Effects of Cooking and Processing Methods on Phenolic Contents and Antioxidant and Anti-Proliferative Activities of Broccoli Florets
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
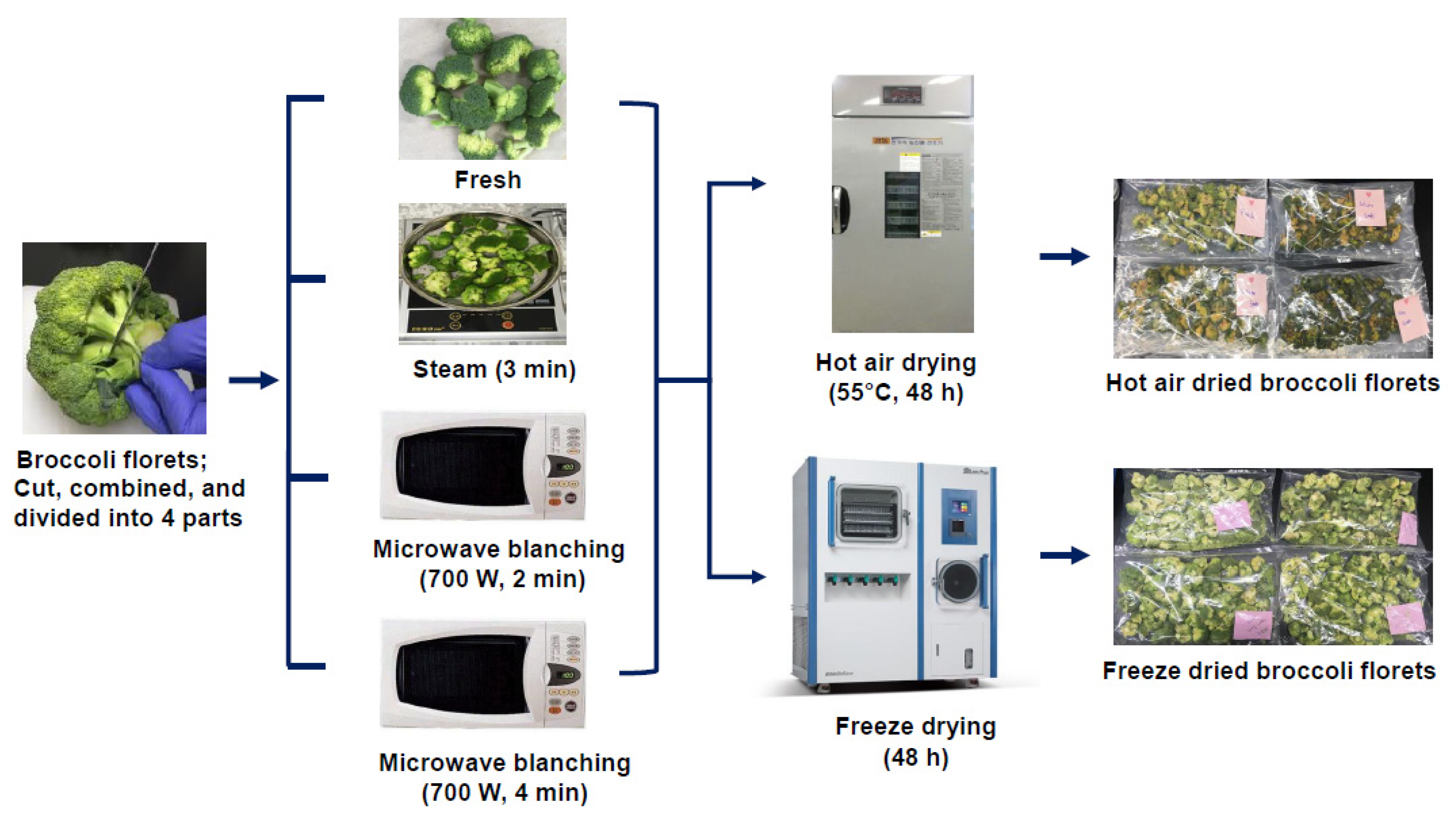
2.2. Cooking Processes
2.2.1. Steaming
2.2.2. Microwave Cooking
2.3. Drying Experiments
2.3.1. Vacuum Freeze-Drying
2.3.2. Hot-Air-Drying
2.4. Extraction of Plant Material
2.5. Determination of Total Polyphenol (TPC) and Flavonoid (TFC) Contents
2.6. Analysis of Antioxidant Activity
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity Based on Electron Spin Resonance (ESR)
2.6.2. Alkyl Radical Scavenging Activity Based on ESR
2.6.3. 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Scavenging Activity Assay
2.7. Cell Culture
2.8. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.9. Measurement of Intracellular ROS Generation
2.10. Quantification of L-Sulforaphane via High-Performance Liquid Chromatography (HPLC)
2.11. Statistical Analysis
3. Results and Discussion
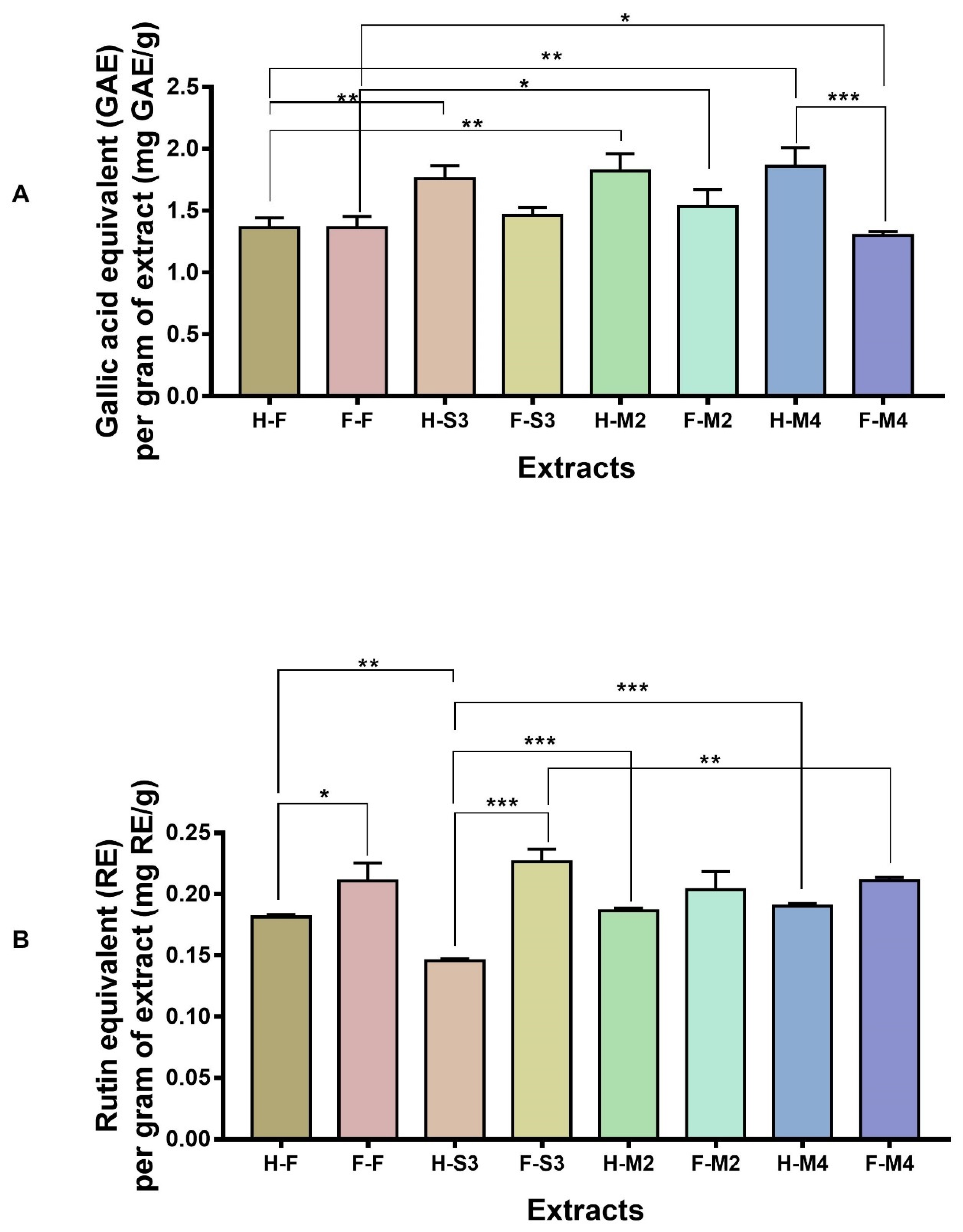
3.1. Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC) of Broccoli Floret Extracts
3.2. Antioxidant Activities
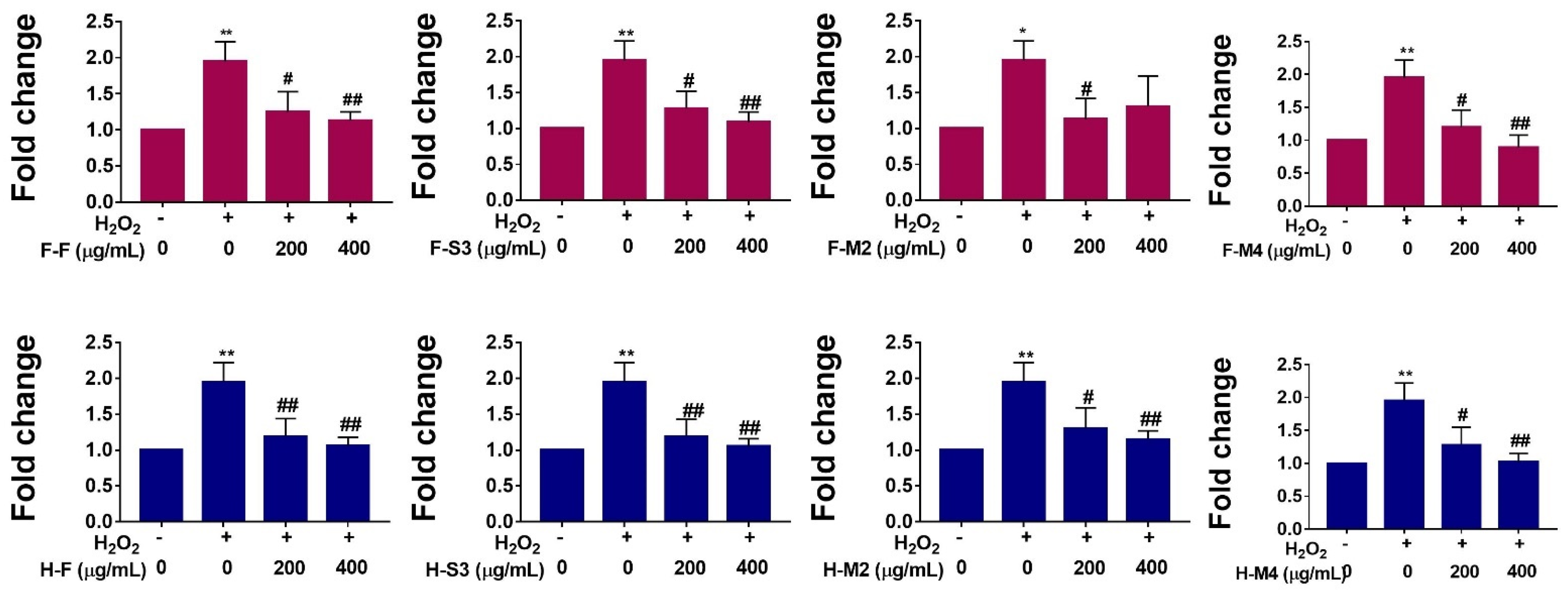
3.3. H2O2-Induced ROS Production
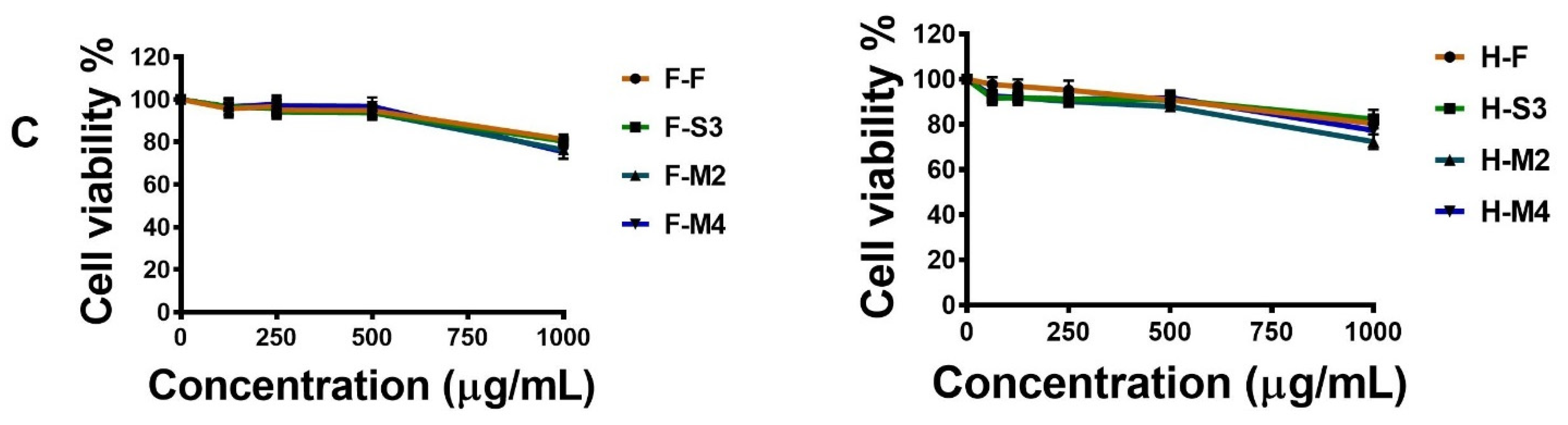
3.4. Effects of Broccoli Extracts on Cell Viability
3.5. L-Sulforaphane Quantification in Broccoli Extracts Using HPLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahn, A.; Rubio, M.P. Evolution of total polyphenols content and antioxidant activity in broccoli florets during storage at different temperatures. J. Food Qual. 2017, 2017, 3742183. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization (FAO). Available online: http://www.fao.org/home/en/ (accessed on 18 February 2021).
- Jeffery, E.H.; Brown, A.F.; Kurilich, A.C.; Keck, A.S.; Matusheski, N.; Klein, B.P.; Juvik, J.A. Variation in content of bioactive components in broccoli. J. Sci. Food Agric. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates–A review. LWT 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Li, Y.; Zhao, C.N.; Liu, Q.; Li, H.B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef]
- Yuan, G.F.; Sun, B.; Yuan, J.; Wang, Q.M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Cao, Y.; Xia, W.; Jiang, Q. Application of simultaneous combination of microwave and steam cooking to improve nutritional quality of cooked purple sweet potatoes and saving time. Innov. Food Sci. Emerg. Technol. 2016, 36, 303–310. [Google Scholar] [CrossRef]
- Md Salim, N.S.; Gariépy, Y.; Raghavan, V. Hot air drying and microwave-assisted hot air drying of broccoli stalk slices (Brassica oleracea L. Var. Italica). J. Food Process. Preserv. 2017, 41, e12905. [Google Scholar] [CrossRef]
- Mahn, A.; Reyes, A. An overview of health-promoting compounds of broccoli (Brassica oleracea var. italica) and the effect of processing. Food Sci. Technol. Int. 2012, 18, 503–514. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Collazo, C.; Aguiló-Aguayo, I. Effects of thermal and non-thermal processing of cruciferous vegetables on glucosinolates and its derived forms. J. Food Sci. Technol. 2018, 55, 1973–1981. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 1–22. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Pak Dek, M.S.; Hairuddin, M.R. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Choi, Y.; Hong, K.H.; Chung, Y.S.; Cho, S.K. Effect of Roasting and Brewing on the Antioxidant and Anti-proliferative Activities of Tartary Buckwheat. Foods 2020, 9, 1331. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.S.; Cho, M.; Choi, H.K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and anti-proliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Nguyen, Y.T.K.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Phenethyl isothiocyanate suppresses stemness in the chemo-and radio-resistant triple-negative breast cancer cell line MDA-MB-231/IR via downregulation of metadherin. Cancers 2020, 12, 268. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R.; Thabrew, I.; Dilip De Silva, E. A study of the potential anticancer activity of Mangifera zeylanica bark: Evaluation of cytotoxic and apoptotic effects of the hexane extract and bioassay-guided fractionation to identify phytochemical constituents. Oncol. Lett. 2016, 11, 1335–1344. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R.; Thabrew, I.; De Silva, E.D. Induction of apoptosis in MCF-7 breast cancer cells by Sri Lankan Endemic Mango (Mangifera zeylanica) fruit peel through oxidative stress and analysis of its phytochemical constituents. J. Food Biochem. 2017, 41, e12294. [Google Scholar] [CrossRef]
- Samarakoon, S.R.; Shanmuganathan, C.; Ediriweera, M.K.; Piyathilaka, P.; Tennekoon, K.H.; Thabrew, I.; Galhena, P.; De Silva, E.D. Anti-hepatocarcinogenic and anti-oxidant effects of mangrove plant Scyphiphora hydrophyllacea. Pharmacogn. Mag. 2017, 13 (Suppl. 1), S76. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Ciska, E.; Pawlak-Lemańska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef]
- Şengül, M.; Yildiz, H.; Kavaz, A. The effect of cooking on total polyphenolic content and antioxidant activity of selected vegetables. Int. J. Food Prop. 2014, 17, 481–490. [Google Scholar] [CrossRef]
- Dolinsky, M.; Agostinho, C.; Ribeiro, D.; Rocha, G.D.S.; Barroso, S.G.; Ferreira, D.; Fialho, E. Effect of different cooking methods on the polyphenol concentration and antioxidant capacity of selected vegetables. J. Culin. Sci. Technol. 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Y.; Haytowitz, D.B.; Chen, P.; Pehrsson, P.R. Effects of domestic cooking on flavonoids in broccoli and calculation of retention factors. Heliyon 2019, 5, e01310. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Halpern, H.J.; Spencer, D.P.; van Polen, J.; Bowman, M.K.; Nelson, A.C.; Dowey, E.M.; Teicher, B.A. Imaging radio frequency electron-spin-resonance spectrometer with high resolution and sensitivity for in-vivo measurements. Rev. Sci. Instrum. 1989, 60, 1040–1050. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R.; Adhikari, A.; Thabrew, I.; De Silva, E.D. Isolation of a new resorcinolic lipid from Mangifera zeylanica Hook. f. bark and its cytotoxic and apoptotic potential. Biomed. Pharmacother. 2017, 89, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Jin, D.E.; Park, C.H.; Seung, T.W.; Choi, S.G.; Heo, H.J. PC12 Cell Protective Effects of Broccoli (Brassica oleracea var. italica) Leaf Fraction against H2O2-induced Oxidative Stress. Korean J. Food Sci. Technol. 2014, 46, 483–488. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Feng, Y.; Liu, N.; Fu, Z.; Wu, J.; Yang, L. Natural ingredients-derived antioxidants attenuate H2O2-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via Keap1/Nrf2 pathway. Free Radic. Biol. Med. 2020, 152, 854–864. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Wang, Q.; Sun, F.; Liu, F. Sulforaphane attenuates H2O2-induced oxidant stress in human trabecular meshwork cells (HTMCs) via the phosphatidylinositol 3-kinase (PI3K)/serine/threonine kinase (Akt)-mediated factor-E2-related factor 2 (Nrf2) signaling activation. Med. Sci. Monit. 2019, 25, 811. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. In vitro assays and techniques utilized in anticancer drug discovery. J. Appl. Toxicol. 2019, 39, 38–71. [Google Scholar] [CrossRef]
- Le, T.N.; Luong, H.Q.; Li, H.P.; Chiu, C.H.; Hsieh, P.C. Broccoli (Brassica oleracea L. var. italica) sprouts as the potential food source for bioactive properties: A comprehensive study on in vitro disease models. Foods 2019, 8, 532. [Google Scholar] [CrossRef]
- Radošević, K.; Srček, V.G.; Bubalo, M.C.; Brnčić, S.R.; Takács, K.; Redovniković, I.R. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Compos. Anal. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Mazarakis, N.; Snibson, K.; Licciardi, P.V.; Karagiannis, T.C. The potential use of l-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence. Clin. Nutr. 2020, 39, 664–675. [Google Scholar] [CrossRef]
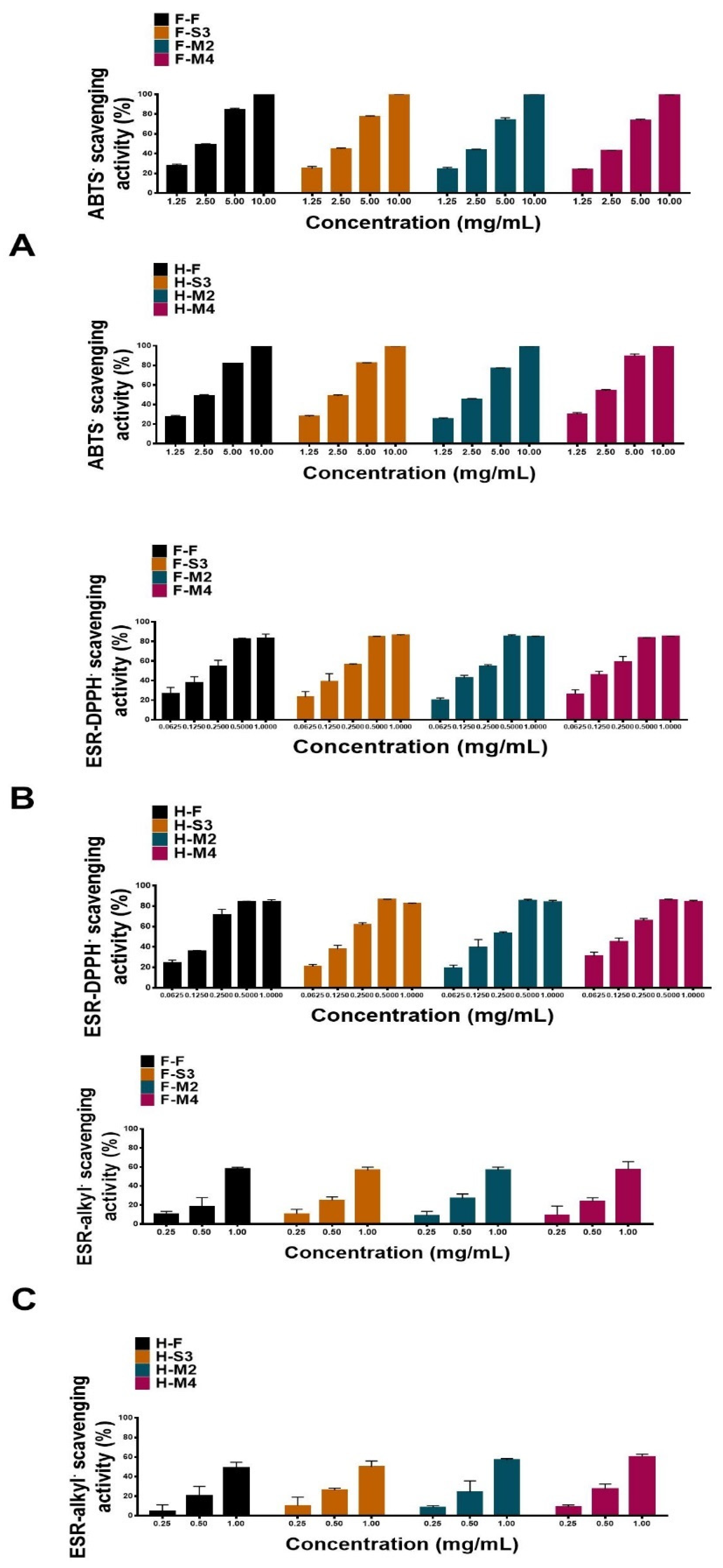

| ABTS• | ESR-DPPH• | ESR-alkyl• | |
|---|---|---|---|
| H-F | 1.98 | 0.15 | 1.49 |
| H-S3 | 1.97 | 0.17 | 1.26 |
| H-M2 | 2.23 | 0.18 | 1.17 |
| H-M4 | 1.67 | 0.13 | 1.03 |
| F-F | 1.92 | 0.17 | 1.22 |
| F-S3 | 2.23 | 0.16 | 1.12 |
| F-M2 | 2.35 | 0.17 | 1.11 |
| F-M4 | 2.39 | 0.15 | 1.18 |
| Breast Cancer Cells | F-F | F-S3 | F-M2 | F-M4 | H-F | H-S3 | H-M2 | H-M4 |
|---|---|---|---|---|---|---|---|---|
| MCF-7 | 1381 a ± 10.590 | 651 b ± 17.030 | 447 c ± 4.500 | 1505.3 d ± 3.210 | 1195.3 e ± 54.590 | 1295 f ± 55.360 | 2988 g ± 10.260 | 2365 h ± 15.090 |
| MDA-MB-231 | 1355 a ± 12.500 | 1130 b ± 7.780 | 948 c ± 12.100 | 1846 d ± 4.200 | 570 e ± 11.410 | 820 f ± 15.140 | 723 g ± 13.410 | 747 h ± 15.470 |
| MCF-10A | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Component | F-F | F-S3 | F-M2 | F-M4 | H-F | H-S3 | H-M2 | H-M4 |
|---|---|---|---|---|---|---|---|---|
| L-sulforaphane | 0.11 a ± 0.001 | 0.18 b ± 0.001 | 0.38 c ± 0.004 | 0.003 d,i ± 6.6 × 10−5 | 0.074 e ± 0.001 | 0.011 f ± 0.0003 | 0.005 g,i ± 0.0003 | 0.002 h,i ± 3.83 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.Y.; Ediriweera, M.K.; Boo, K.-H.; Kim, C.S.; Cho, S.K. Effects of Cooking and Processing Methods on Phenolic Contents and Antioxidant and Anti-Proliferative Activities of Broccoli Florets. Antioxidants 2021, 10, 641. https://doi.org/10.3390/antiox10050641
Kim HY, Ediriweera MK, Boo K-H, Kim CS, Cho SK. Effects of Cooking and Processing Methods on Phenolic Contents and Antioxidant and Anti-Proliferative Activities of Broccoli Florets. Antioxidants. 2021; 10(5):641. https://doi.org/10.3390/antiox10050641
Chicago/Turabian StyleKim, Hee Young, Meran Keshawa Ediriweera, Kyung-Hwan Boo, Chang Sook Kim, and Somi Kim Cho. 2021. "Effects of Cooking and Processing Methods on Phenolic Contents and Antioxidant and Anti-Proliferative Activities of Broccoli Florets" Antioxidants 10, no. 5: 641. https://doi.org/10.3390/antiox10050641
APA StyleKim, H. Y., Ediriweera, M. K., Boo, K.-H., Kim, C. S., & Cho, S. K. (2021). Effects of Cooking and Processing Methods on Phenolic Contents and Antioxidant and Anti-Proliferative Activities of Broccoli Florets. Antioxidants, 10(5), 641. https://doi.org/10.3390/antiox10050641






