Vitamin E beyond Its Antioxidant Label
Abstract
1. Introduction
2. Methods
3. Structures, Dietary Sources, and Daily Intake
3.1. Structures
3.2. Dietary Sources
3.3. Daily Intake
3.4. Bioavailability and Factors That Influence It
3.4.1. Absorption, Distribution, and Metabolism
3.4.2. Natural vs. Synthetic
3.4.3. Dietary Factors
3.4.4. Physiological and Pathological Factors
3.4.5. Technological Factors
4. Molecular and Cellular Mechanism of Action
5. Preclinical Reports
5.1. Energy Homeostasis/Metabolism-Related Signaling
5.2. Regulatory Effects on Inflammation Pathways
5.3. Anti-Proliferative and Pro-Apoptotic Pathways
6. Effects in Humans Regarding Cardio-Metabolic Health
6.1. Effects in Healthy Volunteers
6.2. Cardiometabolic Diseases
6.3. Neurodegenerative Maladies
6.4. Anti-Aging Effects
6.5. Cancer-Related Reports
7. Outlook and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.J.; Green, P.; John Mann, J.; Rapoport, S.I.; Sublette, M.E. Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. 2015, 1597, 220–246. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M. Vitamin E: Regulatory Role on Signal Transduction. IUBMB Life 2019, 71, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Varghese, Z.; Ruan, X.Z. The molecular pathogenic role of inflammatory stress in dysregulation of lipid homeostasis and hepatic steatosis. Genes Dis. 2014, 1, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Sadeghian, M.; Nazarian, B.; Sarreshtedari, M.; Mozaffari-Khosravi, H.; Maleki, V.; Alizadeh, M.; Shokri, A.; Sadeghi, O. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 17234. [Google Scholar] [CrossRef]
- Leon-Pedroza, J.I.; Gonzalez-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodriguez, D.; Escobedo, G.; CGonzalez-Chavez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cirugía y Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Ungurianu, A.; Margina, D.; Gradinaru, D.; Bacanu, C.; Ilie, M.; Tsitsimpikou, C.; Tsarouhas, K.; Spandidos, D.A.; Tsatsakis, A.M. Lipoprotein redox status evaluation as a marker of cardiovascular disease risk in patients with inflammatory disease. Mol. Med. Rep. 2017, 15, 256–262. [Google Scholar] [CrossRef]
- Ungurianu, A.; Seremet, O.; Gagniuc, E.; Olaru, O.T.; Gutu, C.; Gradinaru, D.; Ionescu-Tirgoviste, C.; Margina, D.; Danciulescu-Miulescu, R. Preclinical and clinical results regarding the effects of a plant-based antidiabetic formulation versus well established antidiabetic molecules. Pharmacol. Res. 2019, 150, 104522. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, L.; Ning, S.; Liu, Z.; Lin, H.; Chen, S.; Zhu, J. Vitamin E intake and risk of stroke: A meta-analysis. Br. J. Nutr. 2018, 120, 1181–1188. [Google Scholar] [CrossRef]
- Amanullah, I.; Khan, Y.H.; Anwar, I.; Gulzar, A.; Mallhi, T.H.; Raja, A.A. Effect of vitamin E in non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomised controlled trials. Postgrad. Med. J. 2019, 95, 601–611. [Google Scholar] [CrossRef]
- Springett, G.M.; Husain, K.; Neuger, A.; Centeno, B.; Chen, D.T.; Hutchinson, T.Z.; Lush, R.M.; Sebti, S.; Malafa, M.P. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E delta-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine 2015, 2, 1987–1995. [Google Scholar] [CrossRef]
- Szymańska, R.; Nowicka, B.; Trela, A.; Kruk, J. Vitamin E: Structure and forms. In Molecular Nutrition: Vitamins; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996. [Google Scholar] [CrossRef]
- Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Vitamin E; European Commission Health Consumer Protection Directorate-General: Brussels, Belgium, 2003; pp. 1–18. [Google Scholar]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. Proc. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- DellaPenna, D. A decade of progress in understanding vitamin E synthesis in plants. Proc. J. Plant Physiol. 2005, 162, 729–737. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, R.R.; Zhang, P.; Xu, Y.; Zhu, J.; Gu, M.H.; Liang, G.H.; Liu, Q.Q. Variation and Distribution of Vitamin E and Composition in Seeds Among Different Rice Varieties. Acta Agron. Sin. 2012. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 2006. [Google Scholar] [CrossRef]
- Dunford, N.T. Wheat Germ Oil. In Gourmet and Health-Promoting Specialty Oils; AOCS Press: Urbana, IL, USA, 2009. [Google Scholar] [CrossRef]
- Masterjohn, C. The Anti-Inflammatory Properties of Safflower Oil and Coconut Oil May be Mediated by Their Respective Concentrations of Vitamin E. J. Am. Coll. Cardiol. 2007. [Google Scholar] [CrossRef]
- Radcliffe, J.D.; Hernandez, L.M. The Vitamin E Content of a Variety of Foods Made Exclusively from Almonds or Containing Almonds. J. Am. Diet. Assoc. 2005. [Google Scholar] [CrossRef]
- Bonku, R.; Yu, J. Health aspects of peanuts as an outcome of its chemical composition. Food Sci. Hum. Wellness 2020. [Google Scholar] [CrossRef]
- Institute of Medicine, U.S. Vitamin E. 2000. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 2 March 2021).
- FDA. Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be ConsuMed. at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. 2017. Available online: https://www.govinfo.gov/content/pkg/FR-2019-12-31/pdf/2019-27868.pdf (accessed on 2 March 2021).
- FDA. Converting Units of Measure for Folate, Niacin, and Vitamins A, D, and E on the Nutrition and Supplement Facts Labels: Guidance for Industry. 2019. Available online: https://www.fda.gov/media/129863/download (accessed on 29 March 2019).
- Dietary Reference Intakes; The National Academies Press: Washington, DC, USA, 2006. [CrossRef]
- EFSA. Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13. [Google Scholar] [CrossRef]
- Bioavailability|Definition of Bioavailability by Merriam-Webster. Available online: https://www.merriam-webster.com/dictionary/bioavailability (accessed on 5 March 2021).
- Desrumaux, C.; Risold, P.Y.; Schroeder, H.; Deckert, V.; Masson, D.; Athias, A.; Laplanche, H.; Le Guern, N.; Blache, D.; Jiang, X.C.; et al. Phospholipid transfer protein (PLTP) deficiency reduces brain vitamin E content and increases anxiety in mice. FASEB J. 2005, 19, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Drouineaud, V.; Lagrost, L.; Klein, A.; Desrumaux, C.; Le Guern, N.; Athias, A.; Menetrier, F.; Moiroux, P.; Sagot, P.; Jimenez, C.; et al. Phospholipid transfer protein deficiency reduces sperm motility and impairs fertility of mouse males. FASEB J. 2006, 20, 794–796. [Google Scholar] [CrossRef]
- Jiang, X.C.; Tall, A.R.; Qin, S.; Lin, M.; Schneider, M.; Lalanne, F.; Deckert, V.; Desrumaux, C.; Athias, A.; Witztum, J.L.; et al. Phospholipid transferprotein deficiency protects circulating lipoproteins from oxidation due to the enhancedaccumulation of vitamin E. J. Biol. Chem. 2002, 277, 31850–31856. [Google Scholar] [CrossRef]
- Kostner, G.M.; Oettl, K.; Jauhiainen, M.; Ehnholm, C.; Esterbauer, H.; Dieplinger, H. Human plasma phospholipid transfer protein accelerates exchange/transfer ofa-tocopherol between lipoproteins and cells. Biochem. J. 1995, 305, 659–667. [Google Scholar] [CrossRef]
- Panagabko, C.; Morley, S.; Hernandez, M.; Cassolato, P.; Gordon, H.; Parsons, R.; Manor, D.; Atkinson, J. Ligand specificity in the CRAL-TRIO protein family. Biochemistry 2003, 42, 6467–6474. [Google Scholar] [CrossRef]
- Brigelius-Flohe’, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Mustacich, D.J.; Bruno, R.S.; Traber, M.G. Vitamin E. Vitam. Horm. 2007, 76. [Google Scholar] [CrossRef]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef]
- Handelman, G.J.; Machlin, L.J.; Fitch, K.; Weiter, J.J.; Dratz, E.A. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J. Nutr. 1985, 115, 807–813. [Google Scholar] [CrossRef]
- Sundl, I.; Resch, U.; Bergmann, A.R.; Roob, J.M.; Winklhofer-Roob, B.M. The decrease in gamma-tocopherol in plasma and lipoprotein fractions levels off within two days of vitamin E supplementation. Ann. N. Y. Acad. Sci. 2004, 1031, 378–380. [Google Scholar] [CrossRef]
- Kluth, D.; Landes, N.; Pfluger, P.; Muller-Schmehl, K.; Weiss, K.; Bumke-Vogt, C.; Ristow, M.; Brigelius-Flohe, R. Modulation of Cyp3a11 mRNA expression by alpha-tocopherol but not gamma-tocotrienol in mice. Free Radic. Biol. Med. 2005, 38, 507–514. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef]
- Mustacich, D.J.; Gohil, K.; Bruno, R.S.; Yan, M.; Leonard, S.W.; Ho, E.; Cross, C.E.; Traber, M.G. Alpha-tocopherol modulates genes involved in hepatic xenobiotic pathways in mice. J. Nutr. Biochem. 2009, 20, 469–476. [Google Scholar] [CrossRef]
- Traber, M.G.; Siddens, L.K.; Leonard, S.W.; Schock, B.; Gohil, K.; Krueger, S.K.; Cross, C.E.; Williams, D.E. Alpha-tocopherol modulates Cyp3a expression, increases gamma-CEHC production, and limits tissue gamma-tocopherol accumulation in mice fed high gamma-tocopherol diets. Free Radic. Biol. Med. 2005, 38, 773–785. [Google Scholar] [CrossRef]
- Brown, B.G.; Zhao, X.Q.; Chait, A.; Fisher, L.D.; Cheung, M.C.; Morse, J.S.; Dowdy, A.A.; Marino, E.K.; Bolson, E.L.; Alaupovic, P.; et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 2001, 345, 1583–1592. [Google Scholar] [CrossRef]
- Cheung, M.C.; Zhao, X.Q.; Chait, A.; Albers, J.J.; Brown, B.G. Antioxidant supplements block the response of HDL to simvastatin-niacin therapy in patients with coronary artery disease and low HDL. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1320–1326. [Google Scholar] [CrossRef]
- Waters, D.D.; Alderman, E.L.; Hsia, J.; Howard, B.V.; Cobb, F.R.; Rogers, W.J.; Ouyang, P.; Thompson, P.; Tardif, J.C.; Higginson, L.; et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. JAMA 2002, 288, 2432–2440. [Google Scholar] [CrossRef]
- Weiser, H.; Vecchi, M. Stereoisomers of α-tocopheryl acetate. II. Biopotencies of all eight stereoisomers, individually or in mixtures, as determined by rat resorption-gestation tests. Int. J. Vitam. Nutr. Res. 1982, 52, 351–370. [Google Scholar]
- Hoppe, P.P.; Krennrich, G. Bioavailability and potency of natural-source and all-racemic α-tocopherol in the human: A dispute. Eur. J. Nutr. 2000. [Google Scholar] [CrossRef]
- Lodge, J.K. Vitamin E bioavailability in humans. Proc. J. Plant Physiol. 2005, 162, 790–796. [Google Scholar] [CrossRef]
- Weiser, H.; Vecchi, M.; Schlachter, M. Stereoisomers of α-tocopheryl acetate. III. Simultaneous determination of resorption-gestation and myopathy in rats as a means of evaluating biopotency ratios of all-rac- and RRR-α-tocopheryl acetate. Int. J. Vitam. Nutr. Res. 1985, 55, 149–185. [Google Scholar]
- Jeanes, Y.M.; Hall, W.L.; Ellard, S.; Lee, E.; Lodge, J.K. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 2004. [Google Scholar] [CrossRef]
- Bruno, R.S.; Leonard, S.W.; Park, I.S.; Zhao, Y.; Traber, M.G. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled α-tocopheryl acetate. Am. J. Clin. Nutr. 2006. [Google Scholar] [CrossRef]
- Vinson, J.A.; Al Kharrat, H.; Andreoli, L. Effect of Aloe vera preparations on the human bioavailability of vitamins C and E. Phytomedicine 2005. [Google Scholar] [CrossRef] [PubMed]
- Kemnic, T.R.; Coleman, M. Vitamin E Deficiency-StatPearls-NCBI Bookshelf; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Desmarchelier, C.; Tourniaire, F.; Nowicki, M.; Bott, R.; Borel, P. How does vitamin E intake correlate with concentrations of tocopherols and their metabolites? Genetic variants involved in interindividual variability in vitamin E bioavailability. Free Radic. Biol. Med. 2015. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Tourniaire, F.; Nowicki, M.; Bott, R.; Borel, P. The interindividual variability in vitamin E bioavailability in healthy male adults is significantly explained by a combination of SNPS in genes involved in vitamin E metabolism. Atherosclerosis 2015. [Google Scholar] [CrossRef]
- Dhakal, S.P.; He, J. Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review. Food Res. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Julianto, T.; Yuen, K.H.; Noor, A.M. Improved bioavailability of vitamin E with a self emulsifying formulation. Int. J. Pharm. 2000. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G.F. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Anandharamakrishnan, C. Enhancement of oral bioavailability of vitamin E by spray-freeze drying of whey protein microcapsules. Food Bioprod. Process. 2016. [Google Scholar] [CrossRef]
- Eid, M.; Sobhy, R.; Zhou, P.; Wei, X.; Wu, D.; Li, B. β-cyclodextrin-soy soluble polysaccharide based core-shell bionanocomposites hydrogel for vitamin E swelling controlled delivery. Food Hydrocoll. 2020. [Google Scholar] [CrossRef]
- Miyoshi, N.; Wakao, Y.; Tomono, S.; Tatemichi, M.; Yano, T.; Ohshima, H. The enhancement of the oral bioavailability of γ-tocotrienol in mice by γ-cyclodextrin inclusion. J. Nutr. Biochem. 2011. [Google Scholar] [CrossRef]
- Varga, Z.; Kosaras, E.; Komodi, E.; Katko, M.; Karpati, I.; Balla, J.; Paragh, G.; Aisa, M.C.; Galli, F. Effects of tocopherols and 2,2’-carboxyethyl hydroxychromans on phorbol-ester-stimulated neutrophils. J. Nutr. Biochem. 2008, 19, 320–327. [Google Scholar] [CrossRef]
- Jiang, Z.; Yin, X.; Jiang, Q. Natural forms of vitamin E and 13’-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J. Immunol. 2011, 186, 1173–1179. [Google Scholar] [CrossRef]
- Yam, M.L.; Abdul Hafid, S.R.; Cheng, H.M.; Nesaretnam, K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids 2009, 44, 787–797. [Google Scholar] [CrossRef]
- Ng, L.T.; Ko, H.J. Comparative effects of tocotrienol-rich fraction, alpha-tocopherol and alpha-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem. 2012, 134, 920–925. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Reis, J.C.; Papasian, C.J.; Morrison, D.C.; Qureshi, N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis. 2010, 9, 143. [Google Scholar] [CrossRef]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q. gamma-Tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPbeta and NF-kappaB in macrophages. J. Nutr. Biochem. 2013, 24, 1146–1152. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q. Vitamin E delta-tocotrienol inhibits TNF-alpha-stimulated NF-kappaB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J. Nutr. Biochem. 2019, 64, 101–109. [Google Scholar] [CrossRef]
- Domazetovic, V.; Falsetti, I.; Viglianisi, C.; Vasa, K.; Aurilia, C.; Stio, M.; Menichetti, S.; Iantomasi, T. Protective Role of Natural and Semi-Synthetic Tocopherols on TNFalpha-Induced ROS Production and ICAM-1 and Cl-2 Expression in HT29 Intestinal Epithelial Cells. Antioxidants 2021, 10, 160. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Modulation of NF-kappaB and Nrf2 control of inflammatory responses in FHs 74 Int. cell line is tocopherol isoform-specific. Am. J. Physiol. Gastrointest Liver Physiol. 2013, 305, G940–G949. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef]
- Kannappan, R.; Yadav, V.R.; Aggarwal, B.B. gamma-Tocotrienol but not gamma-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. J. Biol. Chem. 2010, 285, 33520–33529. [Google Scholar] [CrossRef]
- Wells, S.R.; Jennings, M.H.; Rome, C.; Hadjivassiliou, V.; Papas, K.A.; Alexander, J.S. Alpha-, gamma- and delta-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J. Nutr. Biochem. 2010, 21, 589–597. [Google Scholar] [CrossRef]
- Wang, Y.; Moreland, M.; Wagner, J.G.; Ames, B.N.; Illek, B.; Peden, D.B.; Jiang, Q. Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells. J. Nutr. Biochem. 2012, 23, 602–608. [Google Scholar] [CrossRef]
- Montagnani Marelli, M.; Marzagalli, M.; Moretti, R.M.; Beretta, G.; Casati, L.; Comitato, R.; Gravina, G.L.; Festuccia, C.; Limonta, P. Vitamin E delta-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci. Rep. 2016, 6, 30502. [Google Scholar] [CrossRef]
- Xu, M.; Yang, H.; Zhang, Q.; Lu, P.; Feng, Y.; Geng, X.; Zhang, L.; Jia, X. Alpha-Tocopherol prevents esophageal squamous cell carcinoma by modulating PPARgamma-Akt signaling pathway at the early stage of carcinogenesis. Oncotarget 2017, 8, 95914–95930. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Francois, R.A.; Yamauchi, T.; Perez, M.; Sebti, S.M.; Malafa, M.P. Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol. Cancer Ther. 2011, 10, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Crispen, P.L.; Uzzo, R.G.; Golovine, K.; Makhov, P.; Pollack, A.; Horwitz, E.M.; Greenberg, R.E.; Kolenko, V.M. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: Implications for chemoprevention. Prostate 2007, 67, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hong, J.; Yang, C.S. delta-Tocopherol inhibits receptor tyrosine kinase-induced AKT activation in prostate cancer cells. Mol. Carcinog. 2016, 55, 1728–1738. [Google Scholar] [CrossRef]
- Galli, F.; Stabile, A.M.; Betti, M.; Conte, C.; Pistilli, A.; Rende, M.; Floridi, A.; Azzi, A. The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch. Biochem. Biophys. 2004, 423, 97–102. [Google Scholar] [CrossRef]
- Fontana, F.; Moretti, R.M.; Raimondi, M.; Marzagalli, M.; Beretta, G.; Procacci, P.; Sartori, P.; Montagnani Marelli, M.; Limonta, P. delta-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019, 52, e12576. [Google Scholar] [CrossRef]
- Kaneko, S.; Sato, C.; Shiozawa, N.; Sato, A.; Sato, H.; Virgona, N.; Yano, T. Suppressive Effect of Delta-Tocotrienol on Hypoxia Adaptation of Prostate Cancer Stem-like Cells. Anticancer Res. 2018, 38, 1391–1399. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Different tocopherol isoforms vary in capacity to scavenge free radicals, prevent inflammatory response, and induce apoptosis in both adult- and fetal-derived intestinal epithelial cells. Biofactors 2013, 39, 663–671. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Tocopherol isoforms (alpha-, gamma-, and delta-) show distinct capacities to control Nrf-2 and NfkappaB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol. Cell Biochem. 2015, 404, 123–131. [Google Scholar] [CrossRef]
- Hidalgo, M.; Rodriguez, V.; Kreindl, C.; Porras, O. Biological Redox Impact of Tocopherol Isomers Is Mediated by Fast Cytosolic Calcium Increases in Living Caco-2 Cells. Antioxidants 2020, 9, 155. [Google Scholar] [CrossRef]
- Campbell, S.E.; Stone, W.L.; Whaley, S.G.; Qui, M.; Krishnan, K. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer 2003, 3, 25. [Google Scholar] [CrossRef]
- Idriss, M.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules 2020, 10, 577. [Google Scholar] [CrossRef]
- Patacsil, D.; Tran, A.T.; Cho, Y.S.; Suy, S.; Saenz, F.; Malyukova, I.; Ressom, H.; Collins, S.P.; Clarke, R.; Kumar, D. Gamma-tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. J. Nutr. Biochem. 2012, 23, 93–100. [Google Scholar] [CrossRef]
- Dronamraju, V.; Ibrahim, B.A.; Briski, K.P.; Sylvester, P.W. gamma-Tocotrienol Suppression of the Warburg Effect Is Mediated by AMPK Activation in Human Breast Cancer Cells. Nutr. Cancer 2019, 71, 1214–1228. [Google Scholar] [CrossRef]
- Parajuli, P.; Tiwari, R.V.; Sylvester, P.W. Anticancer Effects of gamma-Tocotrienol Are Associated with a Suppression in Aerobic Glycolysis. Biol. Pharm. Bull. 2015, 38, 1352–1360. [Google Scholar] [CrossRef]
- Xu, W.; Mi, Y.; He, P.; He, S.; Niu, L. gamma-Tocotrienol Inhibits Proliferation and Induces Apoptosis Via the Mitochondrial Pathway in Human Cervical Cancer HeLa Cells. Molecules 2017, 22, 1299. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.; Liao, W.; Wong, W.S. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Lee, H.; Lim, Y. Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J. Nutr. Biochem. 2018, 57, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Juretic, N.; Sepulveda, R.; D’Espessailles, A.; Vera, D.B.; Cadagan, C.; de Miguel, M.; Gonzalez-Manan, D.; Tapia, G. Dietary alpha- and gamma-tocopherol (1:5 ratio) supplementation attenuates adipose tissue expansion, hepatic steatosis, and expression of inflammatory markers in a high-fat-diet-fed murine model. Nutrition 2021, 85, 111139. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Ramalingam, L.; Menikdiwela, K.; Scoggin, S.; Shen, C.L.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Chung, E.; Kalupahana, N.S.; et al. Effects of delta-tocotrienol on obesity-related adipocyte hypertrophy, inflammation and hepatic steatosis in high-fat-fed mice. J. Nutr. Biochem. 2017, 48, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.L.; Chin, K.Y. The Role of Tocotrienol in Protecting Against Metabolic Diseases. Molecules 2019, 24, 923. [Google Scholar] [CrossRef]
- Kim, Y.; Natarajan, S.K.; Chung, S. Gamma-Tocotrienol Attenuates the Hepatic Inflammation and Fibrosis by Suppressing Endoplasmic Reticulum Stress in Mice. Mol. Nutr. Food Res. 2018, 62, e1800519. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int. J. Obes. 2015, 39, 438–446. [Google Scholar] [CrossRef]
- Shen, C.L.; Kaur, G.; Wanders, D.; Sharma, S.; Tomison, M.D.; Ramalingam, L.; Chung, E.; Moustaid-Moussa, N.; Mo, H.; Dufour, J.M. Annatto-extracted tocotrienols improve glucose homeostasis and bone properties in high-fat diet-induced type 2 diabetic mice by decreasing the inflammatory response. Sci. Rep. 2018, 8, 11377. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Li, G.; Lee, M.J.; Liu, A.B.; Yang, Z.; Lin, Y.; Shih, W.J.; Yang, C.S. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free Radic. Biol. Med. 2012, 52, 1151–1158. [Google Scholar] [CrossRef]
- Wagner, J.G.; Birmingham, N.P.; Jackson-Humbles, D.; Jiang, Q.; Harkema, J.R.; Peden, D.B. Supplementation with gamma-tocopherol attenuates endotoxin-induced airway neutrophil and mucous cell responses in rats. Free Radic. Biol. Med. 2014, 68, 101–109. [Google Scholar] [CrossRef]
- McCary, C.A.; Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: Reversibility of alpha-tocopherol and gamma-tocopherol’s effects. J. Immunol. 2011, 186, 3674–3685. [Google Scholar] [CrossRef]
- Lu, G.; Xiao, H.; Li, G.X.; Picinich, S.C.; Chen, Y.K.; Liu, A.; Lee, M.J.; Loy, S.; Yang, C.S. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis 2010, 31, 687–694. [Google Scholar] [CrossRef]
- Chen, J.X.; Liu, A.; Lee, M.J.; Wang, H.; Yu, S.; Chi, E.; Reuhl, K.; Suh, N.; Yang, C.S. δ-and γ-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Mol. Carcinog. 2017, 56, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. gamma-Tocotrienol suppresses growth and sensitises human colorectal tumours to capecitabine in a nude mouse xenograft model by down-regulating multiple molecules. Br. J. Cancer 2016, 115, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Natoli, G.; Ghosh, G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 2006, 25, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Yang, S.J.; Lim, Y. Gamma-tocopherol supplementation ameliorated hyper-inflammatory response during the early cutaneous wound healing in alloxan-induced diabetic mice. Exp. Biol. Med. 2017, 242, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Enkhbaatar, P.; Sousse, L.E.; Sakurai, H.; Rehberg, S.W.; Asmussen, S.; Kraft, E.R.; Wright, C.L.; Bartha, E.; Cox, R.A.; et al. Nebulization with gamma-tocopherol ameliorates acute lung injury after burn and smoke inhalation in the ovine model. Shock 2012, 37, 408–414. [Google Scholar] [CrossRef]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef]
- Lee, H.; Lim, Y. Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice. Nutr. Res. Pract. 2019, 13, 377–383. [Google Scholar] [CrossRef]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef]
- Li, X.H.; Fu, D.; Latif, N.H.; Mullaney, C.P.; Ney, P.H.; Mog, S.R.; Whitnall, M.H.; Srinivasan, V.; Xiao, M. Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica 2010, 95, 1996–2004. [Google Scholar] [CrossRef]
- Shibata, A.; Nakagawa, K.; Kawakami, Y.; Tsuzuki, T.; Miyazawa, T. Suppression of gamma-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J. Agric. Food Chem. 2010, 58, 7013–7020. [Google Scholar] [CrossRef]
- Johnson, A.M.; Kleczko, E.K.; Nemenoff, R.A. Eicosanoids in Cancer: New Roles in Immunoregulation. Front. Pharmacol. 2020, 11, 595498. [Google Scholar] [CrossRef]
- Ju, J.; Hao, X.; Lee, M.J.; Lambert, J.D.; Lu, G.; Xiao, H.; Newmark, H.L.; Yang, C.S. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev. Res. 2009, 2, 143–152. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, Z.; Hall, Y.J.; Jang, Y.; Snyder, P.W.; Bain, C.; Huang, J.; Jannasch, A.; Cooper, B.; Wang, Y.; et al. Gamma-tocopherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice. Free Radic. Biol. Med. 2013, 65, 1069–1077. [Google Scholar] [CrossRef]
- Sanches, L.D.; Santos, S.A.; Carvalho, J.R.; Jeronimo, G.D.; Favaro, W.J.; Reis, M.D.; Felisbino, S.L.; Justulin, L.A., Jr. Protective effect of gamma-tocopherol-enriched diet on N-methyl-N-nitrosourea-induced epithelial dysplasia in rat ventral prostate. Int. J. Exp. Pathol. 2013, 94, 362–372. [Google Scholar] [CrossRef]
- Husain, K.; Centeno, B.A.; Chen, D.T.; Hingorani, S.R.; Sebti, S.M.; Malafa, M.P. Vitamin E delta-tocotrienol prolongs survival in the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) transgenic mouse model of pancreatic cancer. Cancer Prev. Res. 2013, 6, 1074–1083. [Google Scholar] [CrossRef]
- Husain, K.; Centeno, B.A.; Coppola, D.; Trevino, J.; Sebti, S.M.; Malafa, M.P. delta-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget 2017, 8, 31554–31567. [Google Scholar] [CrossRef]
- Huang, Y.; Khor, T.O.; Shu, L.; Saw, C.L.; Wu, T.Y.; Suh, N.; Yang, C.S.; Kong, A.N. A gamma-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J. Nutr. 2012, 142, 818–823. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Liu, A.; Wang, G.; Bosland, M.C.; Yang, C.S. delta-Tocopherol inhibits the development of prostate adenocarcinoma in prostate specific Pten-/- mice. Carcinogenesis 2018, 39, 158–169. [Google Scholar] [CrossRef]
- Huang, P.H.; Chuang, H.C.; Chou, C.C.; Wang, H.; Lee, S.L.; Yang, H.C.; Chiu, H.C.; Kapuriya, N.; Wang, D.; Kulp, S.K.; et al. Vitamin E facilitates the inactivation of the kinase Akt by the phosphatase PHLPP1. Sci. Signal. 2013, 6, ra19. [Google Scholar] [CrossRef]
- Selvaduray, K.R.; Radhakrishnan, A.K.; Kutty, M.K.; Nesaretnam, K. Palm tocotrienols inhibit proliferation of murine mammary cancer cells and induce expression of interleukin-24 mRNA. J. Interferon Cytokine Res. 2010, 30, 909–916. [Google Scholar] [CrossRef]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Chang, F.; Steelman, L.S.; Shelton, J.G.; Lee, J.T.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.; McCubrey, J.A. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int. J. Oncol. 2003, 22, 469–480. [Google Scholar]
- Huang, Y.; Wu, R.; Su, Z.Y.; Guo, Y.; Zheng, X.; Yang, C.S.; Kong, A.N. A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin-dependent kinase inhibitors p21 and p27. J. Nutr. Biochem. 2017, 40, 155–163. [Google Scholar] [CrossRef]
- Blair, C.A.; Wu, M.; Huynh, T.; Hu, H.; Walia, A.; Yang, C.S.; Zi, X. Delta tocopherol inhibits urothelial tumorigenesis in the UPII mutant Ha-ras transgenic mouse model and induces apoptosis via activation of the ATF4/CHOP-DR5 pathway. Proc. Am. Assoc. Cancer Res. Annu. Meet. 2017, 77, AM2017–AM2256. [Google Scholar]
- Das Gupta, S.; So, J.Y.; Wall, B.; Wahler, J.; Smolarek, A.K.; Sae-Tan, S.; Soewono, K.Y.; Yu, H.; Lee, M.J.; Thomas, P.E.; et al. Tocopherols inhibit oxidative and nitrosative stress in estrogen-induced early mammary hyperplasia in ACI rats. Mol. Carcinog. 2015, 54, 916–925. [Google Scholar] [CrossRef]
- Wada, S.; Naito, Y.; Matsushita, Y.; Nouchi, M.; Kawai, M.; Minami, E.; Aoi, W.; Ikeda, S.; Higashi, A.; Yoshikawa, T. δ-Tocotrienol suppresses tumorigenesis by inducing apoptosis and blocking the COX-2/PGE2 pathway that stimulates tumor–stromal interactions in colon cancer. J. Funct. Foods 2017, 35, 428–435. [Google Scholar] [CrossRef]
- Guan, F.; Li, G.; Liu, A.B.; Lee, M.J.; Yang, Z.; Chen, Y.K.; Lin, Y.; Shih, W.; Yang, C.S. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev. Res. 2012, 5, 644–654. [Google Scholar] [CrossRef]
- Li, G.X.; Lee, M.J.; Liu, A.B.; Yang, Z.; Lin, Y.; Shih, W.J.; Yang, C.S. delta-tocopherol is more active than alpha-or gamma-tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. 2011, 4, 404–413. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 2017, 8, 850–867. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Wagner, J.G.; Kala, A.; Mills, K.; Wells, H.B.; Alexis, N.E.; Lay, J.C.; Jiang, Q.; Zhang, H.; Zhou, H.; et al. Vitamin E, gamma-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic. Biol. Med. 2013, 60, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wiser, J.; Alexis, N.E.; Jiang, Q.; Wu, W.; Robinette, C.; Roubey, R.; Peden, D.B. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008, 45, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Vucinic, L.; Singh, I.; Spargo, F.J.; Hawley, J.A.; Linden, M.D. Gamma tocopherol supplementation prevents exercise induced coagulation and platelet aggregation. Thromb Res. 2010, 125, 196–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, M.; Wallmon, A.; Olsson-Mortlock, C.; Wallin, R.; Saldeen, T. Mixed tocopherols inhibit platelet aggregation in humans: Potential mechanisms. Am. J. Clin. Nutr. 2003, 77, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Pei, R.; Guo, Y.; Ballard, K.D.; Barker, T.; Rogers, V.E.; Parker, B.A.; Taylor, A.W.; Traber, M.G.; Volek, J.S.; et al. gamma-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic. Biol. Med. 2013, 65, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Pedrelli, V.F.; Lauriola, M.M.; Pigatto, P.D. Clinical evaluation of photoprotective effect by a topical antioxidants combination (tocopherols and tocotrienols). J. Eur. Acad Dermatol. Venereol. 2012, 26, 1449–1453. [Google Scholar] [CrossRef]
- Mahalingam, D.; Radhakrishnan, A.K.; Amom, Z.; Ibrahim, N.; Nesaretnam, K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur. J. Clin. Nutr. 2011, 65, 63–69. [Google Scholar] [CrossRef]
- Mah, E.; Noh, S.K.; Ballard, K.D.; Park, H.J.; Volek, J.S.; Bruno, R.S. Supplementation of a gamma-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J. Nutr. Biochem. 2013, 24, 196–203. [Google Scholar] [CrossRef]
- Evans, H.M.; Bishop, K.S. On the Existence of a Hitherto Unrecognized Dietary Factor Essential for Reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef]
- Barua, S.; Junaid, M.A. Lifestyle, pregnancy and epigenetic effects. Epigenomics 2015, 7, 85–102. [Google Scholar] [CrossRef]
- Anderson, K.; Nisenblat, V.; Norman, R. Lifestyle factors in people seeking infertility treatment—A review. Aust. N. Z. J. Obstet Gynaecol. 2010, 50, 8–20. [Google Scholar] [CrossRef]
- Rumiris, D.; Purwosunu, Y.; Wibowo, N.; Farina, A.; Sekizawa, A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens Pregnancy 2006, 25, 241–253. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E inadequacy in humans: Causes and consequences. Adv. Nutr. 2014, 5, 503–514. [Google Scholar] [CrossRef]
- Simsek, M.; Naziroglu, M.; Simsek, H.; Cay, M.; Aksakal, M.; Kumru, S. Blood plasma levels of lipoperoxides, glutathione peroxidase, beta carotene, vitamin A and E in women with habitual abortion. Cell Biochem. Funct 1998, 16, 227–231. [Google Scholar] [CrossRef]
- Bastani, P.; Hamdi, K.; Abasalizadeh, F.; Navali, N. Effects of vitamin E supplementation on some pregnancy health indices: A randomized clinical trial. Int. J. Gen. Med. 2011, 4, 461–464. [Google Scholar] [CrossRef]
- Mohd Mutalip, S.S.; Ab-Rahim, S.; Rajikin, M.H. Vitamin E as an Antioxidant in Female Reproductive Health. Antioxidants 2018, 7, 22. [Google Scholar] [CrossRef]
- Fang, J.C.; Kinlay, S.; Beltrame, J.; Hikiti, H.; Wainstein, M.; Behrendt, D.; Suh, J.; Frei, B.; Mudge, G.H.; Selwyn, A.P.; et al. Effect of vitamins C and E on progression of transplant-associated arteriosclerosis: A randomised trial. Lancet 2002, 359, 1108–1113. [Google Scholar] [CrossRef]
- Salonen, R.M.; Nyyssonen, K.; Kaikkonen, J.; Porkkala-Sarataho, E.; Voutilainen, S.; Rissanen, T.H.; Tuomainen, T.P.; Valkonen, V.P.; Ristonmaa, U.; Lakka, H.M.; et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: The Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation 2003, 107, 947–953. [Google Scholar] [CrossRef]
- Gey, K.F.; Puska, P.; Jordan, P.; Moser, U.K. Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-cultural epidemiology. Am. J. Clin. Nutr. 1991, 53, 326S–334S. [Google Scholar] [CrossRef]
- Herrera, E.; Barbas, C. Vitamin E: Action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Afshar, S.; Mathers, J.C. Effect of vitamin C and vitamin E supplementation on endothelial function: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 113, 1182–1194. [Google Scholar] [CrossRef]
- May, J.M. How does ascorbic acid prevent endothelial dysfunction? Free Radic. Biol. Med. 2000, 28, 1421–1429. [Google Scholar] [CrossRef]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Pashkow, F.J. Oxidative Stress and Inflammation in Heart Disease: Do Antioxidants Have a Role in Treatment and/or Prevention? Int. J. Inflam. 2011, 2011, 514623. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Ward, N.C.; Wu, J.H.; Clarke, M.W.; Puddey, I.B.; Burke, V.; Croft, K.D.; Hodgson, J.M. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. J. Hypertens 2007, 25, 227–234. [Google Scholar] [CrossRef]
- Wu, J.H.; Ward, N.C.; Indrawan, A.P.; Almeida, C.A.; Hodgson, J.M.; Proudfoot, J.M.; Puddey, I.B.; Croft, K.D. Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clin. Chem. 2007, 53, 511–519. [Google Scholar] [CrossRef]
- Said, E.; Mousa, S.; Fawzi, M.; Sabry, N.A.; Farid, S. Combined effect of high-dose vitamin A, vitamin E supplementation, and zinc on adult patients with diabetes: A randomized trial. J. Adv. Res. 2021, 28, 27–33. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Li, P.; Ma, C.; He, S.; Ping, F.; Zhang, H.; Li, W.; Xu, L.; Li, Y. Potential Protective Effect of Dietary Intake of Non-alpha-Tocopherols on Cellular Aging Markers Mediated by Tumor Necrosis Factor-alpha in Prediabetes: A Cross-Sectional Study of Chinese Adults. Oxid. Med. Cell. Longev. 2020, 2020, 7396801. [Google Scholar] [CrossRef]
- Ebrahimi, F.A.; Samimi, M.; Foroozanfard, F.; Jamilian, M.; Akbari, H.; Rahmani, E.; Ahmadi, S.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. The Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Indices of Insulin Resistance and Hormonal Parameters in Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Exp. Clin. Endocrinol. Diabetes 2017, 125, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.H.; Pourghassem Gargari, B.; Izadi, A.; Taghizadeh, S.H.; Parizad, M. Effect of Vitamin E on Serum Levels of Vascular Endothelial Growth Factor and Angiopoietin-1 in Women with Polycystic Ovary Syndrome: A Pilot Randomized, Placebo-Controlled Trial. Int. J. Fertil Steril. 2021, 15, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.R.; Khan, N.; Adams-Huet, B.; Kakarla, N.; Havelock, J.C.; Gell, J. Effect of vitamin E supplementation with and without hormone therapy on circulatory inflammatory markers in postmenopausal women. Fertil. Steril. 2006, 85, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Li, D.; Jialal, I. The effects of alpha tocopherol supplementation on monocyte function. Decreased lipid oxidation, interleukin 1 beta secretion, and monocyte adhesion to endothelium. J. Clin. Investig. 1996, 98, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Van Tits, L.J.; Demacker, P.N.; de Graaf, J.; Hak-Lemmers, H.L.; Stalenhoef, A.F. alpha-tocopherol supplementation decreases production of superoxide and cytokines by leukocytes ex vivo in both normolipidemic and hypertriglyceridemic individuals. Am. J. Clin. Nutr. 2000, 71, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Stanger, O.; Herrmann, W.; Pietrzik, K.; Fowler, B.; Geisel, J.; Dierkes, J.; Weger, M. DACH-LIGA homocystein (german, austrian and swiss homocysteine society): Consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: Guidelines and recommendations. Clin. Chem. Lab. Med. 2003, 41, 1392–1403. [Google Scholar] [CrossRef]
- Floegel, A.; Chung, S.J.; von Ruesten, A.; Yang, M.; Chung, C.E.; Song, W.O.; Koo, S.I.; Pischon, T.; Chun, O.K. Antioxidant intake from diet and supplements and elevated serum C-reactive protein and plasma homocysteine concentrations in US adults: A cross-sectional study. Public Health Nutr. 2011, 14, 2055–2064. [Google Scholar] [CrossRef]
- Saboori, S.; Shab-Bidar, S.; Speakman, J.R.; Yousefi Rad, E.; Djafarian, K. Effect of vitamin E supplementation on serum C-reactive protein level: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2015, 69, 867–873. [Google Scholar] [CrossRef]
- Somogyi, A.; Herold, M.; Kocsis, I.; Nagy, G.; Somfai, G.; Studinger, P. Effect of vitamin E supplementation on the vitamin content of lipoprotein in young men and women. Orv. Hetil. 2005, 146, 1813–1818. [Google Scholar]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Chapman, T.M.; Kim, H.J.; Min, D.B. Prooxidant activity of oxidized alpha-tocopherol in vegetable oils. J. Food Sci. 2009, 74, C536–C542. [Google Scholar] [CrossRef]
- Nadeem, N.; Woodside, J.V.; Kelly, S.; Allister, R.; Young, I.S.; McEneny, J. The two faces of alpha- and gamma-tocopherols: An in vitro and ex vivo investigation into VLDL, LDL and HDL oxidation. J. Nutr. Biochem. 2012, 23, 845–851. [Google Scholar] [CrossRef]
- Winterbone, M.S.; Sampson, M.J.; Saha, S.; Hughes, J.C.; Hughes, D.A. Pro-oxidant effect of alpha-tocopherol in patients with type 2 diabetes after an oral glucose tolerance test--a randomised controlled trial. Cardiovasc. Diabetol. 2007, 6, 8. [Google Scholar] [CrossRef][Green Version]
- Himmelfarb, J.; Phinney, S.; Ikizler, T.A.; Kane, J.; McMonagle, E.; Miller, G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J. Ren. Nutr. 2007, 17, 296–304. [Google Scholar] [CrossRef]
- Tasanarong, A.; Vohakiat, A.; Hutayanon, P.; Piyayotai, D. New strategy of alpha- and gamma-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrol. Dial. Transplant. 2013, 28, 337–344. [Google Scholar] [CrossRef]
- Koay, Y.Y.; Tan, G.C.J.; Phang, S.C.W.; Ho, J.I.; Chuar, P.F.; Ho, L.S.; Ahmad, B.; Abdul Kadir, K. A Phase IIb Randomized Controlled Trial Investigating the Effects of Tocotrienol-Rich Vitamin E on Diabetic Kidney Disease. Nutrients 2021, 13, 258. [Google Scholar] [CrossRef]
- Ascherio, A.; Weisskopf, M.G.; O’Reilly, E.J.; Jacobs, E.J.; McCullough, M.L.; Calle, E.E.; Cudkowicz, M.; Thun, M.J. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann. Neurol. 2005, 57, 104–110. [Google Scholar] [CrossRef]
- Adalier, N.; Parker, H. Vitamin E, Turmeric and Saffron in Treatment of Alzheimer’s Disease. Antioxidants 2016, 5, 40. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S.; Aggarwal, N.T.; Scherr, P.A. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005, 81, 508–514. [Google Scholar] [CrossRef]
- Morris, M.C.; Schneider, J.A.; Li, H.; Tangney, C.C.; Nag, S.; Bennett, D.A.; Honer, W.G.; Barnes, L.L. Brain tocopherols related to Alzheimer’s disease neuropathology in humans. Alzheimers Dement. 2015, 11, 32–39. [Google Scholar] [CrossRef]
- Baldeiras, I.; Santana, I.; Proenca, M.T.; Garrucho, M.H.; Pascoal, R.; Rodrigues, A.; Duro, D.; Oliveira, C.R. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J. Alzheimers Dis. 2008, 15, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Naumovski, N. Tocotrienols, health and ageing: A systematic review. Maturitas 2017, 95, 55–60. [Google Scholar] [CrossRef]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H.; et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. Aging. 2012, 33, 2282–2290. [Google Scholar] [CrossRef]
- Mangialasche, F.; Solomon, A.; Kareholt, I.; Hooshmand, B.; Cecchetti, R.; Fratiglioni, L.; Soininen, H.; Laatikainen, T.; Mecocci, P.; Kivipelto, M. Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp. Gerontol 2013, 48, 1428–1435. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Pavlik, V.N.; Doody, R.S.; Rountree, S.D.; Darby, E.J. Vitamin E use is associated with improved survival in an Alzheimer’s disease cohort. Dement. Geriatr Cogn Disord. 2009, 28, 536–540. [Google Scholar] [CrossRef]
- Arlt, S.; Muller-Thomsen, T.; Beisiegel, U.; Kontush, A. Effect of one-year vitamin C- and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer’s disease. Neurochem. Res. 2012, 37, 2706–2714. [Google Scholar] [CrossRef]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.; Hofman, A.; Stampfer, M.J.; Witteman, J.C.; Breteler, M.M. Dietary antioxidants and long-term risk of dementia. Arch. Neurol. 2010, 67, 819–825. [Google Scholar] [CrossRef]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C.; Cache County Study, G. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef]
- Giraldo, E.; Lloret, A.; Fuchsberger, T.; Vina, J. Abeta and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Biol. 2014, 2, 873–877. [Google Scholar] [CrossRef]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Cook, N.; Manson, J.; Buring, J.E.; Grodstein, F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch. Intern. Med. 2006, 166, 2462–2468. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Abner, E.L.; Schmitt, F.A.; Mendiondo, M.S.; Marcum, J.L.; Kryscio, R.J. Vitamin E and all-cause mortality: A meta-analysis. Curr. Aging Sci. 2011, 4, 158–170. [Google Scholar] [CrossRef]
- Chin, S.F.; Ibahim, J.; Makpol, S.; Abdul Hamid, N.A.; Abdul Latiff, A.; Zakaria, Z.; Mazlan, M.; Mohd Yusof, Y.A.; Abdul Karim, A.; Wan Ngah, W.Z. Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: A randomized controlled study. Nutr. Metab. 2011, 8, 42. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Moncaglieri, F.; Infantino, V.; Naso, M.; Perna, S. Focus on Pivotal Role of Dietary Intake (Diet and Supplement) and Blood Levels of Tocopherols and Tocotrienols in Obtaining Successful Aging. Int. J. Mol. Sci. 2015, 16, 23227–23249. [Google Scholar] [CrossRef]
- Michaelsson, K.; Wolk, A.; Byberg, L.; Arnlov, J.; Melhus, H. Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am. J. Clin. Nutr. 2014, 99, 107–114. [Google Scholar] [CrossRef]
- Holvik, K.; Gjesdal, C.G.; Tell, G.S.; Grimnes, G.; Schei, B.; Apalset, E.M.; Samuelsen, S.O.; Blomhoff, R.; Michaelsson, K.; Meyer, H.E. Low serum concentrations of alpha-tocopherol are associated with increased risk of hip fracture. A NOREPOS study. Osteoporos Int. 2014, 25, 2545–2554. [Google Scholar] [CrossRef]
- D’Adamo, C.R.; Shardell, M.D.; Hicks, G.E.; Orwig, D.L.; Hochberg, M.C.; Semba, R.D.; Yu-Yahiro, J.A.; Ferrucci, L.; Magaziner, J.S.; Miller, R.R. Serum vitamin E concentrations among highly functioning hip fracture patients are higher than in nonfracture controls. Nutr. Res. 2011, 31, 205–214. [Google Scholar] [CrossRef]
- Pette, D.; Spamer, C. Metabolic properties of muscle fibers. Fed Proc. 1986, 45, 2910–2914. [Google Scholar]
- Evans, W.J. Vitamin E, vitamin C, and exercise. Am. J. Clin. Nutr. 2000, 72, 647S–652S. [Google Scholar] [CrossRef]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.N.; Yang, C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010, 31, 533–542. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Selvaduray, K.R.; Abdul Razak, G.; Veerasenan, S.D.; Gomez, P.A. Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: A pilot clinical trial. Breast Cancer Res. 2010, 12, R81. [Google Scholar] [CrossRef] [PubMed]
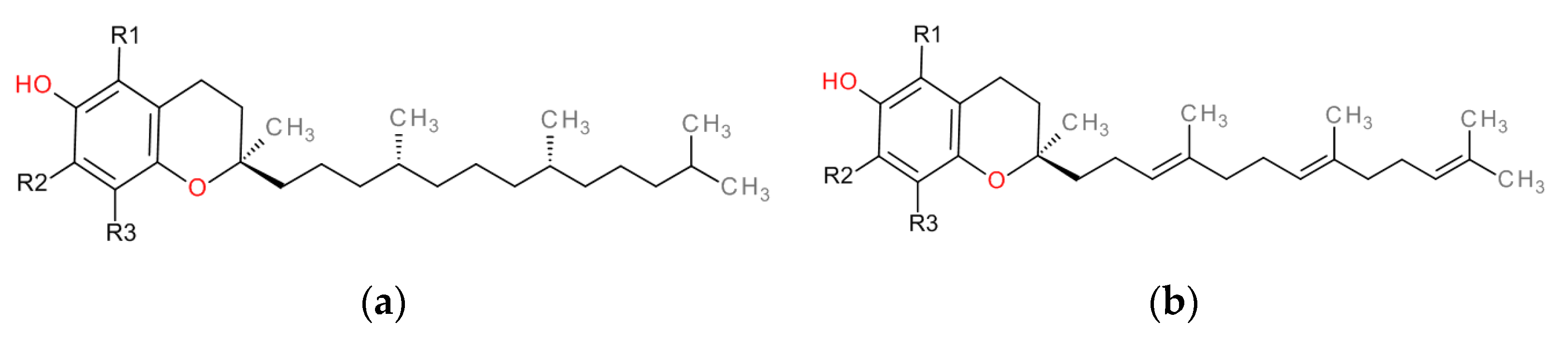
| Tocopherols | Tocotrienols | R1 | R2 | R3 |
|---|---|---|---|---|
| α-tocopherol (α-TF) | α-tocotrienol (α-TT) | CH3 | CH3 | CH3 |
| β-tocopherol (β-TF) | α-tocotrienol (β-TT) | CH3 | H | CH3 |
| γ-tocopherol (γ-TF) | α-tocotrienol (γ-TT) | H | CH3 | CH3 |
| δ-tocopherol (δ-TF) | α-tocotrienol (δ-TT) | H | H | CH3 |
| From | Conversion to Mg α-Tocopherol (Label Claim) |
|---|---|
| 1 mg α-TF | 1 |
| 1 mg RRR-α-TF | 1 |
| 2 mg all-rac-α-TF | 1 |
| 1 U.I. Vitamin E from natural sources (RRR-α-TF) including its ester forms (RRR-α-tocopheryl acetate and RRR-α-tocopheryl succinate) | 0.67 |
| 1 U.I. Vitamin E from synthetic sources (all-rac-α-TF) including its ester forms (all-rac-α-tocopheryl acetate and all rac-α-tocopheryl succinate) | 0.45 |
| Age | 0–6 mo | 7–12 mo | 1–3 y | 4–8 y | 9–13 y | 14–18 y | 18 + y | Pregnancy | Lactation | |
|---|---|---|---|---|---|---|---|---|---|---|
| DRI (mg/day) | EAR | 5 | 6 | 9 | 12 | 12 | 12 | 16 | ||
| RDA | − | − | 6 | 7 | 11 | 15 | 15 | 15 | 19 | |
| AI | 4 | 5 | ||||||||
| UL | 200 | 300 | 600 | 800 | 1000 | 1000 | 1000 | |||
| Age | 7–11 mo | 1–<3 y | 3–<10 y | 10–<18 | 18 + y | ||
|---|---|---|---|---|---|---|---|
| AI | boys | girls | men | women | |||
| 5 * | 6 | 9 | 13 | 11 | 13 | 11 | |
| Cell Line | Design/Treatment | Observed Effects | Reference |
|---|---|---|---|
| Human isolated neutrophils | PMA-stimulated neutrophils model:
|
| [65] |
| Human blood neutrophils or differentiated HL-60 cells |
|
| [66] |
| Raw 264.7 macrophages | LPS-stimulated inflammation model:
|
| [67] |
| Murine peritoneal macrophages | LPS-stimulated inflammation model:
|
| [68] |
| Murine RAW 264.7 cells and peritoneal macrophages (PM, prepared from BALB/c mice) |
|
| [69] |
|
Murine RAW264.7 macrophages Human epithelial cancer cells (A549) |
|
| [70] |
| Raw 264.7 macrophages | LPS-stimulated inflammation model:
|
| [71] |
| Raw 264.7 macrophages | TNF-α-induced NF-κB activation model:
| δ-TT:
| [72] |
| Intestinal epithelial cells (HT29) | TNF-α-induced stress model:
| All tocopherol derivates:
| [73] |
| Fetal-derived intestinal (FHs 74 Int) cells |
|
| [74] |
| Human myeloid KBM-5 cells Human lung adenocarcinoma H1299 cells Human embryonic kidney A293 cells Human breast cancer MCF-7Human multiple myeloma (U266) Head and neck squamous cell carcinoma (SCC4) tumor cells |
|
| [75] |
| Human multiple myeloma (MM) cell lines U266, MM.1R, and MM.1S (dexamethasone-sensitive) and MIA PaCa-2, PC3, and DU-145 cells |
|
| [76] |
| Immortalized human dermal capillary cells (HMEC-1) and HMEC-1A (a subcloned population of pure lymphatic endothelial cells) |
| BEC:
| [77] |
| Human lung epithelial A549 cells |
|
| [78] |
| Melanoma cell lines, BLM and A375 |
|
| [79] |
| Human normal esophageal epithelium cells Het-1A | NMBA-induced carcinogenesis model:
|
| [80] |
| Human pancreatic cancer cells (MiaPaCa-2 and AsPc-1) | For NF-κB activity assessment:
|
| [81] |
| Human prostate cancer cell lines (PC-3, DU-145, LNCaP, and CA-HPV-10) | TNF-α-induced stress model:
|
| [82] |
| Prostate cancer cell line DU145 |
|
| [83] |
| Prostate cancer cell line PC-3 |
|
| [84] |
| Castration-resistant prostate cancer cells (PC3 and DU145) |
|
| [85] |
| Prostate cancer PC3 stem-like cells |
|
| [86] |
| CaCO-2 and primary FHs 74 Int cells intestinal epithelial cell lines |
|
| [87] |
| CaCO-2 cells |
|
| [88] |
| CaCO-2 cells |
|
| [89] |
| SW 480 human colon cancer cell lines |
|
| [90] |
| Breast adenocarcinoma cell lines MDA-MB-231 and MCF7 |
|
| [91] |
| MDA-MB 231 and MCF-7 breast cancer cells |
| γ-TT:
| [92] |
| MDA-MB-231 and MCF-7 and breast cancer cells |
|
| [93] |
| MCF-7 breast cancer cells |
|
| [94] |
| HeLa cells |
|
| [95] |
| Animal Model | Dosage | Duration of Administration | Measured Parameters | Conclusion | Reference |
|---|---|---|---|---|---|
| High-fat diet (HFD) induced hepatic steatosis in male C57BL/6 J mice | α-TF and γ-TF: 0.7 and 3.5 mg/kg/day (1:5 ratio) | 12 weeks |
α-TF and γ-TF:
| In an HFD-setting, a combination of α-TF and γ-TF ameliorated adipocyte enlargement, hepatic steatosis, and inflammation modulated via PPAR-α/NF-κB signaling. | [98] |
| High-fat (45%) diet containing cholesterol (0.2%) in C57BL/6 male mice | γ-TT 0.1% in diet | 5 weeks |
γ-TT:
| γ-TT attenuates hepatic TG accumulation by improving insulin sensitivity and delays progression to NASH by reducing ER stress/hepatic fibrosis axis activation. | [101] |
| Airway inflammation caused by intranasal LPS in male F344 rats | γ-TF at 30 mg/kg (oral gavage), daily and LPS intranasal challenge (0, 5, or 20 µg) | Prior (2 days before) and during LPS challenge | γ-TF:
| Dietary γ-TF inhibited airway neutrophil recruitment and mucus hyperproduction. | [107] |
| Allergy airway inflammation and asthma models in ovalbumin-sensitized and challenged BALB/c mice | α-TF or γ-TF 100 mg/kg, s.c. injection | Prior to and during antigen challenge | γ-TF:
| γ-TF, not α-TF, attenuated airway inflammation. | [108] |
| Alloxan induced diabetes in ICR mice—excisional wounds were made by biopsy punches | γ -TF (35 mg/kg) p.o. 5 times/week | 2 weeks |
γ-TF reduced:
| γ-TF administration prevented diabetes-induced delayed wound healing via the inhibition of NF-κB and the reduction of oxidative stress. | [113] |
| Chemically induced (DSS 2%) colitis in male BALB/c mice | α-TF or γ-TF-rich mix (γ-TF:δ-TF:α-TF, 58:22:11) 0.05% in diet (group A versus group B) | A. 4 week TF-supplementation and 1 week concomitant colitis inductionB. 1 week TF-administration and colitis induction | γ-TF-rich mix and α-TF:
| α-TF- and γ-TF-rich mix significantly reduced diarrhea and fecal bleeding in mice, with superior efficacy in the case of supplementation prior to colitis induction. | [115] |
| Alloxan induced diabetes in ICR mice | γ-TF (35 mg/kg) p.o. | 3 weeks |
γ-TF:
| γ-TF reduces fasting blood glucose levels, ameliorates hyperglycemia-induced hepatic damage, reduces lipid peroxidation and oxidative stress, and inhibits apoptosis. | [116] |
| γ irradiation CD2F1 | δ-TT (400 mg/kg) s.c. | 24 h before and 6 h after total body irradiation at 5 or 8.75 Gy/min |
δ-TT:
| δ-TT protects mouse bone marrow and human CD34+ cells from radiation-induced damage through the ERK activation-associated mTOR survival pathways. | [118] |
| UVB-induced inflammation in HR-1 hairless mice | γ-TT-rich mix (2.3 mg/day) p.o. in corn oil | 14 days | γ-TT:
| γ-TT attenuates UVB-induced inflammation and skin thickening by inhibiting several pro-inflammatory pathways. | [119] |
| Chemically induced (DSS 1.5–2%) colitis in male BALB/c mice | 0.1% γ-TF or γ-TF-rich mix (45% γ-TF, 45% δ-TF, and 10% α-TF) in diet a week prior to DSS administration | 43/62 days | γ-TF:
| An γ-TF-rich, but not γ-TF-rich mix, attenuated moderate colitis induced by one cycle of 1.5% DSS, while neither was protective to severe colitis induced by 3 cycles of 2.5% DSS. | [122] |
| Animal Model | Dosage | Duration of Administration | Measured Parameters | Conclusion | Reference |
|---|---|---|---|---|---|
| Orthotopic xenograft model of human pancreatic ductal adenocarcinoma in mice NIH severe-combined immunodeficient (SCID) nude mice | α-TT, β-TT, γ-TT, and δ-TT: 200 mg/kg and 2/day | 4 weeks | δ-TT:
| δ-TT reduces the growth of pancreatic ductal adenocarcinoma by modulating NF-κB signaling. | [81] |
| Chemically induced (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone) lung tumor in A/J mice | 0.3% γ-TF-rich mix (57% γ-TF, 24% δ-TF, 13% α-TF, and 1.5% β-TF) in diet | 6 weeks | γ-TF-rich mix:
| γ-TF-rich mix significantly reduced tumor volume and tumor weight. | [109] |
| Xenograft tumor growth (human lung cancer H1299 cells) in NCr-nu/nu mice | 6 weeks | 0.3% γ-TF-rich mix in diet significantly lowered the tumor multiplicity. | |||
| Chemically induced (2-amino-1-methyl-6-phenylimidazo (4,5-b) pyridine) prostatic cancer in CYP1A-humanized mice (PhIP) | 0.3% γ-TF-rich mix in diet (mixture of 56.8% γ-TF, 24.3% δ-TF, 13.0% α-TF and 1.5% β-TF) versus 0.2% δ-TF, γ-TF, or α-TF in diet | 41 weeks | γ-TF-rich mix and δ-TF:
| γ-TF-rich mix and δ-TF significantly inhibited the development and severity of mouse prostatic intraepithelial neoplasia, being more effective than γ-TF or α-TF. | [110] |
| Nude mouse xenograft model of human colorectal cancer | 100 mg/kg of γ-TT 5 times/week | 2 weeks | γ-TT:
| γ-TT reduced tumor growth and enhanced the antitumor efficacy of capecitabine, possibly by inhibiting NF-κB signaling. It induced apoptosis, inhibited colony formation, and suppressed key regulators of cell survival, cell proliferation, invasion, angiogenesis, and metastasis. | [111] |
| Orthotopic xenograft model of human pancreatic ductal adenocarcinoma in athymic mice | 200 mg/kg of δ-TT 2/day | 4 weeks | δ-TT:
| δ-TT reduces the growth of pancreatic ductal adenocarcinoma, inhibits pancreatic cancer stem-like cells, and prevents pancreatic cancer metastasis by reducing epithelial-to-mesenchymal transition. | [125] |
| Genetic: Ptenp−/− mice | 0.2% δ-TF or α-TF supplemented in diet | 34 or 28 weeks | δ-TF (not α-TF):
| 0.2% δ-TF, but not α-TF, diet increased apoptosis and reduced Akt activation and cell proliferation. | [127] |
| Orthotopic human colon cancer mouse model (HCCLM3) BALB/c nude mice | 3.25 mg/day of γ-TT 5 days/week | 5 weeks | γ-TT:
| γ-TT reduces the tumor growth, and the tumor-induced angiogenesis by inhibiting AKT/mTOR pathway. | [130] |
| Genetic: UPII mutant Ha-ras transgenic mice | δ-TF 0.2% supplemented in diet | 150 days | δ-TF:
| 0.2% δ-TF diet had an antiproliferative effect and induced apoptosis via the activation of the ATF4/CHOP-DR5 pathway. | [133] |
| Chemically induced (estrogen) mammary hyperplasia in ACI rats | 0.3% γ-TF-rich mix in diet (mixture of 56.1% γ-TF, 22.3% δ-TF, 11.5% α-TF, and 1.2% β-TF) | 14 days | γ-TF-rich mix:
| γ-TF-rich mix exerted cytoprotective action and prevented estrogen-induced mammary hyperplasia. | [134] |
| Chemically induced colon cancer (azoxymethane and DDS) in C57BL/6 mice | 0.1% mixed TTs and TFs in diet (>65% TTs) versus 1% DeltaGold/0.1% in diet (90% δ-TT and 10% γ-TT) | 70 days | δ-TT:
| δ-TT prevented colorectal cancer by inducing apoptosis and blocking the COX-2/PGE2 pathway that stimulates tumor–stromal interactions in colon cancer. | [135] |
| Chemically induced (azoxymethane) induced colon carcinogenesis in F344 Rats | 0.2% δ-TF, γ-TF, or α-TF in diet | 9 weeks | δ-TF treatment:
| δ-TF treatment showed the strongest inhibitory effect, decreasing the numbers of aberrant crypt foci and colon carcinogenesis. | [136] |
| Xenograft tumor growth (human lung cancer H1299 cells) in NCr-nu/nu mice | 0.17% or 0.3% α-TF, δ-TF, γ-TF, or γ-TF-rich mix at diet | 49 days | δ-TF and γ-TF-rich mix:
| Growth inhibition effectiveness: δ-TF 0.3% > γ-TF-rich mix 0.3% > γ-TF 0.3% = δ-TF 0.17%> γ-TF-rich mix 0.17% = γ-TF 0.3% > α-TF 0.17% > α-TF 0.3%, with no significant differences versus control for α-TF. | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. https://doi.org/10.3390/antiox10050634
Ungurianu A, Zanfirescu A, Nițulescu G, Margină D. Vitamin E beyond Its Antioxidant Label. Antioxidants. 2021; 10(5):634. https://doi.org/10.3390/antiox10050634
Chicago/Turabian StyleUngurianu, Anca, Anca Zanfirescu, Georgiana Nițulescu, and Denisa Margină. 2021. "Vitamin E beyond Its Antioxidant Label" Antioxidants 10, no. 5: 634. https://doi.org/10.3390/antiox10050634
APA StyleUngurianu, A., Zanfirescu, A., Nițulescu, G., & Margină, D. (2021). Vitamin E beyond Its Antioxidant Label. Antioxidants, 10(5), 634. https://doi.org/10.3390/antiox10050634







