.; Akhter, S. Cryopreservation of nili-ravi buffalo bull sperm in cryodiluent supplemented with Lolium perenne protein preparations. Cryo-Lett. 2017, 38, 43–50. [Google Scholar]
- Azimi, G.; Farshad, A.; Farzinpour, A.; Rostamzadeh, J.; Sharafi, M. Evaluation of used Purslane extracts in Tris extenders on cryopreserved goat sperm. Cryobiology 2020, 94, 40–48. [Google Scholar] [CrossRef]
- Shikh Maidin, M.; Padlan, M.H.; Azuan, S.A.N.; Jonit, R.; Mohammed, N.H.; Abdullah, R. Supplementation of Nigella sativa oil and honey prolong the survival rate of fresh and post-thawed goat sperms. Trop. Anim. Sci. J. 2018, 41, 94–99. [Google Scholar] [CrossRef]
- Miah, A.G.; Bathgate, R.; Hamano, K.-I.; Salma, U. Effects of pre-freeze Nigella sativa oil supplementation on cryosurvival of ovine spermatozoa. Reprod. Domest. Anim. 2018, 53, 1424–1433. [Google Scholar] [CrossRef]
- Halo, M.; Massanyi, P.; Gren, A.; Lasak, A.; Slanina, T.; Ondruska, L.; Muchacka, R.; Galbavy, D.; Ivanic, P.; Schneir, E.R.; et al. Time and dose-dependent effects of Viscum album quercus on rabbit spermatozoa motility and viability in Vitro. Physiol. Res. 2019, 68, 955–972. [Google Scholar] [CrossRef]
- Allai, L.; Druart, X.; Contell, J.; Louanjli, N.; Moula, A.B.; Badi, A.; Essamadi, A.; Nasser, B.; El Amiri, B. Effect of argan oil on liquid storage of ram semen in Tris or skim milk based extenders. Anim. Reprod. Sci. 2015, 160, 57–67. [Google Scholar] [CrossRef]
- Tvrdá, E.; Michalko, J.; Árvay, J.; Vukovic, N.L.; Ivanišová, E.; Ďuračka, M.; Matušíková, I.; Kačániová, M. Characterization of the Omija (Schisandra chinensis) Extract and Its Effects on the Bovine Sperm Vitality and Oxidative Profile during in Vitro Storage. Evid. Based Complement. Altern. Med. 2020, 2020, 7123780. [Google Scholar] [CrossRef]
- Baiee, F.H.; Wahid, H.; Rosnina, Y.; Ariff, O.; Yimer, N.; Jeber, Z.; Salman, H.; Tarig, A.; Harighi, F. Impact of Eurycoma longifolia extract on DNA integrity, lipid peroxidation, and functional parameters in chilled and cryopreserved bull sperm. Cryobiology 2018, 80, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gale, I.; Gil, L.; Malo, C.; González, N.; Martínez, F. Effect of Camellia sinensis supplementation and increasing holding time on quality of cryopreserved boar semen. Andrologia 2015, 47, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kitaji, H.; Ookutsu, S.; Sato, M.; Miyoshi, K. Preincubation with green tea polyphenol extract is beneficial for attenuating sperm injury caused by freezing-thawing in swine. Anim. Sci. J. 2015, 86, 922–928. [Google Scholar] [CrossRef]
- Park, S.H.; Jeon, Y.; Yu, I.J. Effects of green tea (Camellia sinensis) extract supplementation at different dilution steps on boar sperm cryopreservation and in vitro fertilization. J. Vet. Clin. 2018, 35, 39–45. [Google Scholar] [CrossRef]
- Wittayarat, M.; Kimura, T.; Kodama, R.; Namula, Z.; Chatdarong, K.; Techakumphu, M.; Sato, Y.; Taniguchi, M.; Oto, T. Long-term preservation of chilled canine semen using vitamin C in combination with green tea polyphenol. Cryo-Lett. 2012, 33, 318–326. [Google Scholar]
- Dias, T.R.; Alves, M.G.; Tomás, G.D.; Socorro, S.; Silva, B.M.; Oliveira, P.F. White tea as a promising antioxidant medium additive for sperm storage at room temperature: A comparative study with green tea. J. Agric. Food Chem. 2014, 62, 608–617. [Google Scholar] [CrossRef]
- Mehdipour, M.; Daghigh Kia, H.; Najafi, A.; Vaseghi Dodaran, H.; García-Álvarez, O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology 2016, 73, 297–303. [Google Scholar] [CrossRef]
- Dos Reis, A.N.; Miranda, M.D.S.; Silva, L.K.X.; Da Silva, A.O.A.; De Sousa, J.S.; De Morais, E.; Garcia, A.R.; Domingues, S.F.S.; Da Silva, J.K.D.R.; Ribeiro, H.F.L. Comparative evaluation between the extenders TES-TRIS and ACP-112® and the association of Sálva Marajó oil (Lippia origanoides) in the quality of cryopreserved buffalo sperm. Semin. Agrar. 2017, 38, 3613–3628. [Google Scholar] [CrossRef]
- Asadmobini, A.; Bakhtiari, M.; Khaleghi, S.; Esmaeili, F.; Mostafaei, A. The effect of Tribulus terrestris extract on motility and viability of human sperms after cryopreservation. Cryobiology 2017, 75, 154–159. [Google Scholar] [CrossRef]
- Rahman, S.; Huang, Y.; Zhu, L.; Feng, S.; Khan, I.; Wu, J.; Li, Y.; Wang, X. Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review. Nutrients 2018, 10, 834. [Google Scholar] [CrossRef]
- Wu, M.; Ni, L.; Lu, H.; Xu, H.; Zou, S.; Zou, X. Terpenoids and Their Biological Activities from Cinnamomum: A Review. J. Chem. 2020, 2020, 5097542. [Google Scholar] [CrossRef]
- Woith, E.; Guerriero, G.; Hausman, J.F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant extracellular vesicles and nanovesicles: Focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef]
- Ferramosca, A.; Lorenzetti, S.; Di Giacomo, M.; Lunetti, P.; Murrieri, F.; Capobianco, L.; Dolce, V.; Coppola, L.; Zara, V. Modulation of human sperm mitochondrial respiration efficiency by plant polyphenols. Antioxidants 2021, 10, 217. [Google Scholar] [CrossRef]
- Olson, K.R.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Skora, N.C.; Whelan, J.; Villa, B.P.; Yuan, X.; Mannam, V.; Howard, S.; et al. Green tea polyphenolic antioxidants oxidize hydrogen sulfide to thiosulfate and polysulfides: A possible new mechanism underpinning their biological action. Redox Biol. 2020, 37, 101731. [Google Scholar] [CrossRef]
- Joubert, E.; Beer, D. De Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Patnala, S.; Kanfer, I. Quality control, extraction methods, and standardization: Interface between traditional use and scientific investigation. In Herbal Medicine in Andrology, 1st ed.; Henkel, R., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 6; pp. 175–187. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ros-Santaella, J.L.; Pintus, E. Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants 2021, 10, 772. https://doi.org/10.3390/antiox10050772
Ros-Santaella JL, Pintus E. Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants. 2021; 10(5):772. https://doi.org/10.3390/antiox10050772
Chicago/Turabian StyleRos-Santaella, José Luis, and Eliana Pintus. 2021. "Plant Extracts as Alternative Additives for Sperm Preservation" Antioxidants 10, no. 5: 772. https://doi.org/10.3390/antiox10050772
APA StyleRos-Santaella, J. L., & Pintus, E. (2021). Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants, 10(5), 772. https://doi.org/10.3390/antiox10050772






