The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Different Protein Hydrolysates of Royal Jelly
2.3. The (E)-10-Hydroxydec-2-Enoic Acid (10-HDA) Contents in RJP and RJP Hydrolysates
2.4. Flavonoids and Phenolic Acid Contents in RJP and RJP Hydrolysates
2.5. Amino Acid Composition and Content in RJP and RJP Hydrolysates
2.6. Effect of RJP and RJP Hydrolysates on the Damage to Deoxyribose (Fenton Reaction)
2.7. Effect of RJP and RJP Hydrolysates on 2′-Deoxyguanosine (2′-dG) Oxidation (Fenton Reaction)
2.8. Effect of RJP and RJP Hydrolysates on Bleomycin-Dependent DNA Damage
2.9. Inhibition of Oxidative Damage of Biomolecules by RJP and RJP Hydrolysates
2.10. LDL Preparation and Oxidation
2.10.1. Estimation of the Thiobarbituric Acid Reactive Substance (TBARS)
2.10.2. Conjugated Diene Evolution
2.11. Statistical Analysis
3. Results
3.1. 10-HDA, Flavonoids and Phenolic Acid Contents in RJP and RJP Hydrolysates
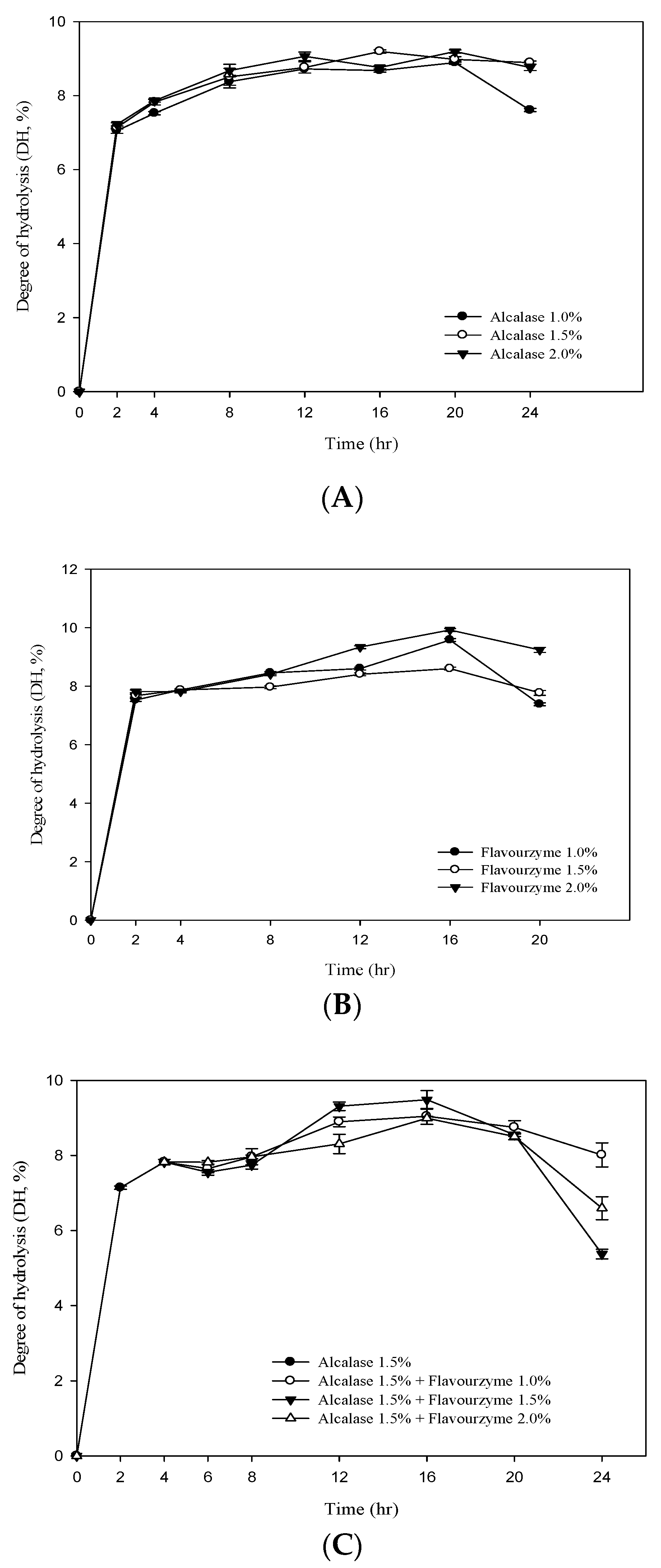
3.2. Degree of Hydrolysis (DH) of RJ Protein
3.3. The Amino Acid Compositions and Content of RJP and RJP Hydrolysates
3.4. Effect of RJP and RJP Hydrolysates on the Fenton Reaction-Induced Oxidative Damage of Deoxyribose
3.5. Effect of RJP and RJP Hydrolysateson the Oxidation of 2′-Deoxyguanosine (2′-dG) to 8-Hydroxy-2′-Deoxyguanosine (8-OH-2′-dG) Induced by the Fenton Reaction
3.6. Effect of RJP and RJP Hydrolysates on Bleomycin-Dependent DNA Damage
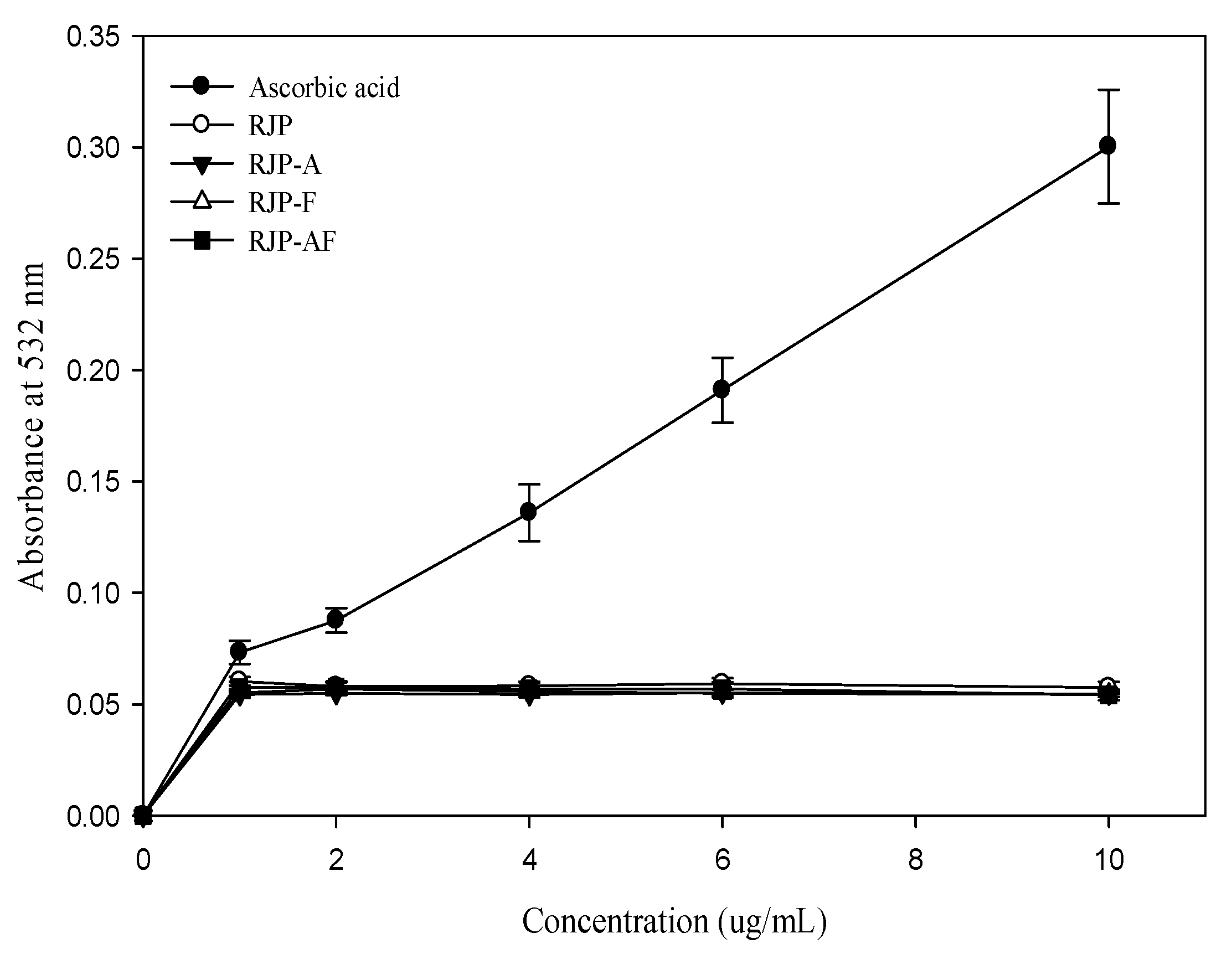
3.7. The Protective Effects and Inhibition of Oxidative Damages of Biomolecules by RJP and RJP Hydrolysates
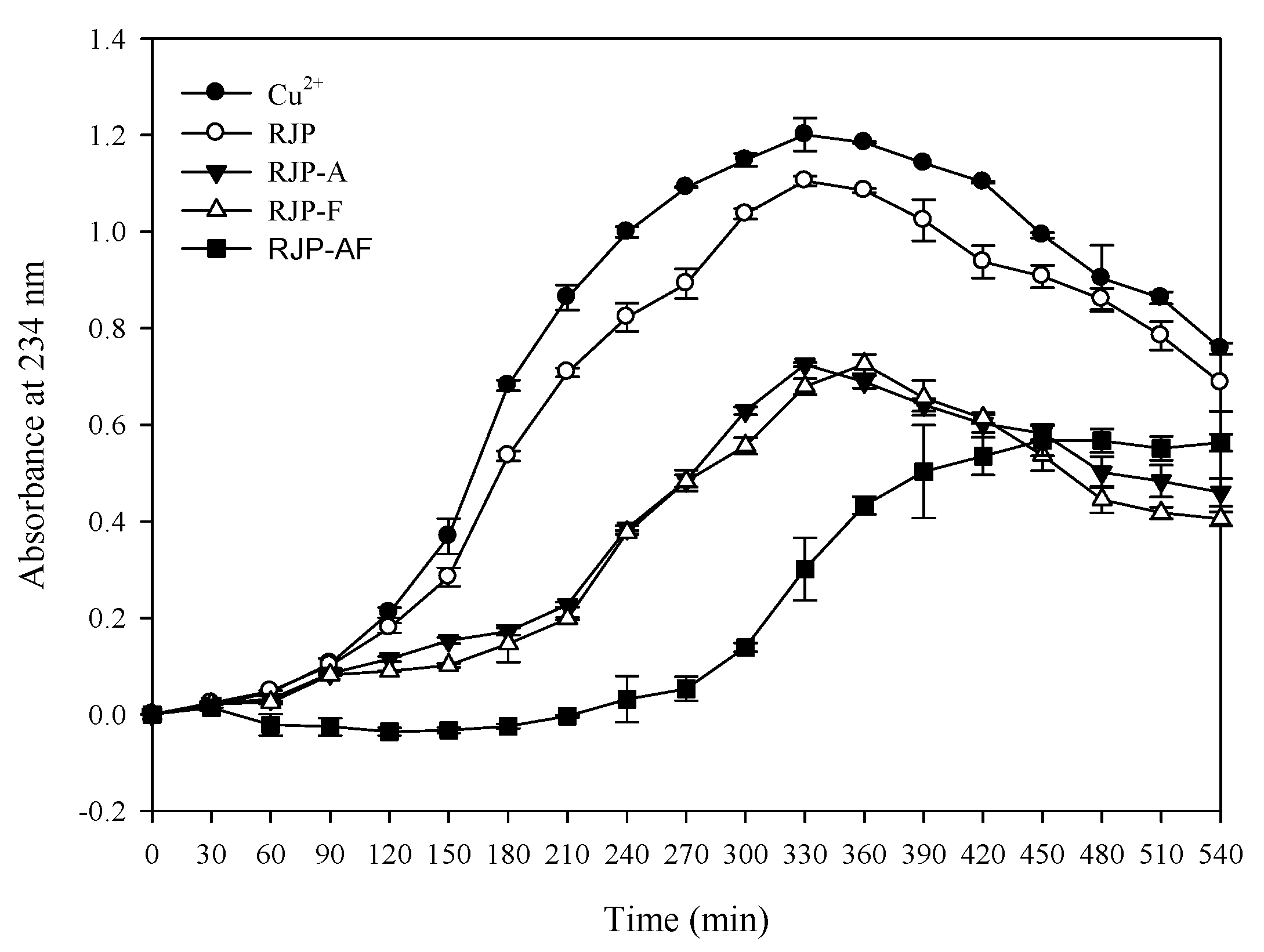
3.8. Effect of RJP and RJP Hydrolysates on the Formation of a Thiobarbituric Acid Reactive Substance (TBARS) and Conjugated Diene Formation by LDL Oxidation Induced by Cu2+
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkalin extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Berner, L.; O’Donnel, J. Functional foods and health claims legistlation: Applications to dairy foods. Int. Dairy J. 1998, 8, 355–362. [Google Scholar] [CrossRef]
- Chiang, S.H.; Wang, S.Y.; Chang, C.Y.; Chen, C.W. Bovine Colostrum Whey Protein Hydrolysates InhibitsCell DNA Damage and LDL Oxidation In Vitro. Molecules 2017, 22, 456. [Google Scholar] [CrossRef]
- Jamnik, P.; Gornanovic, D.; Reaspor, P. Antioxidative action of royal jelly in the yeast cell. Exp. Gerontol. 2007, 42, 594–600. [Google Scholar] [CrossRef]
- Chauvin, R. Traitè de biologie de l’Abeille. Science 1968, 161, 1123–1124. [Google Scholar]
- Fujita, T.; Kozuka-Hata, H.; Ao-Kondo, H.; Kunieda, T.; Oyama, M.; Kubo, T. Proteomic analysis of the Royal Jelly and characterization of the functions of its derivation glands in the honeybee. J. Proteome Res. 2013, 12, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, C.W. Effects of royal jelly extracts on growth inhibition, differentiation human leukemic U937 cells and its immunomodulatory activity. Biocell 2019, 43, 29–41. [Google Scholar]
- Simuth, J. Some properties of the main protein of honeybee (Apis mellifera) Royal Jelly. Apidologie 2001, 32, 69–80. [Google Scholar] [CrossRef]
- Scarselli, R.; Donadio, E.; Giuffrida, M.G.; Fortunato, D.; Conti, A.; Balestreri, E.; Felicioli, R.; Pinzauti, M.; Sabatini, A.G.; Felicioli, A. Toward Royal Jelly proteome. Proteomics 2005, 5, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 2010, 19, 73–79. [Google Scholar] [CrossRef]
- Oka, H.; Emori, Y.; Kobayashi, N.; Hayashi, Y.; Nomoto, K. Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and improvement of Th1/Th2 cell response. Int. Immunopharmacol. 2001, 1, 521–523. [Google Scholar] [CrossRef]
- Okamoto, I. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Nakajima, Y.; Tsuruma, K.; Shimazawa, M.; Mishima, S.; Hara, H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement. Altern. Med. 2009, 9, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kanbur, M.; Eraslan, G.; Beyaz, L. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009, 61, 123–132. [Google Scholar] [CrossRef]
- Shen, L.; Ding, M.; Zhang, L.; Jin, F.; Zhang, W.; Li, D. Expression of Accroyalisin gene from royal jelly of Chinese honeybee in Escherichia coil and its antibacterial activity. J. Agric. Food Chem. 2010, 58, 2266–2273. [Google Scholar] [CrossRef]
- Miyata, Y.; Sakai, H. Anti-Cancer and Protective Effects of Royal Jelly for Therapy-Induced Toxicities in Malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jaio, C.; Wang, T.; Yu, Z. Study on DNA damage induced by the reactive oxygen species generatedin situ based on the multi-walled carbon nanotubes and hemoglobin. J. Electroanal. Chem. 2016, 767, 182–187. [Google Scholar] [CrossRef]
- Chen, C.W.; Chiang, S.H.; Chang, C.Y. The inhibition effect of cell DNA oxidative damage and LDL oxidationby bovine colostrums. Molecules 2016, 21, 1378. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Kaur, H.; Halliwell, B. DNA damage and cancer: Measurement and mechanism. Cancer Lett. 1995, 93, 113–120. [Google Scholar] [CrossRef]
- Oliveira, S.C.B.; Oliveira-Brett, A.M. In situ DNA oxidative damage by electrochemically generated hydroxylfree radicals on a boron-doped diamond electrode. Langmuir 2012, 28, 4896–4901. [Google Scholar] [CrossRef]
- Horsley, E.T.M.; Burkitt, M.J.; Jones, C.M.; Patterson, R.A.; Harris, L.K.; Moss, N.J.; del Rio, J.D.; Leake, D.S. Mechanism of the antioxidant to pro-oxidant switch in the behavior of dehydroascorbate during LDL oxidation by copper(II) ions. Arch. Biochem. Biophys. 2007, 465, 303–314. [Google Scholar] [CrossRef]
- Huxley, P.R.; Neil, H.A. The relation between dietary flavonols and coronary heart disease mortality: A meta-analysis of prospective cohort studied. Eur. J. Clin. Nutr. 2009, 57, 904–908. [Google Scholar] [CrossRef]
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density liporptein oxidation viatheir accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23. [Google Scholar] [CrossRef]
- Schildermann, P.A.E.L.; Ten Hoor, F.; Kleinjas, J.C.S. Induction of oxidative DNA damage and earlylesions in rat gastro-intestinal epithelium in relation to prostaglandin H synthase-mediated metabolism of butylated hydroxyanisole. Food Chem. Toxicol. 1995, 33, 99–109. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 20th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016. [Google Scholar]
- Liao, D.Y.; Chai, Y.C.; Wang, S.H.; Chen, C.W.; Tsai, M.S. Antioxidant activities and contents of flavonoids and phenolic acid of Talinum triangulare extracts and their immunomodulatory effects. J. Food Drug Anal. 2015, 23, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Absin, J.; Becana, M. Complex of iron with phenolic compounds from soybean nodules and other lugume tissues: Pooxidant and antioxidant properyies. Free Radic. Bio. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Murcia, A.; Butler, J.; Halliwell, B. Evaluation of antioxidant and prooxidant actions of gallicacid and its derivatives. J. Agric. Food Chem. 1993, 41, 1880–1885. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 256–275. [Google Scholar] [CrossRef]
- Liu, L.; Wen, W.; Zhang, R.; Wei, Z.; Deng, Y.; Xiao, J.; Zhang, M. Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 2017, 214, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Song, I.B.; Han, H.J.; Lee, N.Y.; Cha, J.Y.; Son, Y.K.; Kwon, J. Antioxidant activity of royal jelly hydrolysatess obtained by enxymeatic treatment. Korean J. Food Sci. Anim. Resour. 2018, 38, 135–142. [Google Scholar]
- Fitzgerald, R.J.; O’cuinn, G. Enzymatic debitteringof food protein hydrolysatess. Biotechnol. Adv. 2006, 24, 234–237. [Google Scholar] [CrossRef]
- Chen, C.W.; Chiang, S.H.; Wang, S.Y.; Lin, Y.T.; Chang, C.Y. Inhibitory Effects of Bovine Colostrum Protein Hydrolysatesson Human Leukemic U937 Cell Growth. J. Food Biochem. 2013, 37, 8–17. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chang, C.Y.; Chen, C.W. Effects of vinegare-egg on growth inhibition, differentiation human leukemic U937 cells and itsimmunomodulatory activity. J. Food Drug Anal. 2018, 26, 731–740. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Weaver, N.; Law, J.H. Heterogeneity of fatty acids from royal jelly. Nature 1960, 188, 938–939. [Google Scholar] [CrossRef]
- Gould, R.L.; Pazdro, R. Impact of supplementary amino acids, micronutrients, and overall diet on glutathione homeostasis. Nutrients 2019, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Antinelli, J.F.; Zeggane, S.; Davico, R.; Rognone, C.; Faucon, J.P.; Lizzani, L. Evaluation of (E)-10-hydroxydec-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past theoxidation-damage base 8-oxo-dG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, M.K.; Ames, B. Assay for 8-hydroxy-2′-deoxyguanosine: A biomarker of in vivo oxidative DNAdamage. Free Radic. Biol. Med. 1991, 10, 211–216. [Google Scholar] [CrossRef]
- Huet, J.; Laval, F. Potentiation of cell killing by inhibitors of poly(adenosine diphosphate-ribose) synthesis inbleomycin-treated Chineses hamster ovary cells. Cancer Res. 1985, 45, 987–991. [Google Scholar]
- Yen, G.C.; Chen, H.Y.; Peng, H.H. Antioxidant and pro-oxidant effects of various tea extracts. J. Agric. Food Chem. 1997, 45, 30–34. [Google Scholar] [CrossRef]
- Bíliková, K.; Mirgorodskaya, E.; Bukovská, G.; Gobom, J.; Lehrach, H.; Šimúth, J. Towards functional proteomics of minority component of honeybee royal jelly: The effect of post-translational modifications on the antimicrobial activity of apalbumin. Proteomics 2009, 9, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Vezeteu, T.V.; Bobiş, O.; Moritz, R.F.A.; Buttstedt, A. Food to some, poison to others-honeybee royal jelly and its growth inhibiting effect on European Foulbrood bacteria. MicrobiologyOpen 2017, 6, e00397. [Google Scholar] [CrossRef] [PubMed]

| Sample Species | 10-HDA Contents (%) | Flavonoids (mg/100 mg) | Phenolic Acids (mg/100 mg) | |||||
|---|---|---|---|---|---|---|---|---|
| Quercetin | Naringin | Hesperetin | Galangin | Chlorogenic Acid | Caffeic Acid | Ferulic Acid | ||
| RJP * | 2.32 ± 0.03 c | 16.13 ± 0.06 b | 0.47 ± 0.00 c | 0.85 ± 0.01 c | 0.51 ± 0.05 b | 37.61 ± 2.16 a | 5.14 ± 0.21 a | 68.42 ± 0.25 b |
| RJP-A | 2.74 ± 0.01 b | 15.96 ± 0.03 b | 0.73 ± 0.02 ab | 1.03 ± 0.01 a | 0.56 ± 0.02 a | 40.33 ± 1.89 a | 4.76 ± 0.29 a | 72.54 ± 0.14 a |
| RJP-F | 2.68 ± 0.02 b | 16.25 ± 0.06 b | 0.66 ± 0.01 b | 0.87 ± 0.00 c | 0.54 ± 0.01 ab | 38.26 ± 3.15 a | 4.89 ± 0.15 a | 74.31 ± 0.22 a |
| RJP-AF | 2.95 ± 0.01 a | 18.44 ± 0.05 a | 0.76 ± 0.01 a | 0.92 ± 0.02 b | 0.57 ± 0.02 a | 39.68 ± 1.43 a | 5.06 ± 0.02 a | 73.22 ± 0.17 a |
| Amino Acid (nmol/mL) | RJP | RJP-A | RJP-F | RJP-AF |
|---|---|---|---|---|
| Aspartic acid | 7.84 ± 025 d* | 28.47 ± 1.04 b | 19.59 ± 0.34 c | 36.88 ± 1.25 a |
| Threonine | 3.41 ± 0.03 d | 40.52 ± 2.21 b | 36.87 ± 0.25 c | 46.92 ± 0.68 a |
| Serine | 29.33 ± 0.16 c | 196.57 ± 12.41 b | 212.67 ± 7.47 a | 224.73 ± 3.25 a |
| Glutamic acid | 4.02 ± 0.22 d | 80.43 ± 2.88 b | 68.59 ± 3.41 c | 109.67 ± 3.84 a |
| Glycine | 40.21 ± 0.31 c | 358.49 ± 14.32 b | 408.72 ± 22.61 a | 411.53 ± 18.41 a |
| Alanine | 35.86 ± 0.37 c | 573.16 ± 27.47 b | 596.42 ± 15.27 ab | 643.76 ± 32.77 a |
| Cysteine | 15.92 ± 0.04 d | 62.87 ± 3.52 a | 48.63 ± 0.54 c | 65.58 ± 1.41 c |
| Valine | 15.88 ± 0.19 c | 45.16 ± 2.29 ab | 42.18 ± 1.36 b | 48.27 ± 0.57 a |
| Methionine | 26.74 ± 0.43 c | 90.73 ± 11.84 b | 116.84 ± 3.57 ab | 113.88 ± 4.05 a |
| Isoleucine | 49.53 ± 1.24 c | 124.22 ± 12.06 a | 103.49 ± 2.95 b | 128.49 ± 9.24 b |
| Leucine | 22.84 ± 0.36 d | 96.51 ± 2.71 a | 78.42 ± 0.67 c | 102.48 ± 2.57 a |
| Tyrosine | 12.51 ± 0.06 c | 65.33 ± 0.95 b | 80.54 ± 0.75 a | 81.26 ± 1.84 a |
| Phenylalanine | 7.13 ± 0.15 c | 89.29 ± 2.31 a | 73.18 ± 3.16 b | 93.67 ± 3.16 a |
| Lysine | 55.97 ± 1.52 c | 465.82 ± 31.01 a | 387.46 ± 22.14 b | 487.93 ± 24.19 a |
| Histidine | 16.47 ± 0.01 c | 78.69 ± 3.92 b | 90.53 ± 4.28 a | 94.73 ± 2.53 a |
| Arginine | 24.38 ± 0.31 c | 84.93 ± 3.08 b | 103.77 ± 3.86 a | 105.22 ± 4.17 a |
| Proine | 9.55 ± 0.24 c | 92.51 ± 2.72 a | 90.19 ± 1.27 b | 95.93 ± 3.48 a |
| EAA ** | 203.32 ± 4.51 c | 1115.87 ± 18.43 a | 1032.74 ± 27.41 b | 1129.59± 15.76 a |
| Addition to RM * | 8-OH-2′-dG (μg/mL) | |||
|---|---|---|---|---|
| RJP | RJP-A | RJP-F | RJP-AF | |
| Blank | 0.224 ± 0.02 b | 0.224 ± 0.02 b | 0.224 ± 0.02 b | 0.224 ± 0.02 b |
| 15 mM ascorbic acid | 3.410 ± 0.18 a | 3.410 ± 0.18 a | 3.410 ± 0.18 a | 3.410 ± 0.18 a |
| 0.125 mg/mL | 0.103 ± 0.002 c | 0 ± 0.00 c | 0.009 ± 0.00 c | 0 ± 0.00 c |
| 0.25 mg/mL | 0.080 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c |
| 0.5 mg/mL | 0.051 ± 0.01 c | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c |
| 1 mg/mL | 0.043 ± 0.01 c | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c |
| 2 mg/mL | 0.015 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c |
| 3 mg/mL | 0.005 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c |
| 4 mg/mL | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 c |
| Addition to RM * | Bleomycin-Fe3+/Asc | Protective Effect of 2′-dG | Fe2+-EDTA/H2O2/Asc | |||
|---|---|---|---|---|---|---|
| Absorbance at 532 nm | Inhibition (%) | 8-OH-2′-dG (μg/mL) | Inhibition (%) | 8-OH-2′-dG (μg/mL) | Inhibition (%) | |
| Ascorbic acid | 0.204 ± 0.01 a ** | 7.46 ± 0.16 a ** | 10.21 ± 0.32 a ** | |||
| RJP | 0.169 ± 0.02 b | 17.16 ± 0.24 c | 2.86 ± 0.04 b | 61.66 ± 0.11 c | 6.84 ± 0.03 b | 33.01 ± 0.53 c |
| RJP-A | 0.141 ± 0.02 bc | 30.88 ± 0.08 ab | 1.83 ± 0.06 c | 75.47 ± 0.25 b | 5.27 ± 0.10 b | 48.38 ± 0.74 b |
| RJP-F | 0.153 ± 0.01 b | 25.0 ± 0.15 b | 1.97 ± 0.03 c | 72.23 ± 0.13 b | 5.73 ± 0.13 b | 43.87 ± 0.23 b |
| RJP-AF | 0.128± 0.02 c | 37.25 ± 0.33 a | 1.42 ± 0.01 d | 80.97 ± 0.34 a | 4.57 ± 0.08 c | 55.24 ± 1.15 a |
| Concentration (mg/mL) | RJP | RJP-A | RJP-F | RJP-AF | RJP | RJP-A | RJP-F | RJP-AF |
|---|---|---|---|---|---|---|---|---|
| TBARS (nmol/mL) | Lag Time (min) * | |||||||
| Blank | 5.41 ± 0.03 aA ** | 5.41 ± 0.03 aA | 5.41 ± 0.03 aA | 5.41 ± 0.03 aA | 90 | 90 | 90 | 90 |
| 0.01 | 4.46 ± 0.01 bA | 4.41 ± 0.02 bB | 4.47 ± 0.02 bB | 4.52 ± 0.01 bB | 90 | 120 | 120 | 210 |
| 0.1 | 4.14 ± 0.02 bA | 4.63 ± 0.01 cB | 4.51 ± 0.01 cB | 4.74 ± 0.02 cB | 120 | 180 | 180 | 270 |
| 1.0 | 3.76 ± 0.03 cB | 4.74 ± 0.03 dB | 4.79 ± 0.02 dB | 4.83 ± 0.01 dB | 150 | 210 | 210 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, S.-H.; Yang, K.-M.; Sheu, S.-C.; Chen, C.-W. The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation. Antioxidants 2021, 10, 580. https://doi.org/10.3390/antiox10040580
Chiang S-H, Yang K-M, Sheu S-C, Chen C-W. The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation. Antioxidants. 2021; 10(4):580. https://doi.org/10.3390/antiox10040580
Chicago/Turabian StyleChiang, Shu-Hua, Kia-Min Yang, Shiann-Cherng Sheu, and Chih-Wei Chen. 2021. "The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation" Antioxidants 10, no. 4: 580. https://doi.org/10.3390/antiox10040580
APA StyleChiang, S.-H., Yang, K.-M., Sheu, S.-C., & Chen, C.-W. (2021). The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation. Antioxidants, 10(4), 580. https://doi.org/10.3390/antiox10040580






