Antioxidant or Apoptosis Inhibitor Supplementation in Culture Media Improves Post-Thaw Recovery of Murine Spermatogonial Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Isolation and Culture of Germ Cells Enriched for SSCs
2.3. Cryopreservation
2.4. Evaluation of the Relative Proliferation Rate after Antioxidant or Apoptosis Inhibitor Treatment
2.5. ROS Generation Assay
2.6. Apoptosis Assay
2.7. Western Blot
2.8. Immunofluorescence
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. In Vivo Transplantation
2.11. Statistical Analysis
3. Results
3.1. Supplementation of Antioxidants or Apoptosis Inhibitors in the Post-Thaw Culture Media Improves the Proliferation Rates of Germ Cells
3.2. Protective Effects of Antioxidants and the Apoptosis Inhibitor in the Post-Thaw Recovery Phase
3.3. Stable Characterization after Post-Thaw Recovery with Antioxidants or Apoptosis Inhibitors
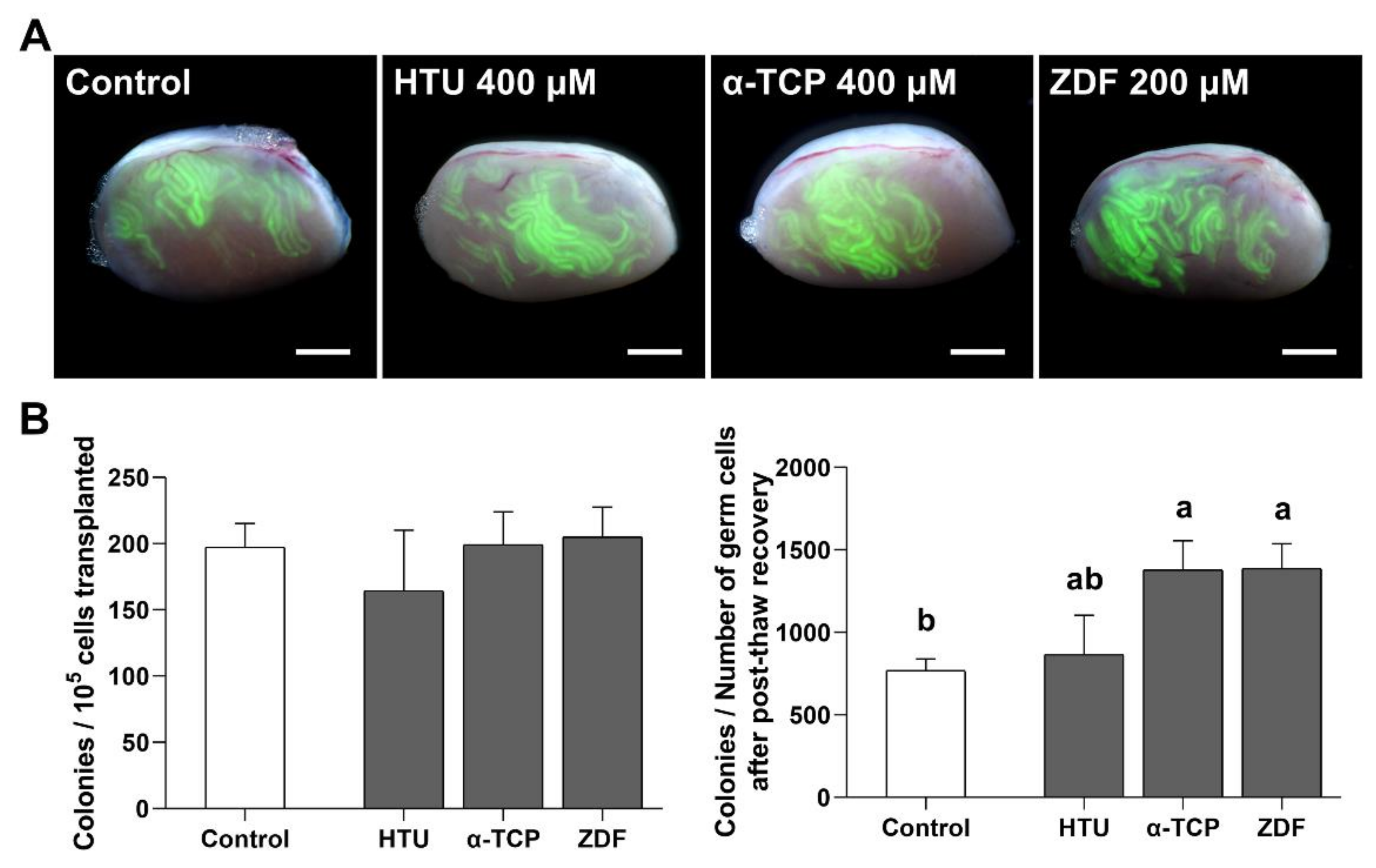
3.4. Functional Activity of SSCs after Post-Thaw Recovery with Antioxidant or Apoptosis Inhibitor Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griswold, M.D.; Oatley, J.M. Concise review: Defining characteristics of mammalian spermatogenic stem cells. Stem Cells 2013, 31, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-Y. Production of Transgenic Animals Using Spermatogonial Stem Cells. Agric. Sci. China 2011, 10, 762–768. [Google Scholar] [CrossRef]
- Oatley, J.M. Recent advances for spermatogonial stem cell transplantation in livestock. Reprod. Fertil. Dev. 2017, 30, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sadri-Ardekani, H.; Atala, A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: From bench to bedside. Stem Cell Res. Ther. 2014, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Len, J.S.; Koh, W.S.D.; Tan, S.X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cowley, S.; Flaim, C.J.; James, W.; Seymour, L.; Cui, Z. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol. Prog. 2010, 26, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Clement, M.V.; Cao, T. Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw survival rate of human embryonic stem cells. Biosci. Rep. 2007, 27, 257–264. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Diogo, P.; Dinis, M.T.; Herráez, M.P.; Sarasquete, C.; Cabrita, E. Incorporation of ascorbic acid and α-tocopherol to the extender media to enhance antioxidant system of cryopreserved sea bass sperm. Theriogenology 2012, 77, 1129–1136. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Diogo, P.; Dinis, M.T.; Soares, F.; Sarasquete, C.; Cabrita, E. Effect of two sulfur-containing amino acids, taurine and hypotaurine in European sea bass (Dicentrarchus labrax) sperm cryopreservation. Cryobiology 2013, 66, 333–338. [Google Scholar] [CrossRef]
- Ha, S.J.; Kim, B.G.; Lee, Y.A.; Kim, Y.H.; Kim, B.J.; Jung, S.E.; Pang, M.G.; Ryu, B.Y. Effect of Antioxidants and Apoptosis Inhibitors on Cryopreservation of Murine Germ Cells Enriched for Spermatogonial Stem Cells. PLoS ONE 2016, 11, e0161372. [Google Scholar] [CrossRef]
- Partyka, A.; Rodak, O.; Bajzert, J.; Kochan, J.; Niżański, W. The Effect of L-Carnitine, Hypotaurine, and Taurine Supplementation on the Quality of Cryopreserved Chicken Semen. Biomed Res. Int. 2017, 2017, 7279341. [Google Scholar] [CrossRef]
- Aliakbari, F.; Gilani, M.A.; Amidi, F.; Baazm, M.; Korouji, M.; Izadyar, F.; Yazdekhasti, H.; Abbasi, M. Improving the Efficacy of Cryopreservation of Spermatogonia Stem Cells by Antioxidant Supplements. Cell Reprogram. 2016, 18, 87–95. [Google Scholar] [CrossRef]
- Aliakbari, F.; Sedighi Gilani, M.A.; Yazdekhasti, H.; Koruji, M.; Asgari, H.R.; Baazm, M.; Izadyar, F.; Kharrazi Nejad, E.; Khanezad, M.; Abbasi, M. Effects of antioxidants, catalase and α-tocopherol on cell viability and oxidative stress variables in frozen-thawed mice spermatogonial stem cells. Artif. Cells Nanomed. Biotechnol. 2017, 45, 63–68. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, M.K.; Song, H.J.; Kang, E.J.; Ock, S.A.; Kumar, B.M.; Balasubramanian, S.; Rho, G.J. Effect of alpha-tocopherol supplementation during boar semen cryopreservation on sperm characteristics and expression of apoptosis related genes. Cryobiology 2009, 58, 181–189. [Google Scholar] [CrossRef]
- Bissoyi, A.; Pramanik, K. Role of the apoptosis pathway in cryopreservation-induced cell death in mesenchymal stem cells derived from umbilical cord blood. Biopreserv. Biobank. 2014, 12, 246–254. [Google Scholar] [CrossRef]
- Coundouris, J.A.; Grant, M.H.; Engeset, J.; Petrie, J.C.; Hawksworth, G.M. Cryopreservation of human adult hepatocytes for use in drug metabolism and toxicity studies. Xenobiotica 1993, 23, 1399–1409. [Google Scholar] [CrossRef]
- Kamalifar, S.; Azarpira, N.; Sadeghi, L.; Ghorbani-Dalini, S.; Nekoei, S.M.; Aghdaie, M.H.; Esfandiari, E.; Azarpira, M.R. ROCK Y-27632 Inhibitor, Ascorbic Acid, and Trehalose Increase Survival of Human Wharton Jelly Mesenchymal Stem Cells After Cryopreservation. Exp. Clin. Transplant. 2020, 18, 505–511. [Google Scholar] [CrossRef]
- Xiang, D.C.; Jia, B.Y.; Fu, X.W.; Guo, J.X.; Hong, Q.H.; Quan, G.B.; Wu, G.Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef]
- Winn, E.; Whitaker, B.D. Quercetin supplementation to the thawing and incubation media of boar sperm improves post-thaw sperm characteristics and the in vitro production of pig embryos. Reprod. Biol. 2020, 20, 315–320. [Google Scholar] [CrossRef]
- Truong, T.T.; Gardner, D.K. Antioxidants increase blastocyst cryosurvival and viability post-vitrification. Hum. Reprod. 2020, 35, 12–23. [Google Scholar] [CrossRef]
- Vanhulle, V.P.; Neyrinck, A.M.; Pycke, J.M.; Horsmans, Y.; Delzenne, N.M. Role of apoptotic signaling pathway in metabolic disturbances occurring in liver tissue after cryopreservation: Study on rat precision-cut liver slices. Life Sci. 2006, 78, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C. Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells. Tissue Cell 2009, 41, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Avarbock, M.R.; Brinster, R.L. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J. Biol. Chem. 2007, 282, 25842–25851. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. Spermatogonial stem cells. Methods Enzymol. 2006, 419, 259–282. [Google Scholar] [PubMed]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef]
- Lee, Y.A.; Kim, Y.H.; Kim, B.J.; Kim, B.G.; Kim, K.J.; Auh, J.H.; Schmidt, J.A.; Ryu, B.Y. Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells. PLoS ONE 2013, 8, e54889. [Google Scholar] [CrossRef][Green Version]
- Nishimura, T.; Duereh, M.; Sugita, Y.; Yoshida, Y.; Higuchi, K.; Tomi, M.; Nakashima, E. Protective effect of hypotaurine against oxidative stress-induced cytotoxicity in rat placental trophoblasts. Placenta 2015, 36, 693–698. [Google Scholar] [CrossRef]
- Lawrence, C.P.; Chow, S.C. Suppression of human T cell proliferation by the caspase inhibitors, z-VAD-FMK and z-IETD-FMK is independent of their caspase inhibition properties. Toxicol. Appl. Pharmacol. 2012, 265, 103–112. [Google Scholar] [CrossRef][Green Version]
- Brinster, R.L. Germline stem cell transplantation and transgenesis. Science 2002, 296, 2174–2176. [Google Scholar] [CrossRef]
- Ogawa, T.; Aréchaga, J.M.; Avarbock, M.R.; Brinster, R.L. Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 1997, 41, 111–122. [Google Scholar]
- Murray, K.A.; Gibson, M.I. Post-Thaw Culture and Measurement of Total Cell Recovery Is Crucial in the Evaluation of New Macromolecular Cryoprotectants. Biomacromolecules 2020, 21, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.P.; Goodman, H.O.; Hurst, C.H.; Shihabi, Z.K.; Jarow, J.P. Hypotaurine in male reproduction. Adv. Exp. Med. Biol. 1992, 315, 437–441. [Google Scholar] [PubMed]
- Jarak, I.; Almeida, S.; Carvalho, R.A.; Sousa, M.; Barros, A.; Alves, M.G.; Oliveira, P.F. Senescence and declining reproductive potential: Insight into molecular mechanisms through testicular metabolomics. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.T.; Mcclure, N.; Lewis, S.E.M. Glutathione and hypotaurine in vitro: Effects on human sperm motility, DNA integrity and production of reactive oxygen species. Mutagenesis 2000, 15, 61–68. [Google Scholar] [CrossRef]
- Celino, F.T.; Yamaguchi, S.; Miura, C.; Ohta, T.; Tozawa, Y.; Iwai, T.; Miura, T. Tolerance of spermatogonia to oxidative stress is due to high levels of Zn and Cu/Zn superoxide dismutase. PLoS ONE 2011, 6, e16938. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox. Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Ryoo, H.D.; Bergmann, A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
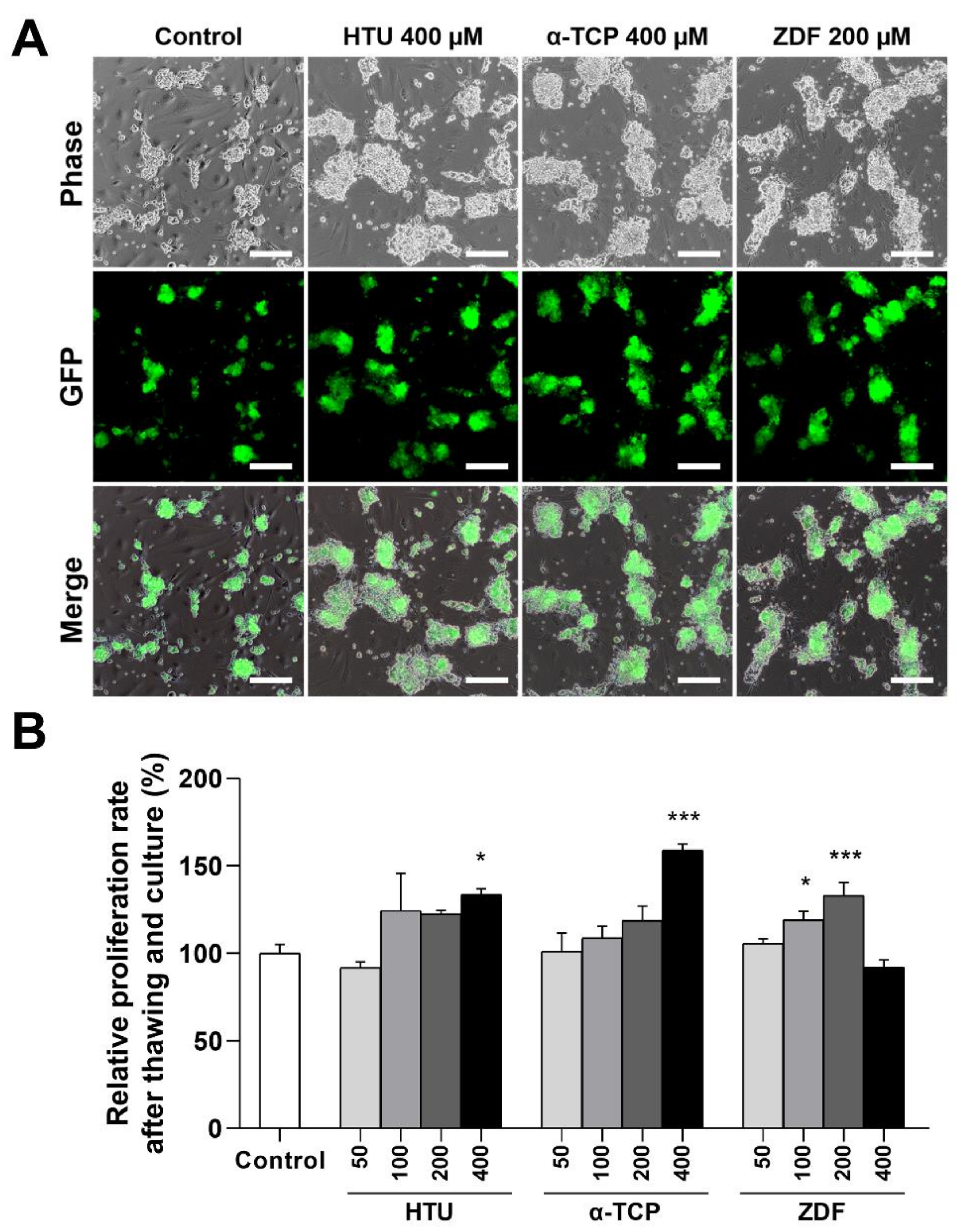
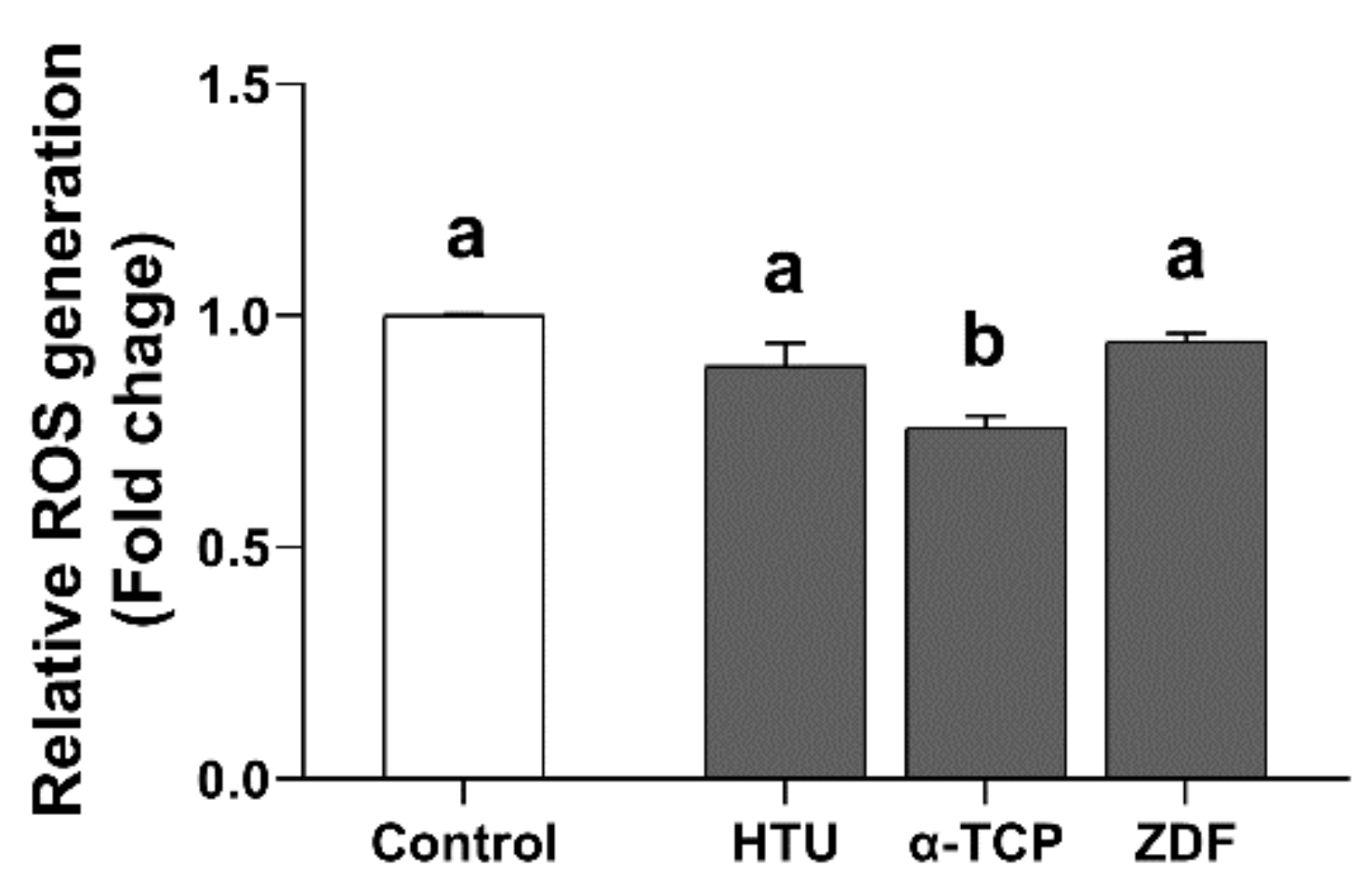
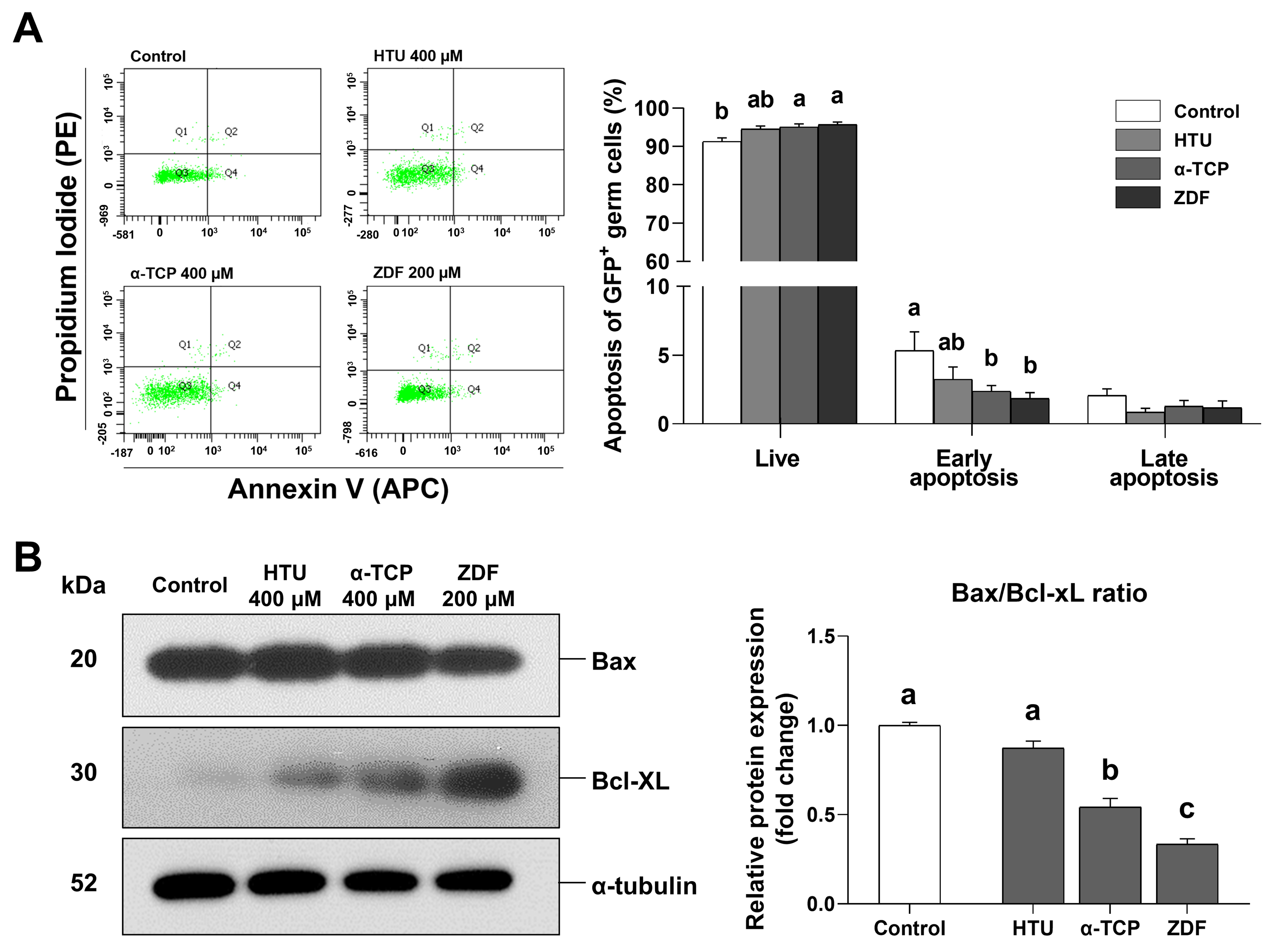
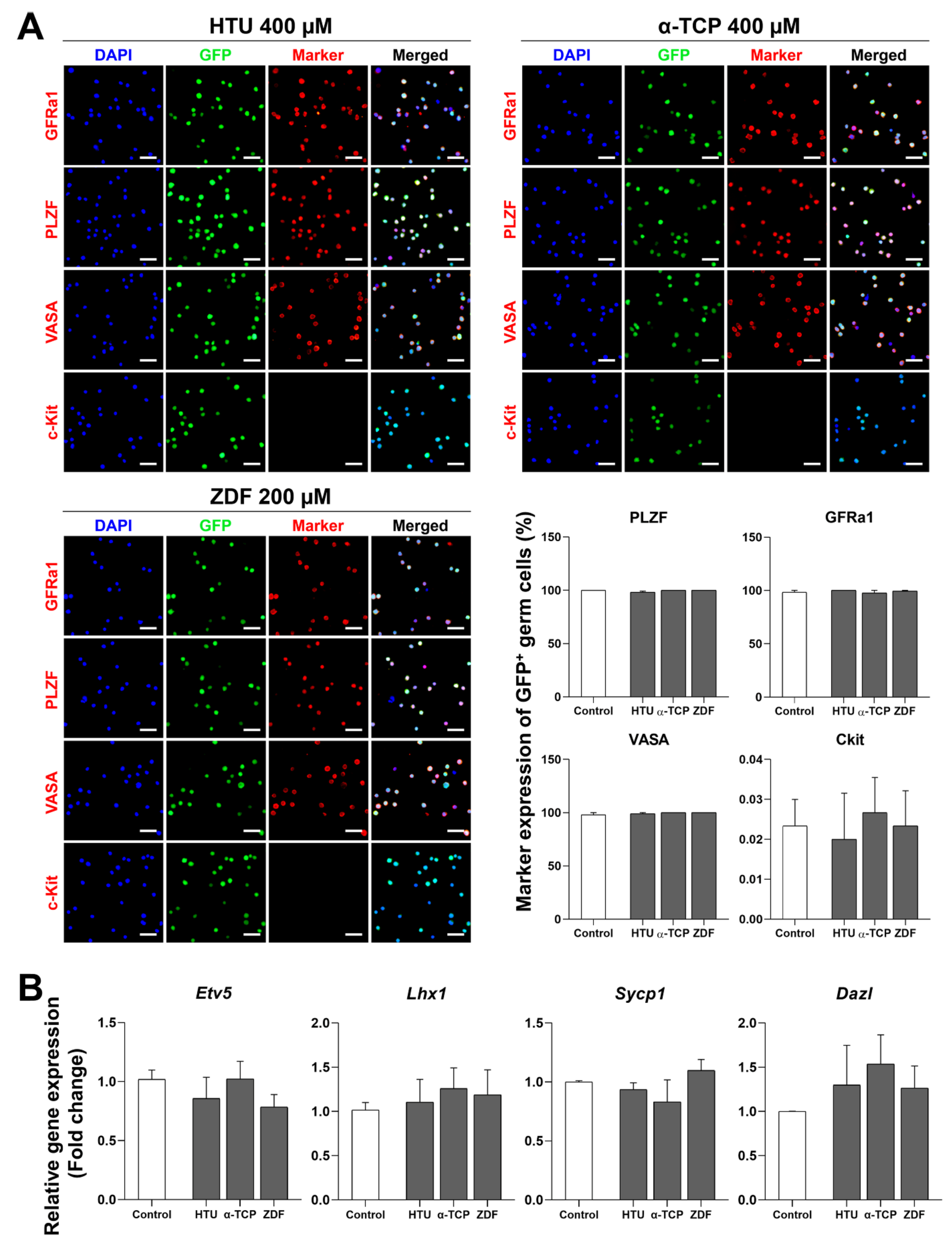
| Ingredients a | Final Concentration |
|---|---|
| Penicillin | 50 unit/mL |
| Streptomycin | 50 μg/mL |
| Bovine serum albumin | 0.2% |
| Iron-saturated transferrin | 10 μg/mL |
| Free fatty acids | 7.8 μEq/L |
| Na2SeO3 | 3 × 10−8 M |
| L-Glutamine | 2 mM |
| 2-Mercaptoethanol | 50 μg |
| Insulin | 5 μg/mL |
| N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) | 10 mM |
| Putrescine | 60 μM |
| Reagent | Property | Final Concentration (µM) | |||
|---|---|---|---|---|---|
| Hypotaurine (HTU) | Antioxidant | 50 | 100 | 200 | 400 |
| α-Tocopherol (α-TCP) | Antioxidant | 50 | 100 | 200 | 400 |
| Z-DEVD-FMK (ZDF) | Apoptosis inhibitor | 50 | 100 | 200 | 400 |
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Accession Number |
|---|---|---|---|
| Gapdh | TGACCCCTTCATTGACCTTC | TACTCAGCACCAGCATCACC | NM_008084.3 |
| Etv5 | CCCGGATGCACTCTTCTCTATG | TCGGATTCTGCCTTCAGGAA | NM_023794 |
| Lhx1 | CCCAGCTTTCCCGAATCCT | GCGGGACGTAAATAAATAAAATGG | NM_008498 |
| Scyp1 | CGCTACAACCACATGCTTCG | GGAACGCTGCTTAGATCTCCTC | NM_011516 |
| Dazl | AATGTTCAGTTCATGATGCTGCTC | TGTATGCTTCGGTCCACAGACT | NM_010021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-E.; Oh, H.-J.; Ahn, J.-S.; Kim, Y.-H.; Kim, B.-J.; Ryu, B.-Y. Antioxidant or Apoptosis Inhibitor Supplementation in Culture Media Improves Post-Thaw Recovery of Murine Spermatogonial Stem Cells. Antioxidants 2021, 10, 754. https://doi.org/10.3390/antiox10050754
Jung S-E, Oh H-J, Ahn J-S, Kim Y-H, Kim B-J, Ryu B-Y. Antioxidant or Apoptosis Inhibitor Supplementation in Culture Media Improves Post-Thaw Recovery of Murine Spermatogonial Stem Cells. Antioxidants. 2021; 10(5):754. https://doi.org/10.3390/antiox10050754
Chicago/Turabian StyleJung, Sang-Eun, Hui-Jo Oh, Jin-Seop Ahn, Yong-Hee Kim, Bang-Jin Kim, and Buom-Yong Ryu. 2021. "Antioxidant or Apoptosis Inhibitor Supplementation in Culture Media Improves Post-Thaw Recovery of Murine Spermatogonial Stem Cells" Antioxidants 10, no. 5: 754. https://doi.org/10.3390/antiox10050754
APA StyleJung, S.-E., Oh, H.-J., Ahn, J.-S., Kim, Y.-H., Kim, B.-J., & Ryu, B.-Y. (2021). Antioxidant or Apoptosis Inhibitor Supplementation in Culture Media Improves Post-Thaw Recovery of Murine Spermatogonial Stem Cells. Antioxidants, 10(5), 754. https://doi.org/10.3390/antiox10050754






