Host Response against Virus Infection in an Insect: Bidensovirus Infection Effect on Silkworm (Bombyx mori)
Abstract
1. Introduction
2. Characterization of Bombyx mori Densovirus and Bidensovirus
3. Pathogenicity of BmDV and BmBDV
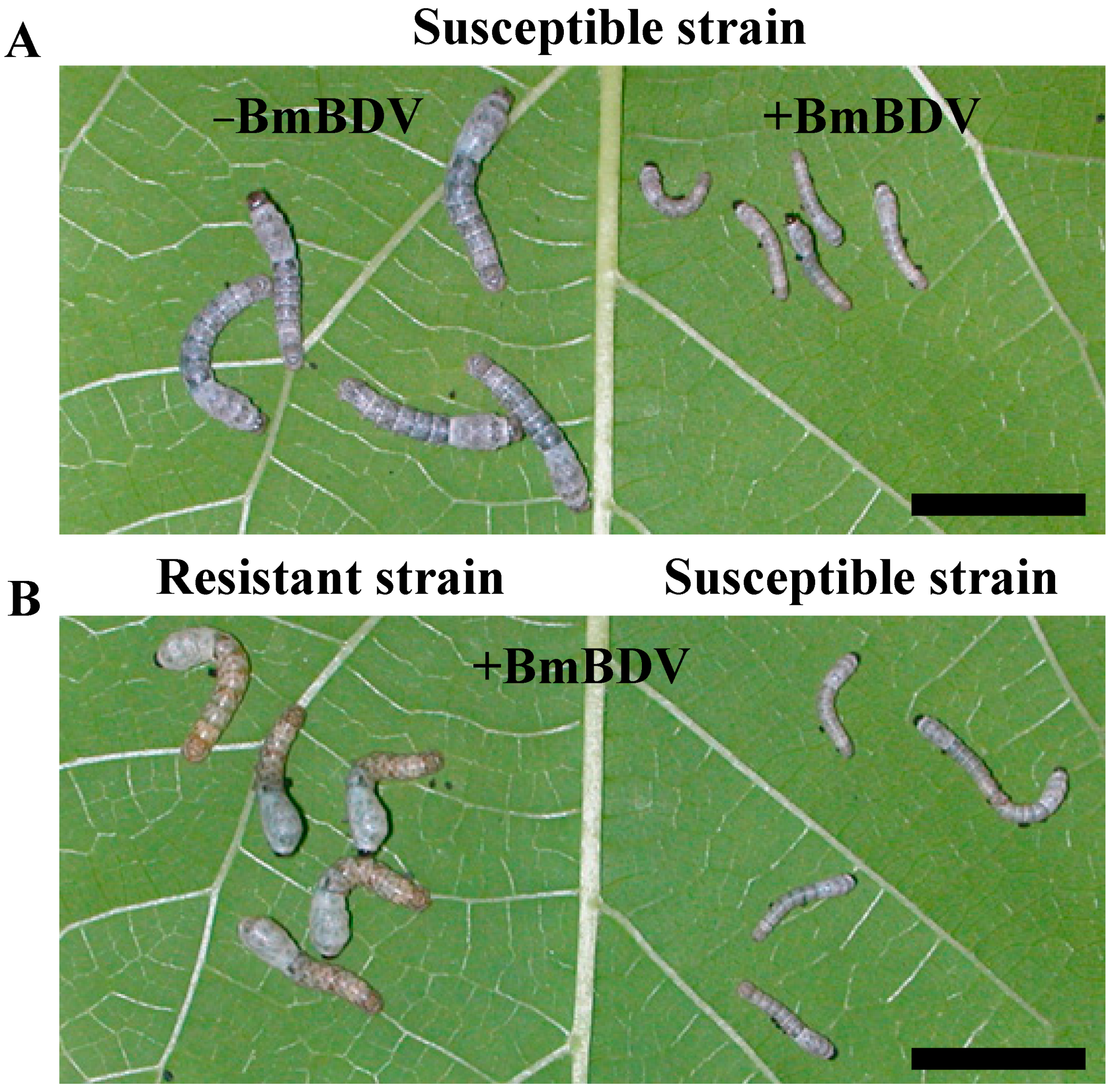
4. Silkworm Resistance to BmDV and BmBDV
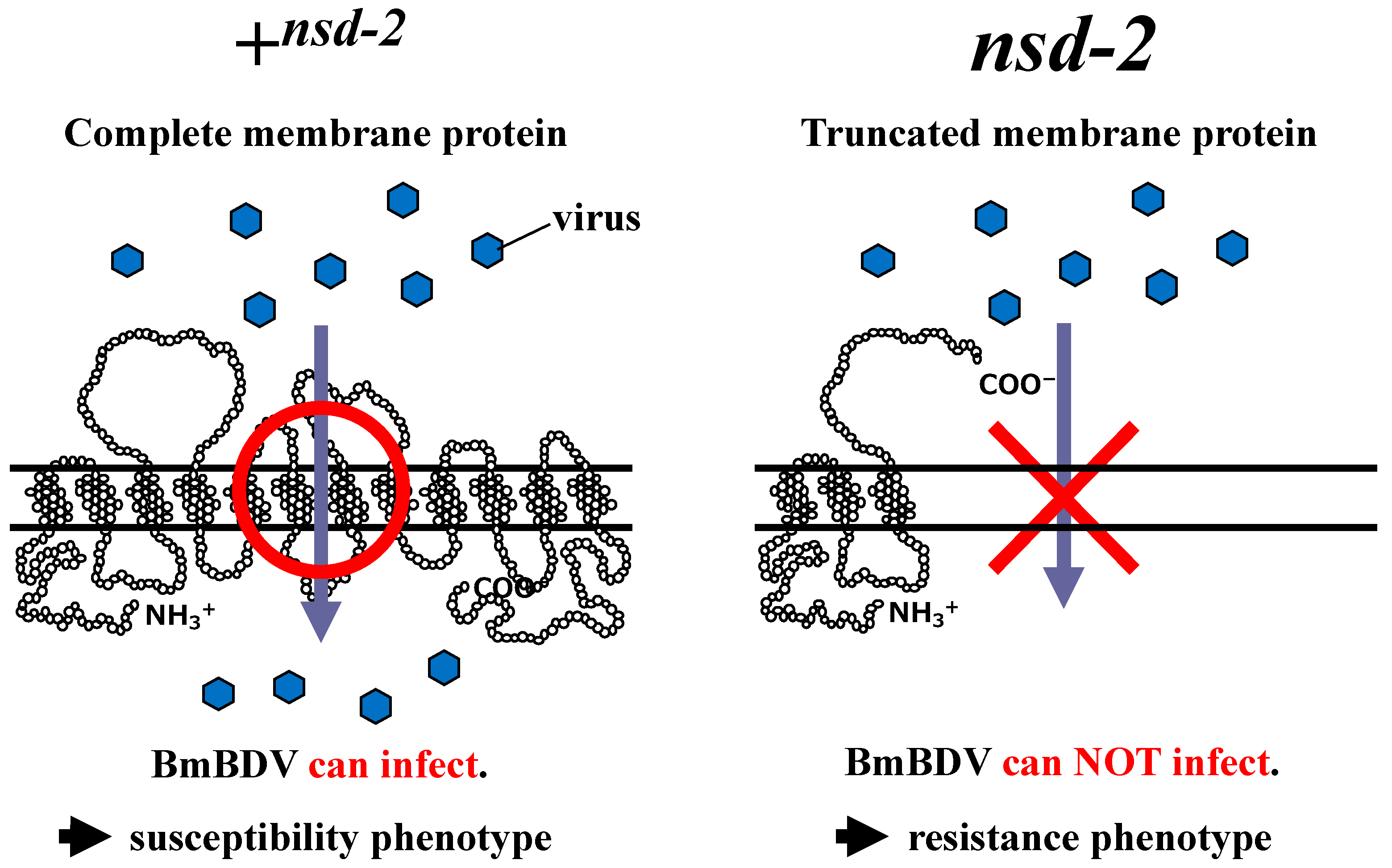
5. Identification of the Gene nsd-2, Responsible for Resistance to BmBDV Infection
6. Putative Function of NSD-2
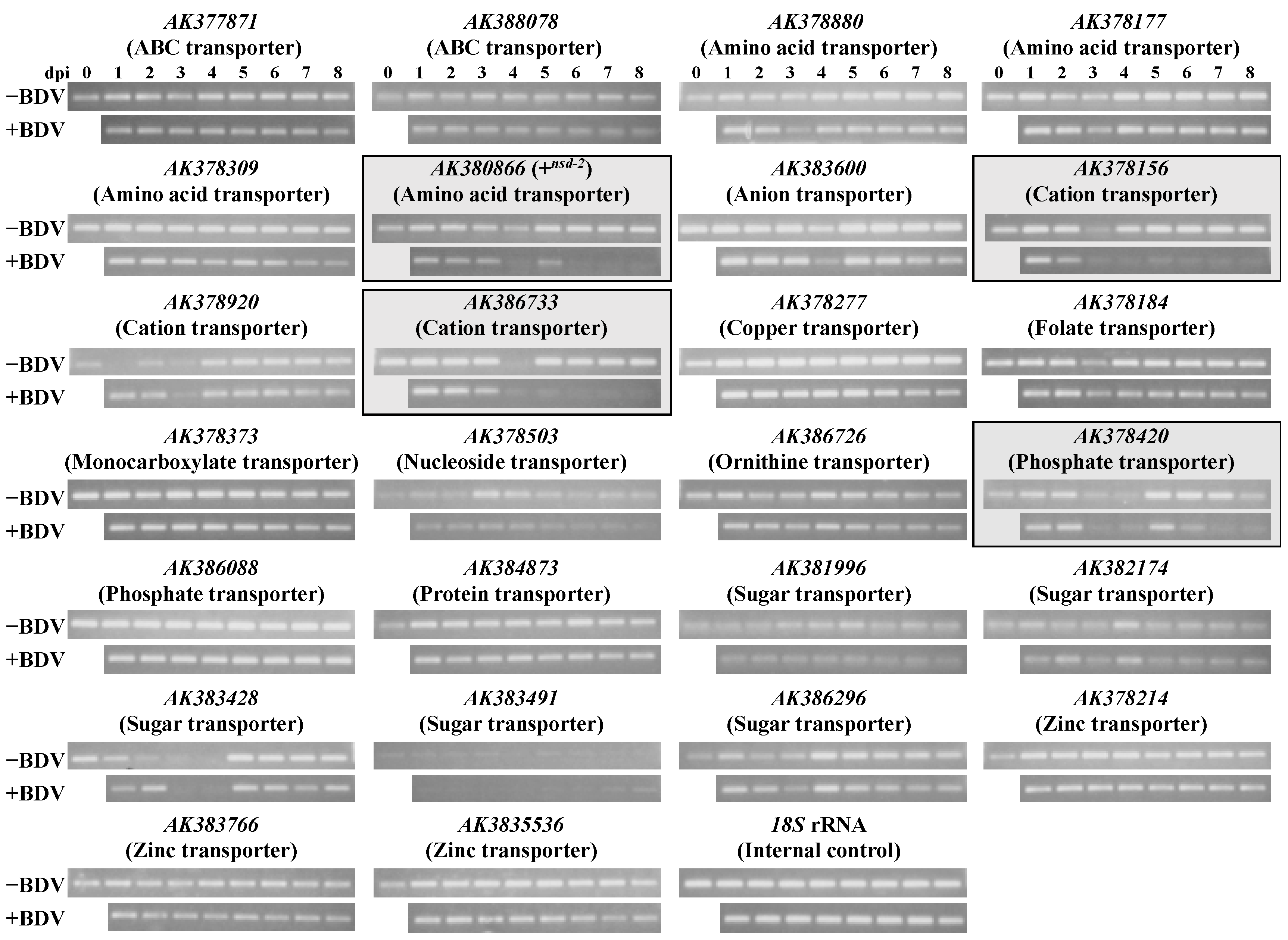
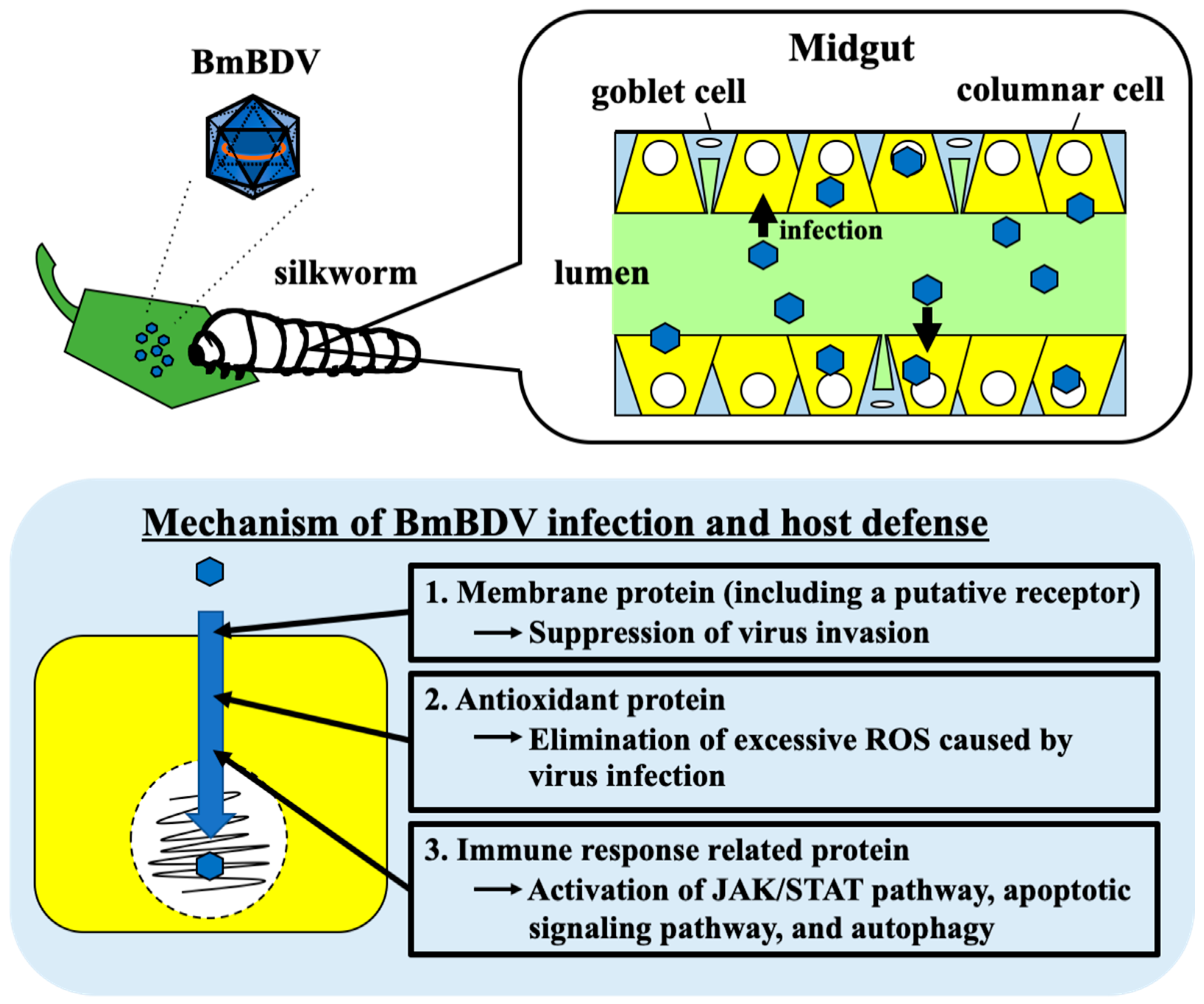
7. The NSD-2 Protein’s Location on Midgut and Relationship with BmBDV Infectivity
8. Influence of the Silkworm by BmBDV
9. Host Response by BmBDV
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71–100. [Google Scholar] [CrossRef]
- Lü, P.; Pan, Y.; Yang, Y.; Zhu, F.; Li, C.; Guo, Z.; Yao, Q.; Chen, K. Discovery of antiviral molecules and their vital functions in Bombyx mori. J. Invertebr. Pathol. 2018, 154, 12–18. [Google Scholar] [CrossRef]
- Ito, K.; Kidokoro, K.; Sezutsu, H.; Nohata, J.; Yamamoto, K.; Kobayashi, I.; Uchino, K.; Kalyebi, A.; Eguchi, R.; Hara, W.; et al. Deletion of a gene encoding an amino acid transporter in the midgut membrane causes resistance to a Bombyx parvolike virus. Proc. Natl. Acad. Sci. USA 2008, 105, 7523–7527. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Kidokoro, K.; Katsuma, S.; Sezutsu, H.; Uchino, K.; Kobayashi, I.; Tamura, T.; Yamamoto, K.; Mita, K.; Shimada, T.; et al. A single amino acid substitution in the Bombyx-specific mucin-like membrane protein causes resistance to Bombyx mori densovirus. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Watanabe, H.; Maeda, S. Genetic resistance to peroral infection with a densonucleosis virus in the silkworm, Bombyx mori. J. Sericult. Sci. Jpn. 1978, 47, 209–214, (In Japanese with English summary). [Google Scholar]
- Seki, H. Mode of inheritance of the resistance to the infection with the densonucleosis virus (Yamanashi isolate) in the silkworm, Bombyx mori. J. Sericult. Sci. Jpn. 1984, 53, 472–475, (In Japanese with English summary). [Google Scholar]
- Watanabe, H.; Kawase, S.; Shimizu, T.; Seki, H. Difference in serological characteristics of densonucleosis viruses in the silkworm, Bombyx mori. J. Sericult. Sci. Jpn. 1986, 55, 75–76. (In Japanese) [Google Scholar]
- Adams, M.J.; Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012). Arch. Virol. 2012, 157, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Pathogenicity of an infectious flacherie virus of the silkworm, Bombyx mori, obtained from sericultural farms in the suburbs of Ina city. J. Sericult. Sci. Jpn. 1975, 44, 45–48. (In Japanese) [Google Scholar]
- Seki, H.; Iwashita, Y. Histopathological features and pathogenicity of a densonucleosis virus of the silkworm, Bombyx mori, isolated from sericultural farms in Yamanashi prefecture. J. Sericult. Sci. Jpn. 1983, 52, 400–405, (In Japanese with English summary). [Google Scholar]
- Wang, Y.J.; Yao, Q.; Chen, K.P.; Wang, Y.; Lu, J.; Han, X. Characterization of the genome structure of Bombyx mori denso-virus (China isolate). Virus Genes 2007, 35, 103–108. [Google Scholar] [CrossRef]
- Gupta, T.; Ito, K.; Kadono-Okuda, K.; Murthy, G.N.; Gowri, E.V.; Ponnuvel, K.M. Characterization and genome comparison of an Indian isolate of Bidensovirus infecting the silkworm Bombyx mori. Arch. Virol. 2018, 163, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Maeda, S.; Matsui, M.; Shimizu, T. Histopathology of the midgut epithelium of the silkworm, Bombyx mori, infected with a newly-isolated virus from the flacherie-diseased larvae. J. Sericult. Sci. Jpn. 1976, 45, 29–34, (In Japanese with English summary). [Google Scholar]
- Ito, K.; Kidokoro, K.; Shimura, S.; Katsuma, S.; Kadono-Okuda, K. Detailed investigation of the sequential pathological changes in silkworm larvae infected with Bombyx densovirus type 1. J. Invertebr. Pathol. 2013, 112, 213–218. [Google Scholar] [CrossRef]
- Watanabe, H.; Kurihara, Y. Comparative histopathology of two densonucleoses in the silkworm, Bombyx mori. J. Invertebr. Pathol. 1988, 51, 287–290. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hashimoto, Y.; Mori, H.; Nagamine, T. Changes in DNA, RNA, Protein and Glycogen in the Midgut of the Silkworm, Bombyx mori (Lepidoptera: Bombydicae), during the Infection with a Densonucleosis Virus. Appl. Entomol. Zoöl. 1986, 21, 486–489. [Google Scholar] [CrossRef][Green Version]
- Ito, K.; Shimura, S.; Katsuma, S.; Tsuda, Y.; Kobayashi, J.; Tabunoki, H.; Yokoyama, T.; Shimada, T.; Kadono-Okuda, K. Gene expression and localization analysis of Bombyx mori bidensovirus and its putative receptor in B. mori midgut. J. Invertebr. Pathol. 2016, 136, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Maeda, S. Genetically determined nonsusceptibility of silkworm Bombyx mori to infection with a denso-nucleosis virus (densovirus). J. Invertebr. Pathol. 1981, 38, 370–373. [Google Scholar] [CrossRef]
- Eguchi, R.; Nagayasu, K.; Ninagi, O.; Hara, W. Genetic analysis on the dominant non-susceptibility to densonucleosis virus type 1 in the silkworm, Bombyx mori. Sanshi Konchu Biotec 2007, 76, 159–163. [Google Scholar]
- Ogoyi, D.O.; Kadono-Okuda, K.; Eguchi, R.; Furuta, Y.; Hara, W.; Nguu, E.K.; Nagayasu, K. Linkage and mapping analysis of a non-susceptibility gene to densovirus (nsd-2) in the silkworm, Bombyx mori. Insect Mol. Biol. 2003, 12, 117–124. [Google Scholar] [CrossRef]
- Qin, J.; Yi, W.Z. Genetic linkage analysis of nsd-Z, the nonsusceptibility gene of Bombyx mori to the Zhenjiang (China) strain densonucleosis virus. Sericologia 1996, 36, 241–244. [Google Scholar]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Eappen, A.; Kamba, M.; Kômoto, N.; Thomas, J.-L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.; Shayakul, C.; Trotti, D.; Sacchi, V.F.; Harvey, W.R.; Hediger, M.A. Cloning and characterization of a potassium-coupled amino acid transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 5395–5400. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.H.; Harvey, W.R.; Stevens, B.R. A Novel Electrogenic Amino Acid Transporter Is Activated by K+ or Na+, Is Alkaline pH-dependent, and Is Cl−-independent. J. Biol. Chem. 2000, 275, 24518–24526. [Google Scholar] [CrossRef]
- Nakagaki, M.; Tsuda, H.; Kajiura, Z.; Takei, R. Localization of DNV infection in the midgut of the silkworm, Bombyx mori. J. Sericult. Sci. Jpn. 1993, 62, 405–411. [Google Scholar]
- Ito, K.; Fujii, T.; Yokoyama, T.; Kadono-Okuda, K. Decrease in the expression level of the gene encoding the putative Bombyx mori bidensovirus receptor during virus infection. Arch. Virol. 2018, 163, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Minami, H.; Suetsugu, Y.; Ohyanagi, H.; Satoh, C.; Antonio, B.; Nagamura, Y.; Kadono-Okuda, K.; Kajiwara, H.; Sezutsu, H.; et al. KAIKObase: An integrated silkworm genome database and data mining tool. BMC Genom. 2009, 10, 486. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Futahashi, R.; Kanamori, H.; Kadono-Okuda, K.; Sasanuma, S.-I.; Narukawa, J.; Ajimura, M.; Jouraku, A.; Namiki, N.; Shimomura, M.; et al. Large Scale Full-Length cDNA Sequencing Reveals a Unique Genomic Landscape in a Lepidopteran Model Insect, Bombyx mori. G3 Genes Genomes Genet. 2013, 3, 1481–1492. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Lu, Y.; Gong, Y.; Zhu, M.; Chen, F.; Liang, Z.; Zhu, L.; Kuang, S.; Hu, X.; et al. Immune signaling pathways activated in response to different pathogenic micro-organisms in Bombyx mori. Mol. Immunol. 2015, 65, 391–397. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, H.; Xia, Q.; Jiang, L.; Zhao, P. Transcriptome analysis of the immune response of silkworm at the early stage of Bombyx mori bidensovirus infection. Dev. Comp. Immunol. 2020, 106, 103601. [Google Scholar] [CrossRef]
- Yang, F.; Li, S.; Li, F.; Xiang, J. A cuticle protein from the Pacific white shrimp Litopenaeus vannamei involved in WSSV infection. Dev. Comp. Immunol. 2018, 81, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Müller, F. Reactive oxygen intermediates and human immunodeficiency virus (HIV) infection. Free. Radic. Biol. Med. 1992, 13, 651–657. [Google Scholar] [CrossRef]
- Peterhans, E.; Grob, M.; Burge, T.; Zanoni, R. Virus-Induced Formation of Reactive Oxygen Intermediates in Phagocytic Cells. Free. Radic. Res. Commun. 1987, 3, 39–46. [Google Scholar] [CrossRef]
- Vierucci, A.; De Martino, M.; Graziani, E.; Rossi, M.E.; London, W.T.; Blumberg, B.S. A Mechanism for Liver Cell Injury in Viral Hepatitis: Effects of Hepatitis B Virus on Neutrophil Function in Vitro and in Children with Chronic Active Hepatitis. Pediatric Res. 1983, 17, 814–820. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yang, X.L.; Baltimore, D. Dissecting Fas signaling with an altered specificity death-domain mutant: Requirement of FADD binding for apoptosis but not Jun N-terminal kinase activation. Proc. Natl. Acad. Sci. USA 1999, 96, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Imai, Y.; Nakayama, H.; Takahashi, K.; Takio, K.; Takahashi, R. A Serine Protease, HtrA2, Is Released from the Mitochondria and Interacts with XIAP, Inducing Cell Death. Mol. Cell 2001, 8, 613–621. [Google Scholar] [CrossRef]
- Vučić, D.; Seshagiri, S.; Miller, L.K. Characterization of reaper- and FADD-induced apoptosis in a lepidopteran cell line. Mol. Cell. Biol. 1997, 17, 667–676. [Google Scholar] [CrossRef][Green Version]
- Kadono-Okuda, K.; Ito, K.; Nurthy, G.N.; Sivaprasad, V.; Ponnuvel, K.M. Molecular mechanism of densovirus resistance in silkworm, Bombyx mori. Sericologia 2014, 54, 1–10. [Google Scholar]
- Bao, Y.-Y.; Tang, X.-D.; Lv, Z.-Y.; Wang, X.-Y.; Tian, C.-H.; Xu, Y.P.; Zhang, C.-X. Gene expression profiling of resistant and susceptible Bombyx mori strains reveals nucleopolyhedrovirus-associated variations in host gene transcript levels. Genomics 2009, 94, 138–145. [Google Scholar] [CrossRef]
- Gao, K.; Deng, X.-Y.; Qian, H.-Y.; Qin, G.; Guo, X.-J. Digital Gene Expression analysis in the midgut of 4008 silkworm strain infected with cytoplasmic polyhedrosis virus. J. Invertebr. Pathol. 2014, 115, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Shimada, T.; Kobayashi, M.; Kang, W. Identification of differentially expressed host genes in Bombyx mori nucleopolyhedrovirus infected cells by using subtractive hybridization. Appl. Entomol. Zoöl. 2007, 42, 151–159. [Google Scholar] [CrossRef]
- Sagisaka, A.; Fujita, K.; Nakamura, Y.; Ishibashi, J.; Noda, H.; Imanishi, S.; Mita, K.; Yamakawa, M.; Tanaka, H. Genome-wide analysis of host gene expression in the silkworm cells infected with Bombyx mori nucleopolyhedrovirus. Virus Res. 2010, 147, 166–175. [Google Scholar] [CrossRef]
- Wu, P.; Wang, X.; Qin, G.X.; Liu, T.; Jiang, Y.F.; Li, M.W.; Guo, X.J. Microar-ray analysis of the gene expression profile in the midgut of silkworm infected with cytoplasmic polyhedrosis virus. Mol. Biol. Rep. 2011, 38, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Guo, R.; Kumar, D.; Ma, H.Y.; Liu, J.B.; Hu, X.L.; Cao, G.L.; Xue, R.Y.; Gong, C.L. Identification, gene expression and immune function of the novel Bm-STAT gene in virus-infected Bombyx mori. Gene 2016, 577, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, L.; Shi, H.; Xia, H.; Gao, L.; Lian, C.; Chen, L.; Yao, Q.; Chen, K.; Liu, X. Microarray analysis of gene expression profile in resistant and susceptible Bombyx mori strains reveals resistance-related genes to nucleopolyhedrovirus. Genomics 2013, 101, 256–262. [Google Scholar] [CrossRef]


| Virus Type | Bombyx mori Densovirus (BmDV) | Bombyx mori Bidensovirus (BmBDV) |
|---|---|---|
| Previous classification | Bombyx mori densovirus type-1 (BmDNV-1) | Bombyx mori densovirus type-2 (BmDNV-2) |
| Isolates | Ina isolate (Japan) | Yamanashi isolate (Japan), Saku isolate (Japan), China isolate (China), Zhenjiang isolate (China), Indian isolate (India) |
| Family/Genus | Parvoviridae/Iteradensovirus | Bidnaviridae/Bidensovirus |
| Virion Size | 20 nm | 24 nm |
| Genome Topology | Linear | Linear |
| Genome Type | ssDNA | Segmented ssDNA |
| Genome Size | 5.0 kb | 6 kb; 6.5 kb |
| Number of ORFs | 3 | 6 or 7 |
| Pathogenicity | acute | chronic |
| Virus Affected Tissue | Midgut columnar cell | Midgut columnar cell * |
| Resistance Gene | Nid-1 and nsd-1 | nsd-2 and nsd-z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, K.; Ponnuvel, K.M.; Kadono-Okuda, K. Host Response against Virus Infection in an Insect: Bidensovirus Infection Effect on Silkworm (Bombyx mori). Antioxidants 2021, 10, 522. https://doi.org/10.3390/antiox10040522
Ito K, Ponnuvel KM, Kadono-Okuda K. Host Response against Virus Infection in an Insect: Bidensovirus Infection Effect on Silkworm (Bombyx mori). Antioxidants. 2021; 10(4):522. https://doi.org/10.3390/antiox10040522
Chicago/Turabian StyleIto, Katsuhiko, Kangayam M. Ponnuvel, and Keiko Kadono-Okuda. 2021. "Host Response against Virus Infection in an Insect: Bidensovirus Infection Effect on Silkworm (Bombyx mori)" Antioxidants 10, no. 4: 522. https://doi.org/10.3390/antiox10040522
APA StyleIto, K., Ponnuvel, K. M., & Kadono-Okuda, K. (2021). Host Response against Virus Infection in an Insect: Bidensovirus Infection Effect on Silkworm (Bombyx mori). Antioxidants, 10(4), 522. https://doi.org/10.3390/antiox10040522






