Antiglycating Effect of Phenolics from the Chilean Currant Ribes cucullatum under Thermal Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fruit Collection and Polyphenol-Enriched Extract (PEE) Preparation
2.3. Sarcoplasmic Protein Extraction
2.4. Incubation of Sarcoplasmic Proteins with Glyoxal and PEE from R. cucullatum
2.5. Quantification of Lysine Residues in Proteins Exposed to Glyoxal and PEE from R. cucullatum
2.6. Quantification of Arginine Residues in Proteins Exposed to Glyoxal and PEE from R. cucullatum
2.7. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.8. Immunochemical Detection and Quantification of Carboxymethyl-Lysine (CML)
2.9. Oxygen Consumption Analyses
2.10. Identification and Quantification of Main Phenolic Compounds Present in the PEE from R. cucullatum Exposed to Thermal Processing
2.11. Protein-Quinone Conjugation Determination by Means of Redox Cycling Staining
2.12. Determination of Lipid-Derived Electrophiles Content after Simulated Gastric Digestion
2.13. Statistical Analyses
3. Results
3.1. Characterization of Phenolic Compounds from Ribes cucullatum by Means of HPLC-DAD Analyses
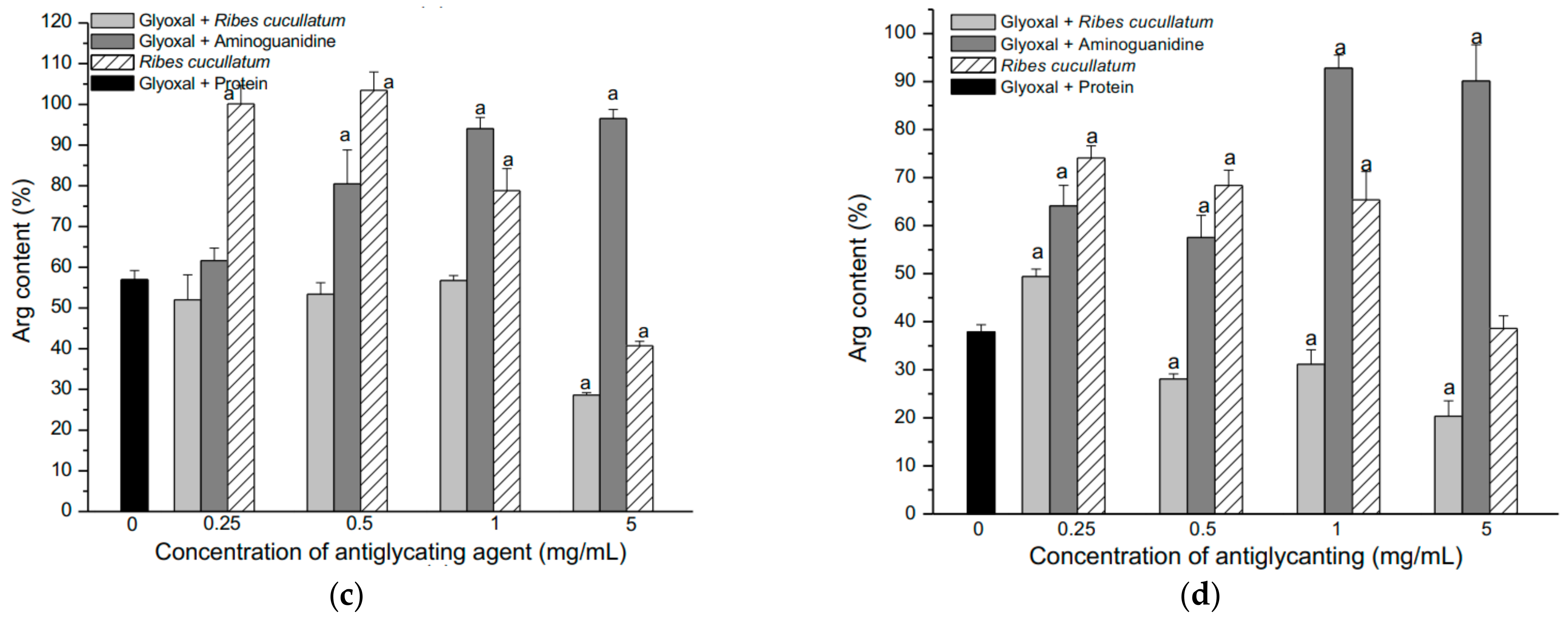
3.2. Evaluation of Antiglycating Activity of the PEE from R. cucullatum
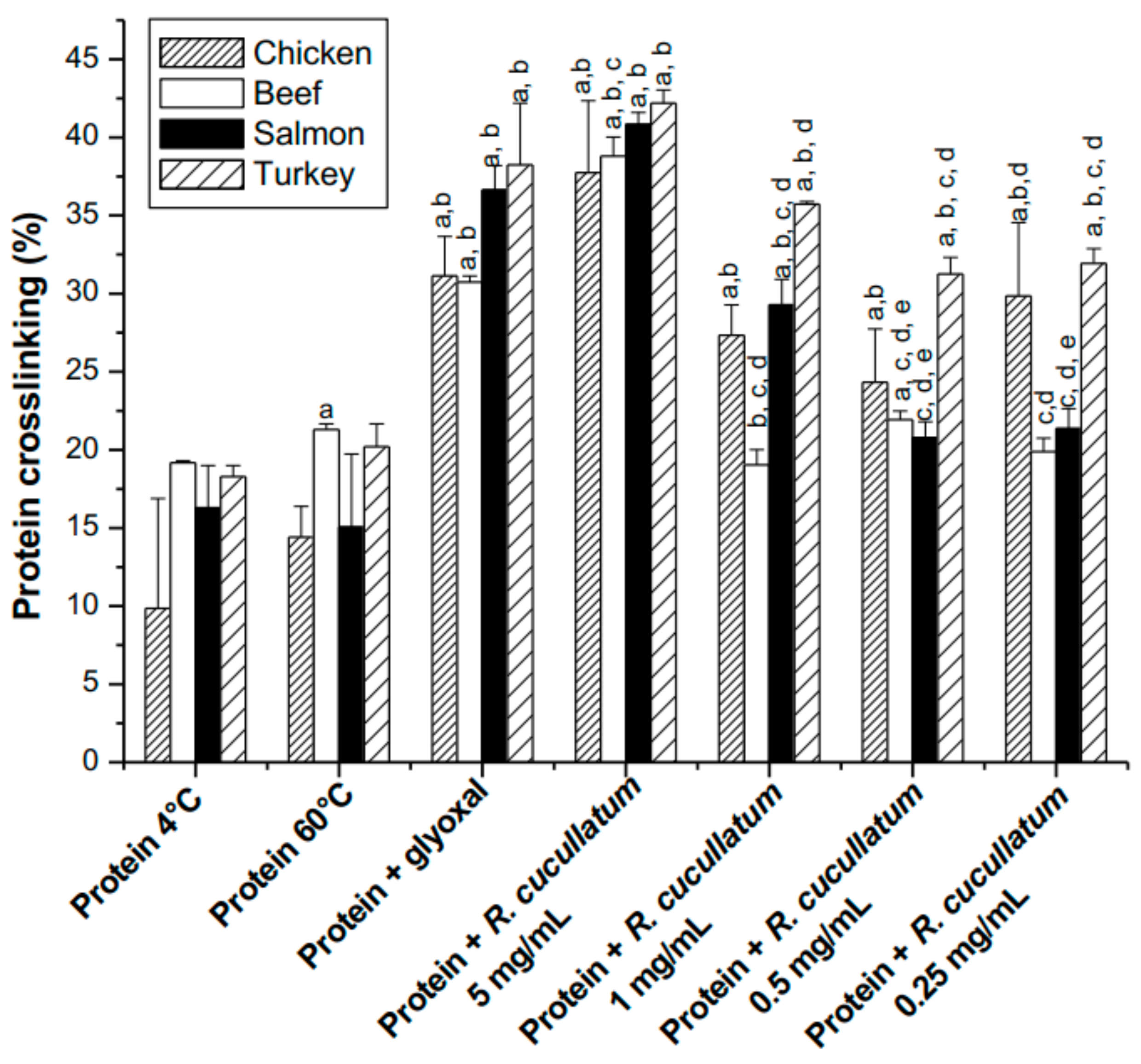
3.3. Proteomic and HPLC Analyses of the Effects Induced by PEE from R. cucullatum under Thermal Degradation Conditions in Meat Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- De Bry, L. Anthropological Implications of the Maillard Reaction: An Insight. In Maillard Reactions in Chemistry, Food and Health; Labuza, T.P., Reineccius, G.A., Monnier, V.M., O’Brien, J., Baynes, J.W., Eds.; Woodhead Publishing: Cambridge, UK, 2005; pp. 28–36. [Google Scholar]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.; Friguet, B.; Silva, E. Photosensitizing Activity of Endogenous Eye Lens Chromophores: An Attempt to Unravel Their Contributions to Photo-Aging and Cataract Disease. Photochem. Photobiol. 2015, 91, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, B.; Luth, H.J.; Haferburg, D.; Boeck, K.; Arendt, T.; Munch, G. Methylglyoxal, glyoxal, and their detoxification in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2005, 1043, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef]
- Koschinsky, T.; He, C.J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef]
- Baye, E.; Kiriakova, V.; Uribarri, J.; Moran, L.J.; de Courten, B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017, 7, 2266. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Muthenna, P.; Akileshwari, C.; Reddy, G.B. Ellagic acid, a new antiglycating agent: Its inhibition of N-(carboxymethyl)lysine. Biochem. J. 2012, 442, 221–230. [Google Scholar] [CrossRef]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.; Johns, T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Antioxidant and anti–glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Zhang, Y.; Rahnama, N.; Zerlin, A.; Thames, G.; Tseng, C.H.; Heber, D. Decrease of postprandial endothelial dysfunction by spice mix added to high-fat hamburger meat in men with Type 2 diabetes mellitus. Diabetic Med. 2013, 30, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Avila, F.; Echeverria, G.; Perez, D.; Trejo, S.; Leighton, F. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxid. Med. Cell. Longev. 2017, 2017, 8361493. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodríguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Theoduloz, C.; Burgos-Edwards, A.; Schmeda-Hirschmann, G.; Jiménez-Aspee, F. Effect of polyphenols from wild Chilean currants (Ribes spp.) on the activity of intracellular antioxidant enzymes in human gastric AGS cells. Food Biosci. 2018, 24, 80–88. [Google Scholar] [CrossRef]
- Eady, M.; Samuel, D.; Bowker, B. Effect of pH and postmortem aging on protein extraction from broiler breast muscle. Poult. Sci. 2014, 93, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Nazar, D.S.; Persson, G.; Olin, T.; Waters, S.; Decken, A.v.d. Sarcoplasmic and myofibrillar proteins in white trunk muscle of salmon (Salmo salar) after estradiol treatment. Comp. Biochem. Physiol. B Comp. Biochem. 1991, 98, 109–114. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Fuentes, E.; Avila, F.; Alarcon, M.; Palomo, I. Roles of Phenolic Compounds in the Reduction of Risk Factors of Cardiovascular Diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef]
- Sirota, R.; Gorelik, S.; Harris, R.; Kohen, R.; Kanner, J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol. Nutr. Food Res. 2013, 57, 916–919. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Huang, I.M.; Hwang, L.S.; Ho, C.-T.; Li, S.; Lo, C.-Y. Anthocyanins in blackcurrant effectively prevent the formation of advanced glycation end products by trapping methylglyoxal. J. Funct. Foods 2014, 8, 259–268. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J. Agric. Food Chem. 2014, 62, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of Quercetin and Its Methylglyoxal Adducts on the Formation of α-Dicarbonyl Compounds in a Lysine/Glucose Model System. J. Agric. Food Chem. 2017, 65, 2233–2239. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; MCucci, A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Pormetter, L.; Mettke, J.; Ávila, F.; Schmeda-Hirschmann, G. Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect. Molecules 2019, 24, 4028. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Antileo-Laurie, J.; Theoduloz, C.; Jiménez-Aspee, F.; Avila, F.; Burgos-Edwards, A.; Olate-Olave, V. Phenolic composition, antioxidant capacity and α-glucosidase inhibitory activity of raw and boiled Chilean Araucaria araucana kernels. Food Chem. 2021, 350, 129241. [Google Scholar] [CrossRef] [PubMed]
- Milić, B.L.; Djilas, S.M.; Čanadanović-Brunet, J.M. Antioxidative activity of phenolic compounds on the metal-ion breakdown of lipid peroxidation system. Food Chem. 1998, 61, 443–447. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox. Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed Sweet Corn Has Higher Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov. Food Sci. Em. Technol. 2009, 10, 16–22. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef]
- Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef]
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Hagglund, P.; Mariotti, M.; Davies, M.J. Identification and characterization of protein cross-links induced by oxidative reactions. Expert Rev. Proteomics 2018, 15, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Raffel, R.A.; Souid, A.K.; Goodisman, J. Kinetic studies on enzyme-catalyzed reactions: Oxidation of glucose, decomposition of hydrogen peroxide and their combination. Biophys. J. 2009, 96, 2977–2988. [Google Scholar] [CrossRef] [PubMed]
- Montana, M.P.; Blasich, N.; Haggi, E.; Garcia, N.A. Oxygen uptake in the vitamin B-sensitized photo-oxidation of tyrosine and tryptophan in the presence of uracil: Kinetics and mechanism. Photochem. Photobiol. 2009, 85, 1097–1102. [Google Scholar] [CrossRef]
- Ávila, F.; Ravello, N.; Zanocco, A.L.; Gamon, L.F.; Davies, M.J.; Silva, E. 3-Hydroxykynurenine bound to eye lens proteins induces oxidative modifications in crystalline proteins through a type I photosensitizing mechanism. Free Radic. Biol. Med. 2019, 141, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal Degradation of Acylated and Nonacylated Anthocyanins. J. Food Sci. 2006, 71, C504–C512. [Google Scholar] [CrossRef]
- Kader, F.; Haluk, J.-P.; Nicolas, J.-P.; Metche, M. Degradation of Cyanidin 3-Glucoside by Blueberry Polyphenol Oxidase: Kinetic Studies and Mechanisms. J. Agric. Food Chem. 1998, 46, 3060–3065. [Google Scholar] [CrossRef]
- Yin, J.; Hedegaard, R.V.; Skibsted, L.H.; Andersen, M.L. Epicatechin and epigallocatechin gallate inhibit formation of intermediary radicals during heating of lysine and glucose. Food Chem. 2014, 146, 48–55. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef]
- Labenski, M.T.; Fisher, A.A.; Lo, H.H.; Monks, T.J.; Lau, S.S. Protein electrophile-binding motifs: Lysine-rich proteins are preferential targets of quinones. Drug Metab. Dispos. 2009, 37, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jongberg, S.; Andersen, M.L.; Davies, M.J.; Lund, M.N. Quinone-induced protein modifications: Kinetic preference for reaction of 1,2-benzoquinones with thiol groups in proteins. Free Radic. Biol. Med. 2016, 97, 148–157. [Google Scholar] [CrossRef] [PubMed]
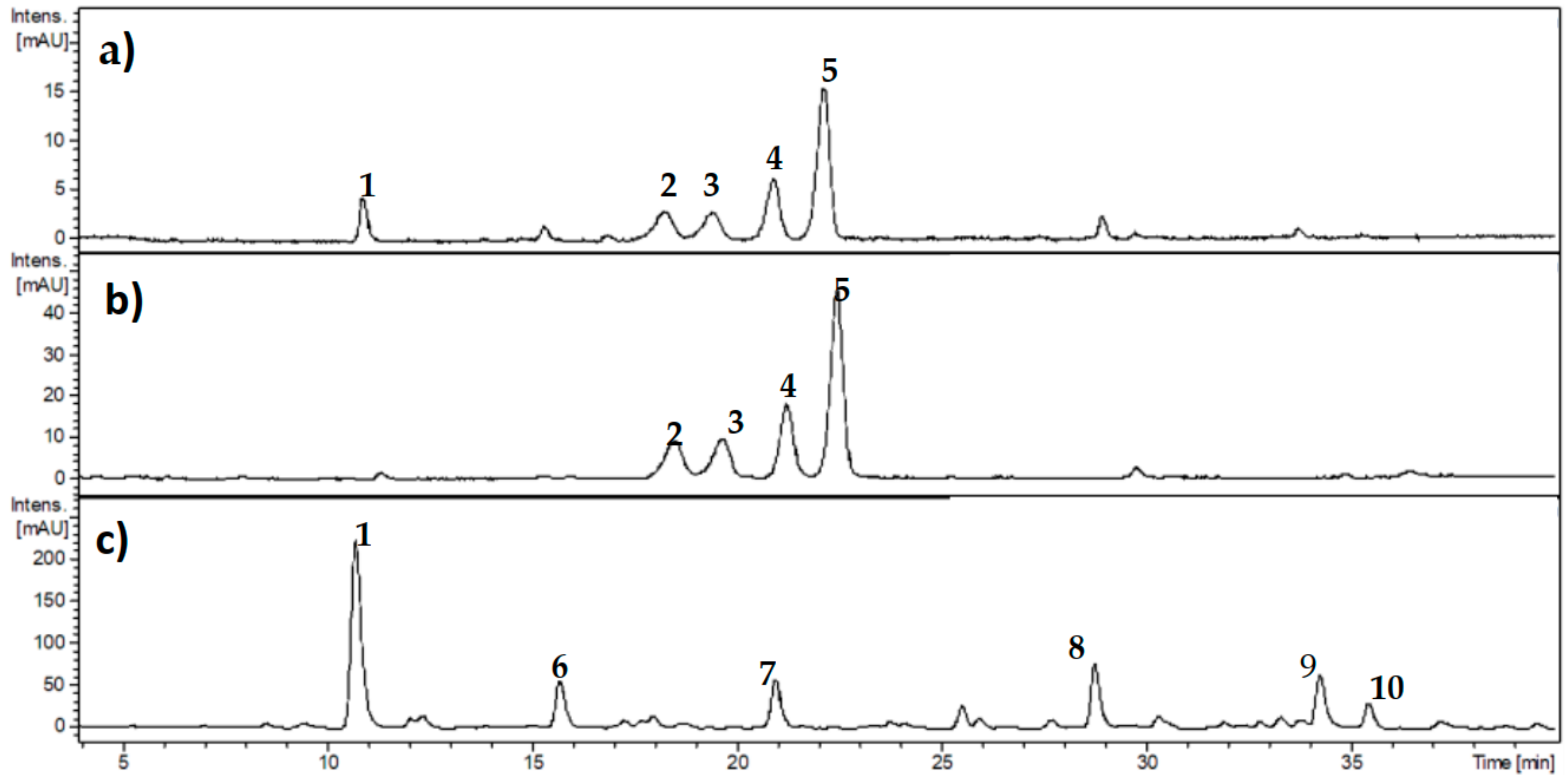
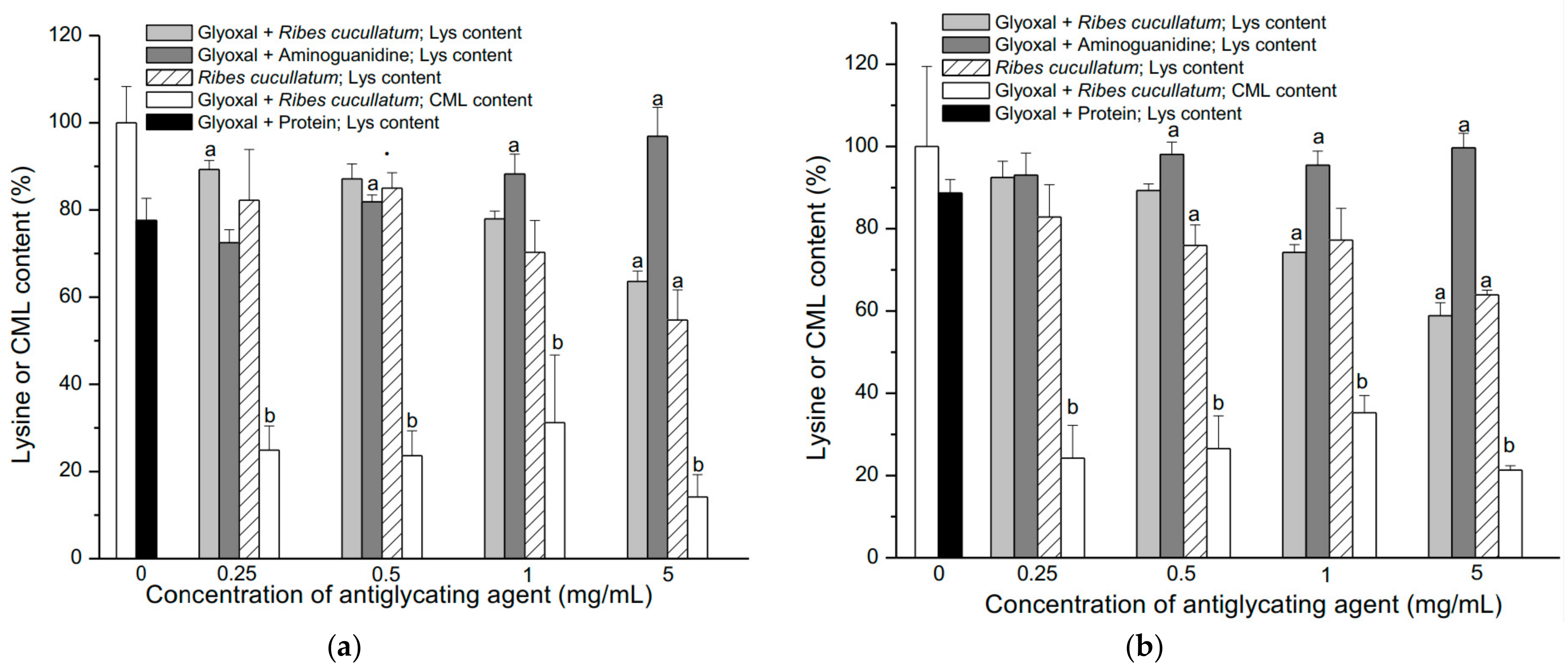
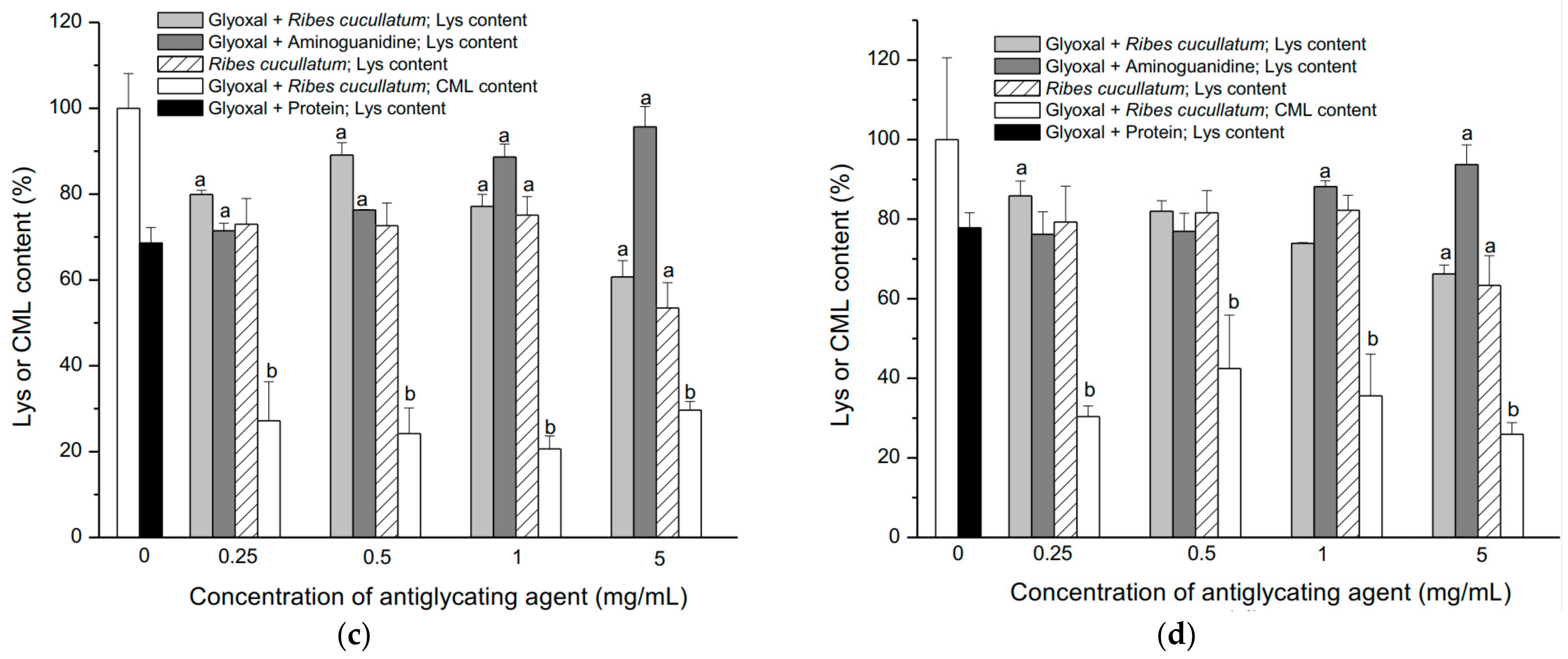
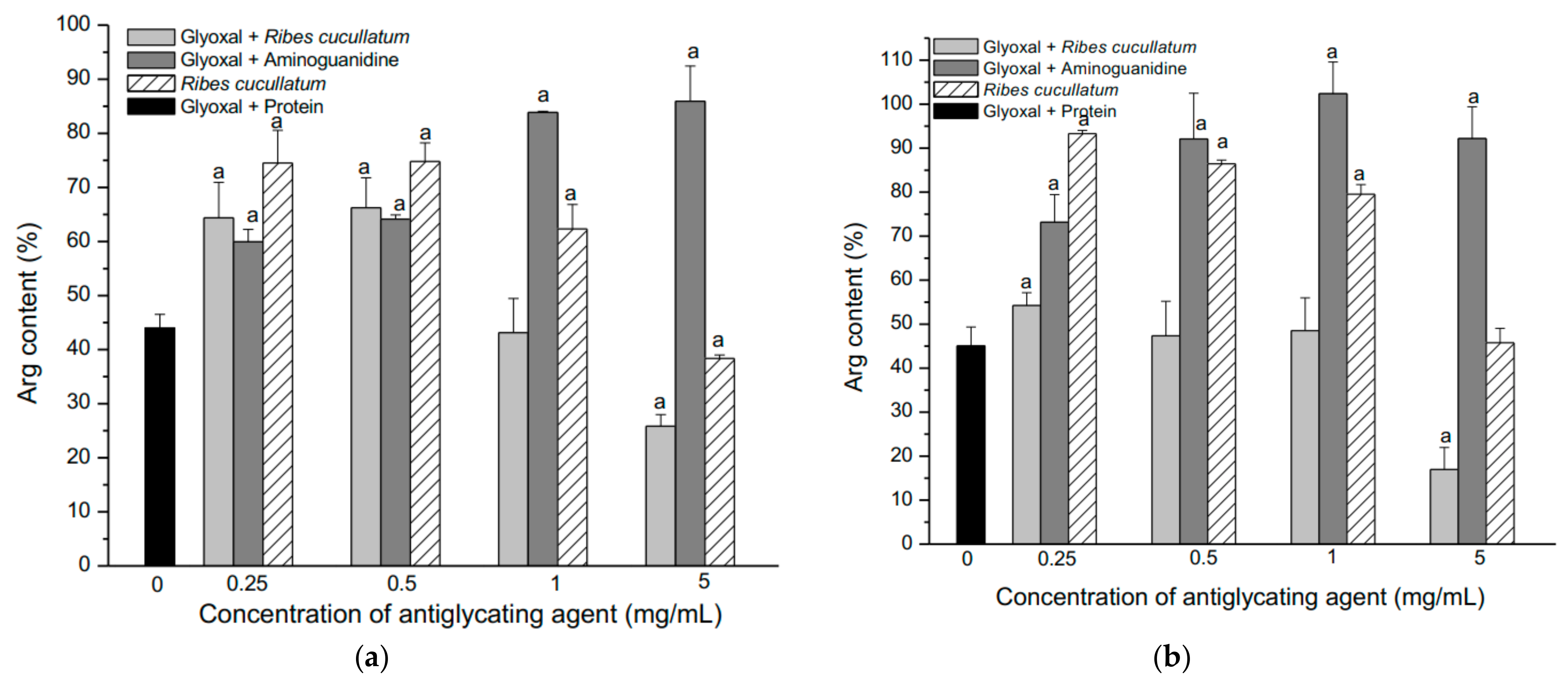


| Sample | IC50 (µg/mL) |
|---|---|
| R. cucullatum PEE | 63.7 ± 6.3 a |
| R. cucullatum PEE 60 °C * | 88.1 ± 2.5 b |
| R. cucullatum CF | 80.2 ± 3.6 a |
| R. cucullatum AF | 88.1 ± 5.8 b |
| Aminoguanidine ** | 329.5 ± 3.8 b |
| Compound | PEE + Protein at 4 °C (µg/g) | PEE + Protein at 60 °C (µg/g) |
|---|---|---|
| Delphinidin-3-glucoside | 52.8 ± 0.5 | 20.3 ± 0.2 a |
| Delphinidin-3-rutinoside | 61.5 ± 0.1 | 21.4 ± 0.2 a |
| Cyanidin-3-glucoside | 54.7 ± 0.7 | 23.0 ± 0.4 a |
| Cyanidin-3-rutinoside | 158.9 ± 0.9 | 62.9 ± 0.5 a |
| Chlorogenic acid | 1090.5 ± 2.8 | 782.0 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila, F.; Ravello, N.; Manriquez, C.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; Theoduloz, C. Antiglycating Effect of Phenolics from the Chilean Currant Ribes cucullatum under Thermal Treatment. Antioxidants 2021, 10, 665. https://doi.org/10.3390/antiox10050665
Ávila F, Ravello N, Manriquez C, Jiménez-Aspee F, Schmeda-Hirschmann G, Theoduloz C. Antiglycating Effect of Phenolics from the Chilean Currant Ribes cucullatum under Thermal Treatment. Antioxidants. 2021; 10(5):665. https://doi.org/10.3390/antiox10050665
Chicago/Turabian StyleÁvila, Felipe, Natalia Ravello, Camila Manriquez, Felipe Jiménez-Aspee, Guillermo Schmeda-Hirschmann, and Cristina Theoduloz. 2021. "Antiglycating Effect of Phenolics from the Chilean Currant Ribes cucullatum under Thermal Treatment" Antioxidants 10, no. 5: 665. https://doi.org/10.3390/antiox10050665
APA StyleÁvila, F., Ravello, N., Manriquez, C., Jiménez-Aspee, F., Schmeda-Hirschmann, G., & Theoduloz, C. (2021). Antiglycating Effect of Phenolics from the Chilean Currant Ribes cucullatum under Thermal Treatment. Antioxidants, 10(5), 665. https://doi.org/10.3390/antiox10050665






