The Role of Toxic Metals and Metalloids in Nrf2 Signaling
Abstract
:1. Introduction
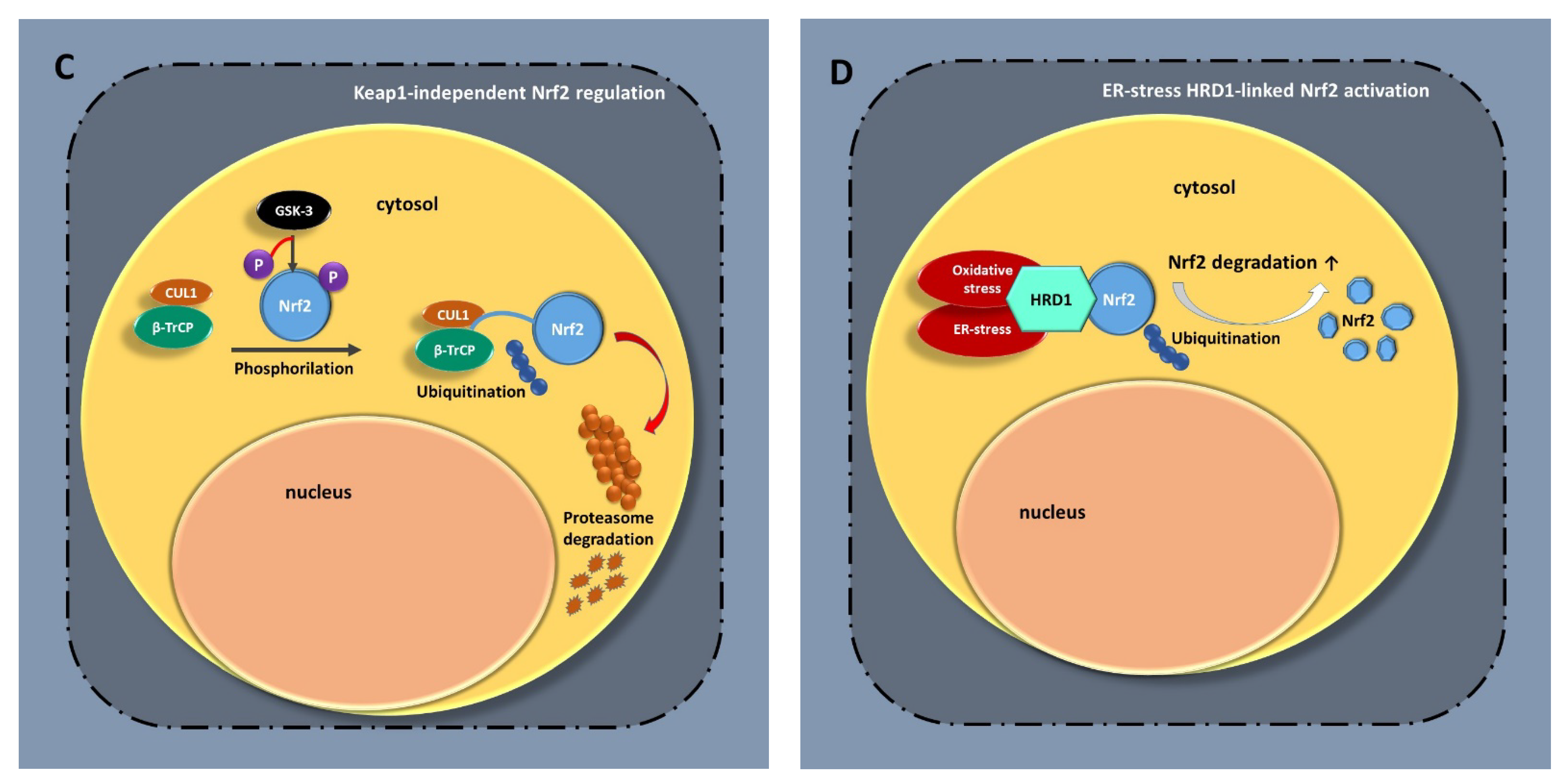
1.1. Nrf2 Signaling
1.2. Toxic Metals and Metalloids
2. Determining Toxic Elements Role in Nrf2 Signaling
2.1. Cadmium-Associated Changes in Nrf2 Signaling
2.2. Lead-Associated Changes in Nrf2 Signaling
2.3. Arsenic-Associated Changes in Nrf2 Signaling
2.4. Mercury-Associated Changes in Nrf2 Signaling
2.5. Nickel-Associated Changes in Nrf2 Signaling
2.6. Chromium-Associated Changes in Nrf2 Signaling
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- He, X.; Lin, G.X.; Chen, M.G.; Zhang, J.X.; Ma, Q. Protection against Chromium (VI)-Induced Oxidative Stress and Apoptosis by Nrf2. Recruiting Nrf2 into the Nucleus and Disrupting the Nuclear Nrf2/Keap1 Association. Toxicol. Sci. 2007, 98, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-M.; Ma, J.-Q.; Xie, W.-R.; Liu, S.-S.; Feng, Z.-J.; Zheng, G.-H.; Wang, A.-M. Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-κB pathway. Food Chem. Toxicol. 2015, 82, 19–26. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Osburn, W.; Kensler, T. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat. Res. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Li, S.; Lv, Y.; Yang, D.; Li, J.; Yang, Q.; Wu, P.; Lv, Z.; Zhang, Z. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019, 10, 5555–5565. [Google Scholar] [CrossRef]
- Yang, D.; Han, B.; Baiyun, R.; Lv, Z.; Wang, X.; Li, S.; Lv, Y.; Xue, J.; Liu, Y.; Zhang, Z. Sulforaphane Attenuates Hexavalent Chromium-Induced Cardiotoxicity via Activation of the Sesn2/AMPK/Nrf2 Signaling Pathway. Metallomics 2020. [Google Scholar] [CrossRef] [PubMed]
- Baiyun, R.; Li, S.; Liu, B.; Lu, J.; Lv, Y.; Xu, J.; Wu, J.; Li, J.; Lv, Z.; Zhang, Z. Luteolin-mediated PI3K/AKT/Nrf2 signaling pathway ameliorates inorganic mercury-induced cardiac injury. Ecotoxicol. Environ. Saf. 2018, 161, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Wang, X.-J.; Zhao, F.; Villeneuve, N.F.; Wu, T.; Jiang, T.; Sun, Z.; White, E.; Zhang, D.D. A Noncanonical Mechanism of Nrf2 Activation by Autophagy Deficiency: Direct Interaction between Keap1 and p62. Mol. Cell. Biol. 2010, 30, 3275–3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, A.; Zheng, Y.; Tao, S.; Wang, H.; Whitman, S.A.; White, E.; Zhang, D.D. Arsenic Inhibits Autophagic Flux, Activating the Nrf2-Keap1 Pathway in a p62-Dependent Manner. Mol. Cell. Biol. 2013, 33, 2436–2446. [Google Scholar] [CrossRef] [Green Version]
- Copple, I.M.; Lister, A.; Obeng, A.D.; Kitteringham, N.R.; Jenkins, R.E.; Layfield, R.; Foster, B.J.; Goldring, C.E.; Park, B.K. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. J. Biol. Chem. 2010, 285, 16782–16788. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhao, F.; Gao, B.; Tan, C.; Yagishita, N.; Nakajima, T.; Wong, P.K.; Chapman, E.; Fang, D.; Zhang, D.D. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014, 28, 708–722. [Google Scholar] [CrossRef] [Green Version]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.; Beer, H.-D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, D.; Biswas, J.; Bishayee, A. Nrf2-mediated redox signaling in arsenic carcinogenesis: A review. Arch. Toxicol. 2013, 87, 383–396. [Google Scholar] [CrossRef]
- Yang, B.; Yin, C.; Zhou, Y.; Wang, Q.; Jiang, Y.; Bai, Y.; Qian, H.; Xing, G.; Wang, S.; Li, F.; et al. Curcumin protects against methylmercury-induced cytotoxicity in primary rat astrocytes by activating the Nrf2/ARE pathway independently of PKCδ. Toxicology 2019, 425, 152248. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Buha, A.; Telkoparan-akillilar, P.; Cevik, D.; Kouretas, D.; Veskoukis, A.; Skaperda, Z.; Tsatsakis, A.; Wallace, D.; Suzen, S.; et al. Potential applications of NRF2 modulators in cancer therapy. Antioxidants 2020, 9, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauletto, M.; Tolosi, R.; Giantin, M.; Guerra, G.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Insights into Aflatoxin B1 Toxicity in Cattle: An In Vitro Whole-Transcriptomic Approach. Toxins 2020, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, J.; Tai, W.; Deng, S.; Li, T.; Wu, W.; Pu, L.; Fan, D.; Lei, W.; Zhang, T.; et al. High-Dose Paraquat Induces Human Bronchial 16HBE Cell Death and Aggravates Acute Lung Intoxication in Mice by Regulating Keap1/p65/Nrf2 Signal Pathway. Inflammation 2019, 42, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Han, B.; Wu, P.; Li, S.; Lv, Y.; Lu, J.; Yang, Q.; Li, J.; Zhu, Y.; Zhang, Z. Dibutyl phthalate induces allergic airway inflammation in rats via inhibition of the Nrf2/TSLP/JAK1 pathway. Environ. Pollut. 2020, 267, 115564. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Diwan, B.A.; Sun, Y.; Liu, J.; Qu, W.; He, Y.; Styblo, M.; Waalkes, M.P. Arsenic-induced malignant transformation of human keratinocytes: Involvement of Nrf2. Free Radic. Biol. Med. 2008, 45, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Albarakati, A.J.A.; Baty, R.S.; Aljoudi, A.M.; Habotta, O.A.; Elmahallawy, E.K.; Kassab, R.B.; Abdel Moneim, A.E. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol. Biol. Rep. 2020, 47, 2591–2603. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Ma, X.; Xu, X.; Du, Y.; Lv, Q.; Li, X.; Wu, Y.; Sun, H.; Yu, L.; et al. Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J. Inorg. Biochem. 2019, 197, 110698. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Yang, S.-H.; Long, M.; Yu, L.-H.; Li, L.; Li, P.; Zhang, Y.; Guo, Y.; Gao, F.; Liu, M.-D.; He, J.-B. Sulforaphane Prevents Testicular Damage in Kunming Mice Exposed to Cadmium via Activation of Nrf2/ARE Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangapandiyan, S.; Ramesh, M.; Miltonprabu, S.; Hema, T.; Jothi, G.B.; Nandhini, V. Sulforaphane potentially attenuates arsenic-induced nephrotoxicity via the PI3K/Akt/Nrf2 pathway in albino Wistar rats. Environ. Sci. Pollut. Res. 2019, 26, 12247–12263. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-H.; Yu, L.-H.; Li, L.; Guo, Y.; Zhang, Y.; Long, M.; Li, P.; He, J.-B. Protective Mechanism of Sulforaphane on Cadmium-Induced Sertoli Cell Injury in Mice Testis via Nrf2/ARE Signaling Pathway. Molecules 2018, 23, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Xu, Z.; Li, H.; Guo, M.; Yang, T.; Feng, S.; Xu, B.; Deng, Y. Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and Nrf2-ARE pathway activation. Hum. Exp. Toxicol. 2017, 36, 949–966. [Google Scholar] [CrossRef]
- Mahalanobish, S.; Saha, S.; Dutta, S.; Sil, P.C. Mangiferin alleviates arsenic induced oxidative lung injury via upregulation of the Nrf2-HO1 axis. Food Chem. Toxicol. 2019, 126, 41–55. [Google Scholar] [CrossRef]
- Kim, J.; Keum, Y.-S. NRF2, a Key Regulator of Antioxidants with Two Faces towards Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Niu, Q.; Hu, Y.; Feng, G.; Wang, H.; Li, S. Proanthocyanidins Antagonize Arsenic-Induced Oxidative Damage and Promote Arsenic Methylation through Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Wallace, D.R.; Taalab, Y.M.; Heinze, S.; Tariba Lovaković, B.; Pizent, A.; Renieri, E.; Tsatsakis, A.; Farooqi, A.A.; Javorac, D.; Andjelkovic, M.; et al. Toxic-Metal-Induced Alteration in miRNA Expression Profile as a Proposed Mechanism for Disease Development. Cells 2020, 9, 901. [Google Scholar] [CrossRef] [Green Version]
- Martí-Cid, R.; Llobet, J.M.; Castell, V.; Domingo, J.L. Dietary Intake of Arsenic, Cadmium, Mercury, and Lead by the Population of Catalonia, Spain. Biol. Trace Elem. Res. 2008, 125, 120–132. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Djordjevic, A.B.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef] [Green Version]
- Djordjevic, V.R.; Wallace, D.R.; Schweitzer, A.; Boricic, N.; Knezevic, D.; Matic, S.; Grubor, N.; Kerkez, M.; Radenkovic, D.; Bulat, Z.; et al. Environmental cadmium exposure and pancreatic cancer: Evidence from case control, animal and in vitro studies. Environ. Int. 2019, 128, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.R.; Buha Djordjevic, A. Heavy metal and pesticide exposure: A mixture of potential toxicity and carcinogenicity. Curr. Opin. Toxicol. 2020, 19, 72–79. [Google Scholar] [CrossRef]
- Buha, A.; Dukić-Ćosić, D.; Ćurčić, M.; Bulat, Z.; Antonijević, B.; Moulis, J.M.; Goumenou, M.; Wallace, D. Emerging links between cadmium exposure and insulin resistance: Human, animal, and cell study data. Toxics 2020, 8, 63. [Google Scholar] [CrossRef]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of cadmium thyroid disrupting effects and mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity-An overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Mutter, J.; Aaseth, J. Metal chelators and neurotoxicity: Lead, mercury, and arsenic. Arch. Toxicol. 2017, 91, 3787–3797. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic toxicity: Molecular targets and therapeutic agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Crisponi, G.; Nurchi, V.M.; Buha Djordjevic, A.; Aaseth, J. A Review on Coordination Properties of Thiol-Containing Chelating Agents Towards Mercury, Cadmium, and Lead. Molecules 2019, 24, 3247. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, A. Carcinogenicity of metal compounds: Possible role of DNA repair inhibition. Toxicol. Lett. 1998, 102–103, 235–239. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Sabolić, I. Common Mechanisms in Nephropathy Induced by Toxic Metals. Nephron Physiol. 2006, 104, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Đukić-Ćosić, D.; Baralić, K.; Javorac, D.; Djordjevic, A.B.; Bulat, Z. An overview of molecular mechanisms in cadmium toxicity. Curr. Opin. Toxicol. 2020, 19, 56–62. [Google Scholar] [CrossRef]
- Repić, A.; Bulat, P.; Antonijević, B.; Antunović, M.; Džudović, J.; Buha, A.; Bulat, Z. The influence of smoking habits on cadmium and lead blood levels in the Serbian adult people. Environ. Sci. Pollut. Res. 2020, 27, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-H.; Li, P.; Yu, L.-H.; Li, L.; Long, M.; Liu, M.-D.; He, J.-B. Sulforaphane Protect Against Cadmium-Induced Oxidative Damage in mouse Leydigs cells by Activating Nrf2/ARE Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 630. [Google Scholar] [CrossRef] [Green Version]
- Buha, A.; Jugdaohsingh, R.; Matovic, V.; Bulat, Z.; Antonijevic, B.; Kerns, J.G.; Goodship, A.; Hart, A.; Powell, J.J. Bone mineral health is sensitively related to environmental cadmium exposure- experimental and human data. Environ. Res. 2019, 176, 108539. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. 2012;100(PT C):11. IARC Monogr. Eval. Carcinog. Risks Humans 2012, 100, 11. [Google Scholar]
- Wallace, D.; Spandidos, D.; Tsatsakis, A.; Schweitzer, A.; Djordjevic, V.; Djordjevic, A. Potential interaction of cadmium chloride with pancreatic mitochondria: Implications for pancreatic cancer. Int. J. Mol. Med. 2019, 44, 145–156. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Lombaert, N.; Lison, D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ. Int. 2016, 86, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimonte, V.M.; Besharat, Z.M.; Antonioni, A.; Cella, V.; Lenzi, A.; Ferretti, E.; Migliaccio, S. The endocrine disruptor cadmium: A new player in the pathophysiology of metabolic diseases. J. Endocrinol. Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive functions and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Tariba Lovaković, B. Cadmium, arsenic, and lead: Elements affecting male reproductive health. Curr. Opin. Toxicol. 2020, 19, 7–14. [Google Scholar] [CrossRef]
- Silva, N.; Peiris-John, R.; Wickremasinghe, R.; Senanayake, H.; Sathiakumar, N. Cadmium a metalloestrogen: Are we convinced? J. Appl. Toxicol. 2012, 32, 318–332. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J.; Ge, J.; Wang, L.-L.; Li, N.; Sun, X.-T.; Cao, H.-B.; Li, J.-L. Selenium triggers Nrf2-mediated protection against cadmium-induced chicken hepatocyte autophagy and apoptosis. Toxicol. Vitr. 2017, 44, 349–356. [Google Scholar] [CrossRef]
- Wang, L.; Gallagher, E.P. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol. Appl. Pharmacol. 2013, 266, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.-G.; Wang, X.-Y.; Wang, J.-H.; Fan, R.-F.; Wang, L. Trehalose prevents cadmium-induced hepatotoxicity by blocking Nrf2 pathway, restoring autophagy and inhibiting apoptosis. J. Inorg. Biochem. 2019, 192, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Y.; Lu, Z.; Guo, W.; Tumen, B.; He, Y.; Chen, C.; Hu, S.; Xu, K.; Wang, Y.; et al. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway. Int. J. Environ. Res. Public Health 2019, 17, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, K.-C.; Wang, Z.-Y.; Tang, K.-K.; Zhu, Y.-S.; Fan, R.-F. Trehalose suppresses cadmium-activated Nrf2 signaling pathway to protect against spleen injury. Ecotoxicol. Environ. Saf. 2019, 181, 224–230. [Google Scholar] [CrossRef]
- Lawal, A.O.; Ellis, E.M. Nrf2-mediated adaptive response to cadmium-induced toxicity involves protein kinase C delta in human 1321N1 astrocytoma cells. Environ. Toxicol. Pharmacol. 2011, 32, 54–62. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, M.G.; Ma, Q. Activation of Nrf2 in Defense against Cadmium-Induced Oxidative Stress. Chem. Res. Toxicol. 2008, 21, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, Y.; Kimura, T.; Itagaki, A.; Yamamoto, C.; Taguchi, K.; Yamamoto, M.; Kumagai, Y.; Kaji, T. Partial contribution of the Keap1–Nrf2 system to cadmium-mediated metallothionein expression in vascular endothelial cells. Toxicol. Appl. Pharmacol. 2016, 295, 37–46. [Google Scholar] [CrossRef]
- Wu, K.C.; Liu, J.J.; Klaassen, C.D. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol. Appl. Pharmacol. 2012, 263, 14–20. [Google Scholar] [CrossRef]
- Wang, Y.; Mandal, A.K.; Son, Y.-O.; Pratheeshkumar, P.; Wise, J.T.F.; Wang, L.; Zhang, Z.; Shi, X.; Chen, Z. Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol. Appl. Pharmacol. 2018, 353, 23–30. [Google Scholar] [CrossRef]
- Xu, J.; Wise, J.T.F.; Wang, L.; Schumann, K.; Zhang, Z.; Shi, X. Dual Roles of Oxidative Stress in Metal Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 345–376. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, Y.R. The protective role of Nrf2 in cadmium-induced DNA damage. Mol. Cell. Toxicol. 2011, 7, 61–66. [Google Scholar] [CrossRef]
- Almeer, R.; Soliman, D.; Kassab, R.; AlBasher, G.; Alarifi, S.; Alkahtani, S.; Ali, D.; Metwally, D.; Abdel Moneim, A. Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway. Int. J. Mol. Sci. 2018, 19, 3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotaibi, M.F.; Al-Joufi, F.; Abou Seif, H.S.; Alzoghaibi, M.A.; Djouhri, L.; Ahmeda, A.F.; Mahmoud, A.M. Umbelliferone Inhibits Spermatogenic Defects and Testicular Injury in Lead-Intoxicated Rats by Suppressing Oxidative Stress and Inflammation, and Improving Nrf2/HO-1 Signaling. Drug Des. Devel. Ther. 2020, 14, 4003–4019. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Jiang, A.; Li, X.; Chang, W.; Chen, J.; Ye, F. Metformin alleviates lead-induced mitochondrial fragmentation via AMPK/Nrf2 activation in SH-SY5Y cells. Redox Biol. 2020, 36, 101626. [Google Scholar] [CrossRef] [PubMed]
- Javorac, D.; Signević, A.B.; Signelković, M.; Tatović, S.; Baralić, K.; Antonijević, E.; Kotur-Stevuljević, J.; Signukić-Äosić, D.; Antonijević, B.; Bulat, Z. Redox and essential metal status in the brain of Wistar rats acutely exposed to a cadmium and lead mixture. Arh. Hig. Rada Toksikol. 2020, 71, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Aglan, H.S.; Gebremedhn, S.; Salilew-Wondim, D.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Regulation of Nrf2 and NF-κB during lead toxicity in bovine granulosa cells. Cell Tissue Res. 2020, 380, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Tian, Z.-K.; Zhang, Y.-J.; Ming, Q.-L.; Ma, J.-Q.; Ji, L.-P. Effects of Gastrodin against Lead-Induced Brain Injury in Mice Associated with the Wnt/Nrf2 Pathway. Nutrients 2020, 12, 1805. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, X.; Li, L.; Lyu, L.; Yuan, J.; Chen, J. The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem. Toxicol. 2015, 86, 191–201. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, H.; Liu, B.; Baiyun, R.; Li, S.; Lv, Y.; Li, D.; Qiao, S.; Tan, X.; Zhang, Z. Grape seed procyanidin extract protects against Pb-induced lung toxicity by activating the AMPK/Nrf2/p62 signaling axis. Food Chem. Toxicol. 2018, 116, 59–69. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Huang, S.; Chen, L.; Fan, G.; Wang, C. The chronic effects of low lead level on the expressions of Nrf2 and Mrp1 of the testes in the rats. Environ. Toxicol. Pharmacol. 2013, 35, 109–116. [Google Scholar] [CrossRef]
- AL-Megrin, W.A.; Alomar, S.; Alkhuriji, A.F.; Metwally, D.M.; Mohamed, S.K.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Luteolin protects against testicular injury induced by lead acetate by activating the Nrf2/ HO -1 pathway. IUBMB Life 2020, 72, 1787–1798. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Xiao, Q.; Li, D. Daphnetin activates the Nrf2-dependent antioxidant response to prevent arsenic-induced oxidative insult in human lung epithelial cells. Chem. Biol. Interact. 2019, 302, 93–100. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Zeng, T.; Liu, P.; Wei, Y.; Dodson, M.; Chapman, E.; Zhang, D.D. Chronic arsenic exposure enhances metastatic potential via NRF2-mediated upregulation of SOX9. Toxicol. Appl. Pharmacol. 2020, 402, 115138. [Google Scholar] [CrossRef] [PubMed]
- Aono, J.; Yanagawa, T.; Itoh, K.; Li, B.; Yoshida, H.; Kumagai, Y.; Yamamoto, M.; Ishii, T. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem. Biophys. Res. Commun. 2003, 305, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Nakaso, K.; Kitayama, M.; Ishii, T.; Bannai, S.; Yanagawa, T.; Kimura, K.; Nakashima, K.; Ohama, E.; Yamada, K. Effects of kainate-mediated excitotoxicity on the expression of rat counterparts of A170 and MSP23 stress proteins in the brain. Mol. Brain Res. 1999, 69, 155–163. [Google Scholar] [CrossRef]
- He, X.; Chen, M.G.; Lin, G.X.; Ma, Q. Arsenic Induces NAD(P)H-quinone Oxidoreductase I by Disrupting the Nrf2·Keap1·Cul3 Complex and Recruiting Nrf2·Maf to the Antioxidant Response Element Enhancer. J. Biol. Chem. 2006, 281, 23620–23631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Hou, Y.; Li, L.; Yang, Y.; Jia, J.; Hong, Z.; Li, T.; Xu, Y.; Fu, J.; Sun, Y.; et al. Nrf2 deficiency aggravates the increase in osteoclastogenesis and bone loss induced by inorganic arsenic. Toxicol. Appl. Pharmacol. 2019, 367, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, C.; Yao, M.; Wang, L.; Liang, B.; Zhang, B.; Huang, X.; Zhang, A. The PKCδ-Nrf2-ARE signalling pathway may be involved in oxidative stress in arsenic-induced liver damage in rats. Environ. Toxicol. Pharmacol. 2018, 62, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, Q.; Fu, Y.; Wadgaonkar, P.; Zhang, W.; Almutairy, B.; Xu, L.; Rice, M.; Qiu, Y.; Thakur, C.; et al. Nrf2 and HIF1α converge to arsenic-induced metabolic reprogramming and the formation of the cancer stem-like cells. Theranostics 2020, 10, 4134–4149. [Google Scholar] [CrossRef]
- Wu, X.; Sun, R.; Wang, H.; Yang, B.; Wang, F.; Xu, H.; Chen, S.; Zhao, R.; Pi, J.; Xu, Y. Enhanced p62-NRF2 Feedback Loop due to Impaired Autophagic Flux Contributes to Arsenic-Induced Malignant Transformation of Human Keratinocytes. Oxid. Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Trinh, E.; Qiang, L.; Xie, L.; Hu, W.-Y.; Prins, G.; Pi, J.; He, Y.-Y. Arsenic Induces p62 Expression to Form a Positive Feedback Loop with Nrf2 in Human Epidermal Keratinocytes: Implications for Preventing Arsenic-Induced Skin Cancer. Molecules 2017, 22, 194. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.G.; El-Gareib, A.W. Gestational Arsenic Trioxide Exposure Acts as a Developing Neuroendocrine-Disruptor by Downregulating Nrf2/PPARγ and Upregulating Caspase-3/NF-ĸB/Cox2/BAX/iNOS/ROS. Dose-Response 2019, 17, 1559325819858266. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, J.; Li, L.; Li, Y.; Lv, H.; Xu, Y.; Sun, G.; Pi, J. Effects of Nrf2 deficiency on arsenic metabolism in mice. Toxicol. Appl. Pharmacol. 2017, 337, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Kanda, H.; Shinkai, Y.; Toyama, T. The Role of the Keap1/Nrf2 Pathway in the Cellular Response to Methylmercury. Oxid. Med. Cell. Longev. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, X.; Yang, D.; Lu, J.; Liu, B.; Baiyun, R.; Zhang, Z. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget 2017, 8, 40982–40993. [Google Scholar] [CrossRef] [Green Version]
- Unoki, T.; Akiyama, M.; Kumagai, Y.; Gonçalves, F.M.; Farina, M.; da Rocha, J.B.T.; Aschner, M. Molecular Pathways Associated With Methylmercury-Induced Nrf2 Modulation. Front. Genet. 2018, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Toyama, T.; Shinkai, Y.; Yasutake, A.; Uchida, K.; Yamamoto, M.; Kumagai, Y. Isothiocyanates Reduce Mercury Accumulation via an Nrf2-Dependent Mechanism during Exposure of Mice to Methylmercury. Environ. Health Perspect. 2011, 119, 1117–1122. [Google Scholar] [CrossRef]
- Zeng, L.; Zheng, J.-L.; Wang, Y.-H.; Xu, M.-Y.; Zhu, A.-Y.; Wu, C.-W. The role of Nrf2/Keap1 signaling in inorganic mercury induced oxidative stress in the liver of large yellow croaker Pseudosciaena crocea. Ecotoxicol. Environ. Saf. 2016, 132, 345–352. [Google Scholar] [CrossRef]
- Kim, H.L.; Seo, Y.R. Molecular and genomic approach for understanding the gene-environment interaction between Nrf2 deficiency and carcinogenic nickel-induced DNA damage. Oncol. Rep. 2012, 28, 1959–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (IARC). Nickel Nickel Compounds, 100C ed.; IARC: Lyon, France, 2018; Volume 1–42. [Google Scholar]
- Lewis, J.B.; Messer, R.L.; McCloud, V.V.; Lockwood, P.E.; Hsu, S.D.; Wataha, J.C. Ni(II) activates the Nrf2 signaling pathway in human monocytic cells. Biomaterials 2006, 27, 5348–5356. [Google Scholar] [CrossRef]
- Jiménez-Vidal, L.; Espitia-Pérez, P.; Torres-Ávila, J.; Ricardo-Caldera, D.; Salcedo-Arteaga, S.; Galeano-Páez, C.; Pastor-Sierra, K.; Espitia-Pérez, L. Nuclear factor erythroid 2–related factor 2 and its relationship with cellular response in nickel exposure: A systems biology analysis. BMC Pharmacol. Toxicol. 2019, 20, 78. [Google Scholar] [CrossRef]
- Shaw, P.; Sen, A.; Mondal, P.; Dey Bhowmik, A.; Rath, J.; Chattopadhyay, A. Shinorine ameliorates chromium induced toxicity in zebrafish hepatocytes through the facultative activation of Nrf2-Keap1-ARE pathway. Aquat. Toxicol. 2020, 228, 105622. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Chromium (VI) Compounds; World Health Organization: Lyon, France, 2018; Volume 1–42. [Google Scholar]
- Jindal, R.; Handa, K. Hexavalent chromium-induced toxic effects on the antioxidant levels, histopathological alterations and expression of Nrf2 and MT2 genes in the branchial tissue of Ctenopharyngodon idellus. Chemosphere 2019, 230, 144–156. [Google Scholar] [CrossRef]
- Kalayarasan, S.; Sriram, N.; Sureshkumar, A.; Sudhandiran, G. Chromium (VI)-induced oxidative stress and apoptosis is reduced by garlic and its derivative S- allylcysteine through the activation of Nrf2 in the hepatocytes of Wistar rats. J. Appl. Toxicol. 2008, 28, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Mondal, P.; Bandyopadhyay, A.; Chattopadhyay, A. Environmentally relevant concentration of chromium activates Nrf2 and alters transcription of related XME genes in liver of zebrafish. Chemosphere 2019, 214, 35–46. [Google Scholar] [CrossRef] [PubMed]

| Type | Cell Culture/ Species | Treatment Doses | Duration | Effects on Nrf2 Signaling | Ref. |
|---|---|---|---|---|---|
| In vitro | Nrf2 knockout (Nrf2-/-) mouse embryonic fibroblasts (MEF) cells | 2, 5, 10, 50, and 100 μM CdCl2 | 5 h | Increase in the ROS production and increased sensitivity to Cd-induced cell death in Nrf2 knockout (Nrf2-/-) MEF cells. | [75] |
| rat proximal tubular (rPT) cells | 2.5 µM Cd | 12 h | Oxidative stress via Nrf2 antioxidant pathway. Enhanced Nrf2 nuclear translocation. Downmodulation of Keap1. Activated Nrf2 target genes, including detoxifying enzymes (NQO1 and HO-1). Autophagosome accumulation. | [69] | |
| astrocytoma cell line 1321N1 | 5 and 10 µM Cd | 24 h | Increased levels of NQO1 and HO-1 mRNA. Increased nuclear accumulation of Nrf2. Connection between phospholipase C activation and Nrf2 signaling. | [74] | |
| RKO human colon carcinoma cell line | 0, 5, 10, 20, 40, 80, 160 and 320 μM CdCl2 | 24 h | DNA damage and increased intracellular ROS generation in Nrf2 lacking RKO cells. Induction of micronuclei (MN)—hallmark of carcinogenicity in Cd-exposed Nrf2 deficient cells. Nrf2—important role in suppression of Cd-induced carcinogenicity. | [80] | |
| bovine aortic endothelial cells | 0.5, 1, 2, 5 µM CdCl2 | 24 h | Modification of cysteine residues in Keap1 and Nrf2 activation. Up-regulation of metallothionein. Participation of Keap1–Nrf2 system in the modulation of metallothionein-1/2 expression. | [76] | |
| BEAS-2BR lung cells | 5 or 20 µM CdCl2 | 24 h | Autophagy deficiency, accumulation of autophagosomes, and increased p62. Nrf2-p62 positive feedback mechanism. Constitutive Nrf2 activation increases anti-apoptotic proteins, Bcl-2 and Bcl-xl. Apoptosis resistance. | [78] | |
| In vivo | zebrafish | 0, 11, and 110 μg·L−1 CdCl2 | 24 h | Dose-dependent induction of Nrf2-regulated antioxidant genes. Increased glutathione S-transferase pi, glutamate–cysteine ligase catalytic subunit, HO-1 and peroxiredoxin 1 mRNA. | [70] |
| mice | 4 mg/kg b.w. CdCl2 i.p. | single dose | Activated NF-κB, NLRP3, and MAPKs signaling pathways in liver. Inhibition of Nrf2, HO-1, and activation of NF-κB, NLRP3, and MAPKs contribute to liver injury. | [72] | |
| mice | 3.5 mg/kg b.w. CdCl2 i.p. | single dose | Nrf2 activation prevents Cd-induced oxidative stress and liver injury through induction of genes involved in antioxidant defense rather than genes that scavenge Cd (metallothioneins). | [77] | |
| mice | 6.5 mg/kg b.w. CdCl2 i.p. | 7 days | Impaired expression of Nrf2 gene in testes. | [81] | |
| mice | 2.3 mg/kg b.w. CdCl2 i.p. | 10 days | Reduced mRNA and protein expression of mouse testicular Nrf2. Decreased expression of Nrf2 downstream genes, GSH-Px, glutamyl cysteine synthetase (GCS), HO-1, NQO1. | [32] | |
| rats | 20 mg/L CdCl2 Drinking water | 8 weeks | Increased Nrf2 nuclear translocation. Elevated expression of Nrf2-downstream targets in rat liver. Cd-elevated protein levels of hepatic antioxidant enzymes. | [71] | |
| rats | 20 mg/L CdCl2 Drinking water | 8 weeks | Increased Nuclear translocation of Nrf2 in spleen. Induction of apoptosis and inhibition of autophagy. | [73] |
| Type of Study | Cell Culture/ Species | Treatment Concentration/Dose | Duration | Effects on Nrf2 Signaling | Ref. |
|---|---|---|---|---|---|
| In vitro | bovine granulosa cells | 1, 2, 3, 5, and 10 μg/mL | 2 h | Oxidative stress that attenuates cell proliferation and alters cell cycle progression. Apoptosis through disrupted Nrf2/NF-κB interaction. Decrease in Nrf2. Concomitant downregulation of both SOD and CAT. | [85] |
| SH-SY5Y cells | 1, 5, 25 or 125 μM Pb (CH3COO)2 | 24 h | Nrf2/HO-1 signaling pathway as cellular self-defense mechanism protects against Pb-induced oxidative stress. | [83] | |
| SH-SY5Y cells | 125 μM Pb (CH3COO)2 | 3, 6, 12 and 24 h | Rapid increase in Nrf2 nuclear accumulation. Nrf2–ARE binding activities in a ROS-dependent manner. Nrf2 regulated induction of mRNA transcription of HO-1, GSTa1, and NQO1, as well as the protein expression of HO-1 and g-GCS. | [87] | |
| In vivo | rats | 20 mg/kg b.w., Pb (CH3COO)2 i.p. | 7 days | Downmodulation of antioxidant enzyme activity and expression in renal tissue (SOD, CAT, GSH-Px). High MDA levels. Downregulation of Nfe212 and Homx1 mRNA expression. Increased inflammatory markers (TNF-α, IL-1β and NO). Upregulated synthesis of apoptotic related proteins. Downregulated anti-apoptotic protein expression. | [28] |
| rats | 20 mg/kg b.w. Pb (CH3COO)2 i.p. | 7 days | Pb (CH3COO)2 deactivated Nrf2 and HO-1 in the testicular tissue. Overactivation of nuclear factor kappa B (NF-κB) by free radical overproduction, increased the level of Keap1, leading to Nrf2 impairment and decrease in its antioxidant effect. | [90] | |
| rats | 50 mg/kg b.w. Pb (CH3COO)2 Oral gavage | 4 weeks | Downregulated gene expression of testicular Nrf2, NQO-1, and HO-1. Oxidative damage, inflammation, and cell death. | [82] | |
| mice | 250 mg/L Pb (CH3COO)2 Drinking water | 4 weeks | Apoptosis of neurons in hippocampus tissue. Oxidative stress, inflammation, and apoptosis by inhibiting the activations of Nrf2 HO-1 in rat brain. Decreased nuclear translocation of Nrf2 and the protein expressions of HO-1 and NQO1. | [81] | |
| rats | 2500 ppm Pb (CH3COO)2 Drinking water | 5 weeks | AMPK/Nrf2/p62 signaling protects the lung from oxidative stress, inflammation, and apoptosis. | [88] | |
| rats | 0, 0.3, and 0.9 g/L Pb (CH3COO)2 Drinking water | 6 months | Significant increases in the expressions of Mrp1 and Nrf2 in rat testes at both administered dose levels. Increased nuclear translocation of Nrf2. Dose-dependent decrease in GST and GSH. Mrp1—important roles in lead detoxification by Nrf2. | [89] |
| Type of Study | Cell Culture/ Species | Treatment Doses | Duration | Effects on Nrf2 Signaling | Ref. |
|---|---|---|---|---|---|
| mouse hepa1c1c7 cells | 2.5 and 10 µM NaAsO2 | 5 h | Nrf2 is required for induction of detoxification gene, NQO1. As extended the t1⁄2 of Nrf2 by inhibiting the Keap1–Cul3-dependent ubiquitination and proteasomal Nrf2 turnover. As did not disrupt the Nrf2–Keap1–Cul3 association in the cytoplasm, but it induced Nrf2 dissociation from Keap1 and Cul3 and dimerization of Nrf2 with a Maf protein (Maf G/Maf K) in nucleus. | [98] | |
| In vitro | bronchial epithelial cell line BEAS-2B | 0, 0.25, 0.5, 1, 2, 4 µM As3+(inorganic) | 8 h | Binding of Nrf2 and/or HIF1α on the genome. Amplified Nrf2 enrichment peaks in intergenic region, promoter and gene body. Mutual transcriptional regulation between Nrf2 and HIF1α. Nrf2 activation is an initiating signal for As-induced HIF1α activation. | [98] |
| L-02 cells | 25 μM NaAsO2 | 24 h | Decreased Nrf2 and its downstream genes expression. | [38] | |
| non-small cell lung cancer (NSCLC) | 0.5 μM NaAsO2 | 3 months | Chronic As exposure enhances the invasive and migratory capacity of immortalized lung epithelial cells via Nrf2-dependent upregulation of SRY-box 9 (SOX9), transcription factor linked with cell proliferation, epithelial-mesenchymal transition, and metastasis. Hyperactivation of Nrf2 gene via knockout of Keap1 contributes to cell proliferation. | [92] | |
| human HaCaT keratinocytes | 4 and 8 μM NaAsO2 | 28 weeks | As induces p62 expression to form a positive feedback loop with Nrf2. | [100] | |
| human HaCaT keratinocytes | 100 nM NaAsO2 | 28 weeks | Increased intracellular glutathione and elevated expression of Nrf2 and its target genes. Generalized apoptotic resistance. Diminished Nrf2-mediated antioxidant response induced by acute exposure to high doses of arsenite. Biomarkers for malignant transformation, MMP-9, and cytokeratins, are potentially regulated by Nrf2. Constitutive Nrf2 activation may be involved in arsenic skin carcinogenesis. | [27] | |
| human keratinocytes (HaCaT) | 100 nM or 200 nM NaAsO2 | 4 h | Silencing NRF2 abrogated the increase in mRNA and protein levels of p62 and malignant phenotypes induced by arsenite | [99] | |
| MC3T3-E1 osteoblasts | 800 µM NaAsO2 | 16 h | Nrf2 activation. Transcriptional activation of target genes encoding HO-1, Prx I, and A170. | [93] | |
| In vivo | Nrf2-WT and Nrf2-KO mice | 5 mg NaAsO2 Oral gavage/ 20 ppm NaAsO2 Drinking water | single dose/ 6 weeks | Increased basal transcript levels of GSTa1 and significantly lower GST mu 1 (Gstm1) in liver of Nrf2-KO mice compared to Nrf2-WT control. | [102] |
| mice | 10 mg/kg b.w. NaAsO2 Drinking water | 3 months | An upregulated expression of Nrf2 protein in mice lungs. Nrf2 has a pivotal role to maintain the endogenous redox balance. Induced synthesis of antioxidants SOD2 and HO-1. | [36] | |
| mice (Nrf2+/+ and Nrf2−/−) | 5 ppm NaAsO2 Drinking water | 4 months | Decrease in the bone volume in mice lacking Nrf2. Lack of Nrf2 increases As-induced ROS levels and phosphorylation of p38. | [96] | |
| rats | 5 mg/kg b.w. NaAsO2 Oral gavage | 28 days | Increased levels of ROS, 8-hydroxydeoxyguanosine (8-OHdG) and lipid peroxidation in kidney. Decreased levels of enzymatic and non-enzymatic antioxidants. Increase in apoptotic markers, DNA damage, TUNEL-positive cells, and dark staining of ICAM-1 in renal tissue with decreased PI3K/Akt/Nrf2 gene expression. | [33] | |
| rats | 100 mg/L Drinking water /25, 50, 100 mg/kg as food | 90 days | Oxidative stress in rat liver related to the PKCδ-Nrf2-ARE signaling pathway. Nrf2 was associated with upregulation of the transcriptional expression of SOD1 and GSH-Px1 in each arsenic poisoning group. | [97] |
| Type of Study | Cell Culture/ Species | Treatment Doses | Duration | Effects on Nrf2 Signaling | Ref. |
|---|---|---|---|---|---|
| In vitro | rat astrocytes | 5 μM MeHg | 6h | Cytotoxicity by promoting the Nrf2/ARE signaling pathway. | [20] |
| In vivo | yellow croaker Pseudosciaena crocea | 0,32 and 64 μg/L HgCl2 | 96h | A coordinated transcriptional regulation of antioxidant genes, by Nrf2 in liver. A negative relationship between the mRNA levels of Nrf2 and Keap1 indicated that Keap1 may play an important role in switching off the Nrf2 response. | [107] |
| homozygous (–/–) Nrf2-deficient mice (C57BL/6J) and wild-type (+/+) mice | 1 mg/kg MeHg Oral gavage | 22 days | MeHg in Nrf2-deficient mice—induction of hind-limb flaccidity. The body weight decrease of Nrf2-deficient mice. | [106] | |
| rats | 80 mg/L HgCl2 Drinking water | 56 days | Decreased Nrf2 accumulation in the nucleus in the cardiac tissue. Decreased GSH level and GSH/GSSG ratio, increased MDA concentration in the heart. | [10] | |
| rats | 0.6, 1.2, and 2.4 mg/kg HgCl2 i.p. | 3 days | Nrf 2 activation in liver. Upregulation HO-1, and γ-GCS heavy subunit expression. | [35] | |
| rats | 80 mg/L HgCl2 Drinking water | 2 weeks | Increased hepatocyte death attributed to insufficient ROS removal because of a failure in Nrf2 activation. | [104] |
| Type of Study | Cell Culture/ Species | Treatment Doses and Duration | Duration | Effects on Nrf2 Signaling | Ref. |
|---|---|---|---|---|---|
| In vitro | human monocytic cells | 10–30 mM Ni (II) | 6–72 h | Increased whole-cell Nrf2 levels and nuclear translocation of Nrf2. Affected cytokine secretion through Nrf2 pathway modulation. | [110] |
| RKO (ATCC CRL-2577), human colon cancer cells | 20 μM Ni(CH3CO2)2·x H2O | 12 or 24 h | Nrf2 gene silencing exacerbated Ni-induced oxidative stress and DNA damage. | [108] | |
| In vivo | mice | 20 mg/kg/b.w. NiSO4(H2O)6 i.p. | 20 days | DNA methylation and liver inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-κB pathways. | [3] |
| Type of Study | Cell Culture/ Species | Treatment Doses | Duration | Effects on Nrf2 Signaling | Ref. |
|---|---|---|---|---|---|
| in vitro | mouse hepa1c1c7 cells | 2, 5, 10, 50, and 100 M Cr(VI) | 5 h | Elevated ROS production and apoptosis. Protection by Nrf2 correlates with the induction of cytoprotective genes HO-1 and NQO1. Inhibition of ubiquitination of Nrf2 and accumulation of Nrf2 into the nucleus. Nuclear translocation and deubiquitination of Keap1. Transcriptional signaling loop: activation of Nrf2 by Cr, transcription of ARE-driven genes, and reduction of ROS production. | [2] |
| in vivo | zebrafish | 38.16 μg/mL K2CrO4 | 1, 7, 15, 30, or 60 days | Increased Nrf2 in liver both at transcriptional and translational level. Nrf2 translocation into the nucleus. Oxidative stress resulting in lipid peroxidation and extensive changes in tissue ultrastructure. | [116] |
| grass carp Ctenopharyngodon idellus | 5.3 and 10.63 mg/L | 15, 30 or 45 days | Alteration in the gene expression of Nrf2 and Mt2 in gills. Development of oxidative stress. | [114] | |
| rats | 4 mg/kg b.w. K2Cr2O7 i.p. | 35 days | Significantly decrease in Sirt1, Pgc-1α, Nrf2, HO-1, and NQO1 in rat lungs. | [8] | |
| rats | 17 mg/kg b.w. K2Cr2O7 i.p. | single dose | Nrf2 signaling—important mechanism in controlling liver cells susceptibility to ROS-induced cytotoxicty. Nrf2 increase activates antioxidant enzymes. | [115] | |
| rats | 4 mg/kg K2Cr2O7 i.p. | single dose | Decreased expression of P-AMPK/AMPK and Nrf2. Oxidative stress, apoptosis, and the release of inflammatory mediators in the rat heart. | [29] | |
| rats | 4 mg/kg b.w. K2Cr2O7 i.p. | 35 days | Nrf2 pathway—critical protective role against oxidative stress in heart. | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buha, A.; Baralić, K.; Djukic-Cosic, D.; Bulat, Z.; Tinkov, A.; Panieri, E.; Saso, L. The Role of Toxic Metals and Metalloids in Nrf2 Signaling. Antioxidants 2021, 10, 630. https://doi.org/10.3390/antiox10050630
Buha A, Baralić K, Djukic-Cosic D, Bulat Z, Tinkov A, Panieri E, Saso L. The Role of Toxic Metals and Metalloids in Nrf2 Signaling. Antioxidants. 2021; 10(5):630. https://doi.org/10.3390/antiox10050630
Chicago/Turabian StyleBuha, Aleksandra, Katarina Baralić, Danijela Djukic-Cosic, Zorica Bulat, Alexey Tinkov, Emiliano Panieri, and Luciano Saso. 2021. "The Role of Toxic Metals and Metalloids in Nrf2 Signaling" Antioxidants 10, no. 5: 630. https://doi.org/10.3390/antiox10050630
APA StyleBuha, A., Baralić, K., Djukic-Cosic, D., Bulat, Z., Tinkov, A., Panieri, E., & Saso, L. (2021). The Role of Toxic Metals and Metalloids in Nrf2 Signaling. Antioxidants, 10(5), 630. https://doi.org/10.3390/antiox10050630








