The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings
Abstract
1. Introduction
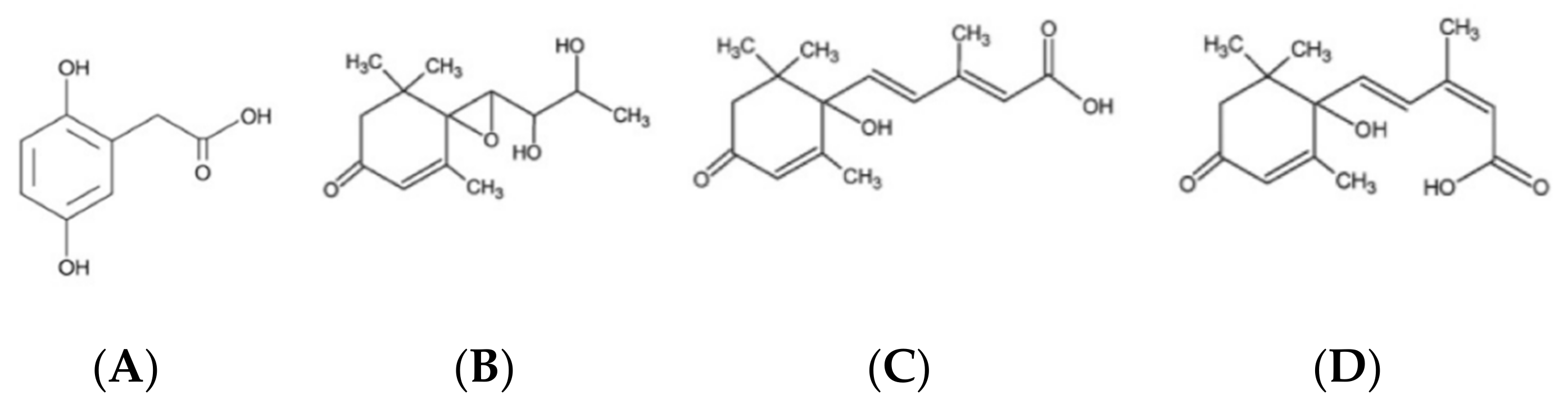
2. Profile and Features
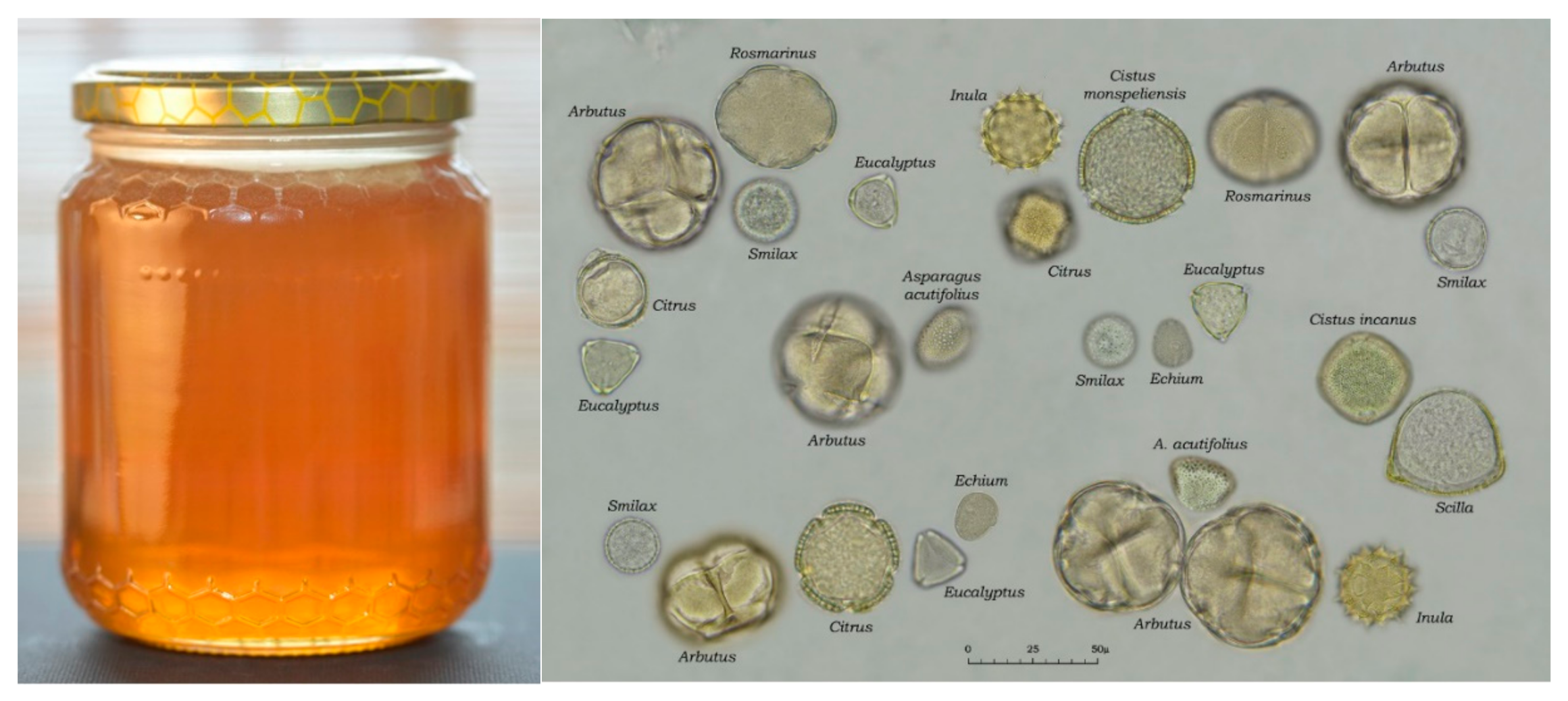
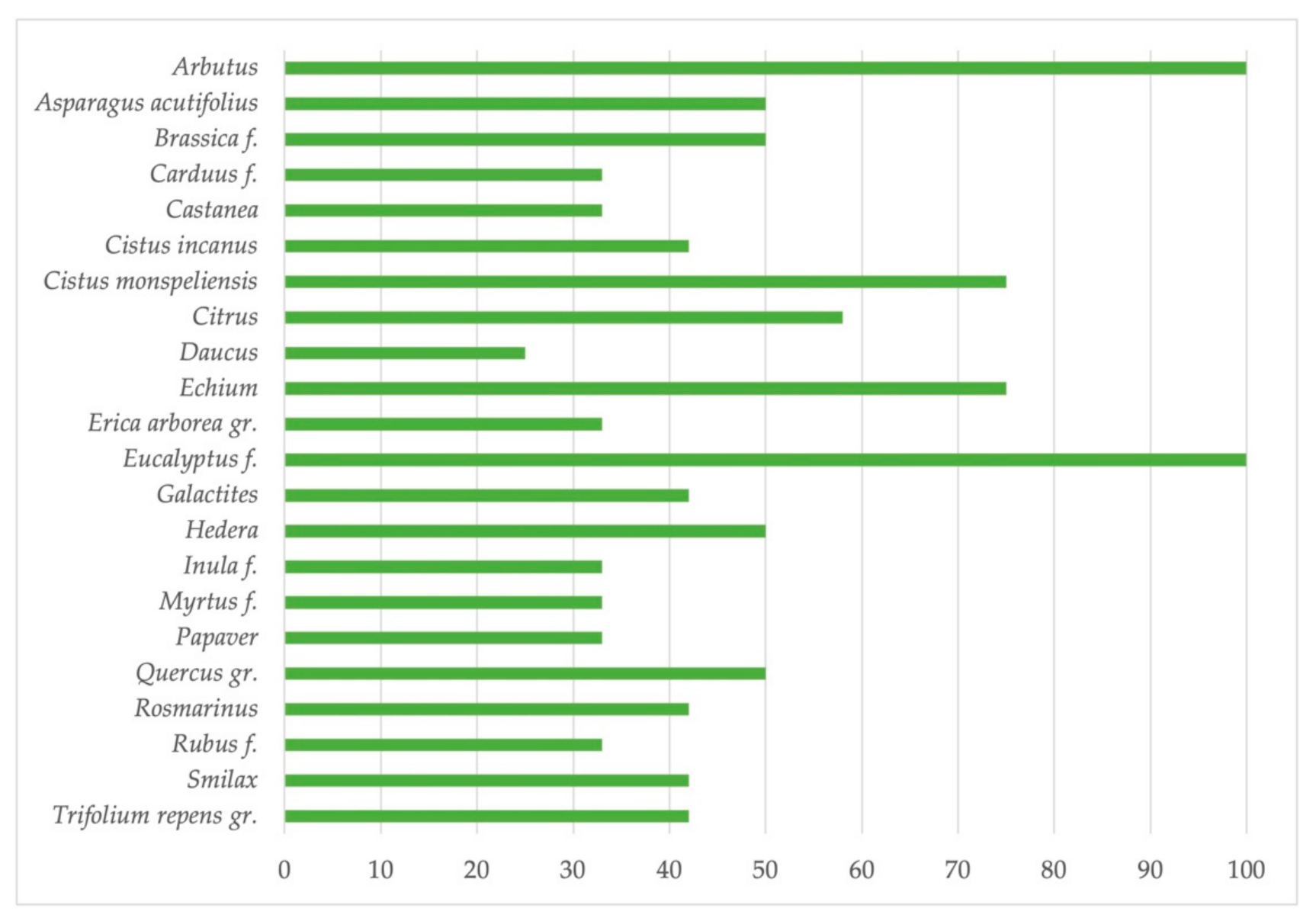
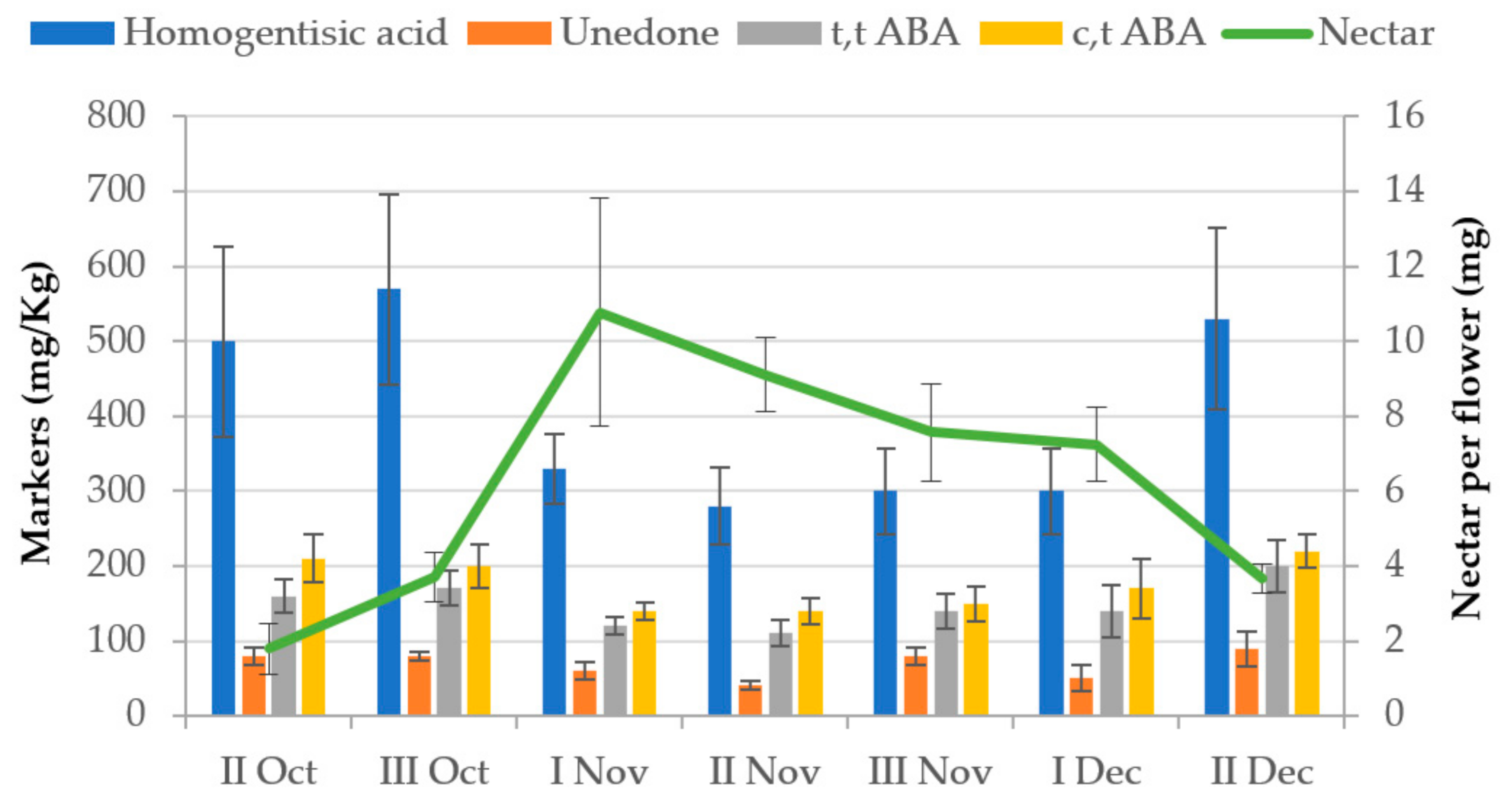
3. Floral Markers
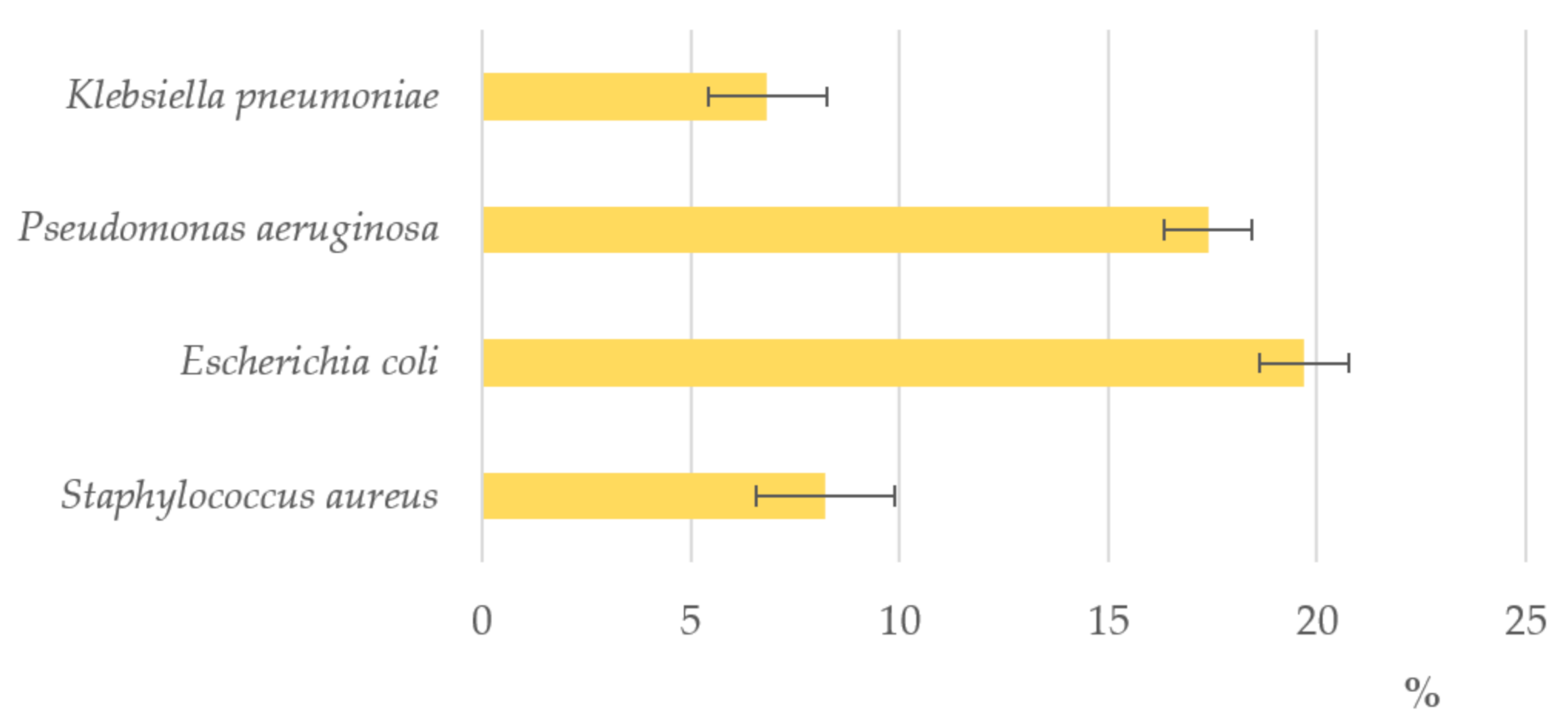
4. Antibacterial Activity
5. Antioxidant Activity and Other Health Benefits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Floris, I.; Prota, R. Notizie di storia e tradizioni dell’apicoltura sarda. Quad. Della Camera Commer. Ind. Artig. Agric. Della Prov. Sassari 1989, 26, 1–14. [Google Scholar]
- Floris, I.; Pusceddu, M.; Francesconi, A.H.D.; Satta, A. Italian Apiculture, a Journey through History and Honey Diversity Sardinian Beekeeping; Floris, I., Ed.; Accademia Nazionale Italiana di Entomologia: Firenze, Italy, 2020; pp. 244–265. [Google Scholar]
- Perroncito. Il Miele Amaro di Sardegna; Avvenire di Sardegna: Cagliari, Italy, 1889. [Google Scholar]
- Sanna, A. Su una qualità di miele della Gallura dal sapore amaro. Ann. Chim. Appl. dell’Università Sassari 1931, 397–402. [Google Scholar]
- Osés, S.M.; Nieto, S.; Rodrigo, S.; Pérez, S.; Rojo, S.; Sancho, M.T.; Fernández-Muiño, M.Á. Authentication of strawberry tree (Arbutus unedo L.) honeys from southern Europe based on compositional parameters and biological activities. Food Biosci. 2020, 38, 100768. [Google Scholar] [CrossRef]
- Floris, I.; Prota, R. Sul miele amaro di Sardegna. Apicolt. Mod. 1989, 80, 55–67. [Google Scholar]
- Floris, I.; Idini, M.G. Nota sugli aspetti inerenti la produzione del miele amaro in Sardegna. Studi Econ. E Dirit. 1992, 2, 375–382. [Google Scholar]
- Floris, I.; Prota, R.; Lentini, A. Flora di interesse apistico della Sardegna (I. Indagine sul potenziale mellifero di Arbutus unedo L. in Sardegna settentrionale). Atti Convegno Murst 40% Sassari 1991, 189–200. [Google Scholar]
- Floris, I.; Vacca, A.; Franco, M.A.; Del Caro, A.; Marras, P.M.; Reniero, F. Fenoli totali e rapport isotopico 13C/12C di mieli uniflorali della Sardegna. Apicoltura 1994, 9, 119–133. [Google Scholar]
- Floris, I.; Satta, A.; Ruiu, L. Honeys of Sardinia (Italy). J. Apic. Res. 2007, 46, 194–204. [Google Scholar] [CrossRef]
- Campus, R.L.; Madau, G.L.; Melis, A. Caratteristiche fisico-chimiche e biochimiche dei mieli della Sardegna. Quad. Della Camera Commer. Ind. Artig. Agric. Della Prov. Sassari 1986, 22, 95–109. [Google Scholar]
- Floris, I.; Farris, G.A.; Papoff, C.M.; Prota, R. Conoscenze attuali sul miele amaro della Sardegna. In Proceedings of the Atti del 1 Congresso Italiano di Scienza e Tecnologia Degli Alimenti, Parma, Italy, 18–20 October 1993. [Google Scholar]
- Papoff, C.M.; Floris, I.; Lampis, A.; Chessa, S. Caratteristiche chimico-fisiche di alcuni mieli monoflorali della Sardegna. L’ape Nostra Amica 1995, 2, 4–8. [Google Scholar]
- Floris, I.; Prota, R.; Marras, P.; Ricciardelli D’Albore, G. Caratterizzazione botanica dei mieli della Sardegna (I. Aspetti generali). Atti Convegno Murst 40% Sassari 1991, 169–188. [Google Scholar]
- Floris, I.; Prota, R.; Fadda, L. Analisi melissopalinologica quantitativa di mieli tipici sardi. Apicolt. Mod. 1996, 87, 161–167. [Google Scholar]
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C.; Ghelli, S. Homogentisic Acid: A Phenolic Acid as a Marker of Strawberry-Tree (Arbutus unedo) Honey. J. Agric. Food Chem. 1999, 47, 4064–4067. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Bifulco, E.; Jerković, I.; Caboni, P.; Cabras, P.; Floris, I. Methyl syringate: A chemical marker of asphodel (Asphodelus microcarpus Salzm. et Viv.) monofloral honey. J. Agric. Food Chem. 2009, 57, 3895–3900. [Google Scholar] [CrossRef]
- Deiana, V.; Tuberoso, C.; Satta, A.; Pinna, C.; Camarda, I.; Spano, N.; Ciulu, M.; Floris, I. Relationship between markers of botanical origin in nectar and honey of the strawberry tree (Arbutus unedo) throughout flowering periods in different years and in different geographical areas. J. Apic. Res. 2015, 54, 342–349. [Google Scholar] [CrossRef]
- Ferreres, F.; Garcı’a-Viguera, C.; Toma´s-Lorente, F.; Tomás-Barberán, F.A. Hesperetin, a marker of the floral origin of citrus honey. J. Agric. Food Chem. 1993, 61, 121–123. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; D’Arcy, B.; Singanusong, R.; Datta, N.; Caffin, N.; Raymont, K. Quantitative high-performance liquid chromatography analyses of flavonoids in Australian Eucalyptus honeys. J. Agric. Food Chem. 2004, 52, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent Advances in the Analysis of Phenolic Compounds in Unifloral Honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Bifulco, E.; Caboni, P.; Cottiglia, F.; Cabras, P.; Floris, I. Floral markers of strawberry tree (Arbutus unedo L.) honey. J. Agric. Food Chem. 2010, 58, 384–389. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 458–496. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural Occurrence of Abscisic Acid in Heather Honey and Floral Nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Kumar, P.; Sindhu, R.K.; Narayan, S.; Singh, I. Honey collected from different floras of Chandigarh Tricity: A comparative study involving physicochemical parameters and biochemical activities. J. Diet. Suppl. 2010, 7, 303–313. [Google Scholar] [CrossRef]
- Agbaje, E.O.; Ogunsanya, T.; Aiwerioba, O.I.R. Conventional use of honey as antibacterial agent. Ann. Afr. Med. 2006, 5, 79–81. [Google Scholar]
- Libonatti, C.; Valera, S.; Basualdo, M. Antibacterial activity of honey: A review of honey around the world. J. Microbiol. Antimicrob. 2014, 6, 51–56. [Google Scholar] [CrossRef]
- Molan, P.C. The antibacterial nature of honey: 1. The nature of antibacterial activity. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Molan, P.C. The antibacterial activity of honey: 2. Variation in the potency of the antibacterial activity. Bee World 1992, 73, 59–76. [Google Scholar] [CrossRef]
- White, J.W.; Stubers, M.H.; Shepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complementary Med. 2003, 9, 267–273. [Google Scholar] [CrossRef] [PubMed]
- White, J.W., Jr.; Subers, M.H. Studies on honey inhibine. 3. Effect of heat. J. Apic. Res. 1964, 3, 45–50. [Google Scholar] [CrossRef]
- White, J.W., Jr.; Subers, M.H. Studies on honey inhibine. 4. Destruction of the peroxide accumulation system by light. J. Food Sci. 1964, 29, 819–828. [Google Scholar] [CrossRef]
- Schepartz, A.I. Honey catalase: Occurance and some kinetic properties. J. Apic. Res. 1966, 5, 167–176. [Google Scholar] [CrossRef]
- Huidobro, J.F.; Sanchez, M.P.; Muniategui, S.; Sancho, M.T. Precise method for the measurement of catalase activity in honey. J. Aoac Int. 2005, 88, 800–804. [Google Scholar] [CrossRef]
- Carter, C.; Thornburg, R.W. Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci. 2004, 9, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Hillwig, M.S.; Liu, X.T.; Liu, G.Y.; Thornburg, R.W.; MacIntosh, G.C. Petunia nectar proteins have ribonuclease activity. J. Exp. Bot. 2010, 61, 2951–2965. [Google Scholar] [CrossRef]
- Hillwig, M.S.; Kanobe, C.; Thornburg, R.W.; MacIntosh, G.C. Identification of S-RNase and peroxidase in petunia nectar. J. Plant Physiol. 2011, 168, 734–738. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Alamanni, M.C.; Juliano, C.; Floris, I.; Marras, P.M. Contributo alla conoscenza dell’attività antibatterica in vitro e dello spettro pollinico del miele amaro di Sardegna. La Riv. Della Soc. Ital. Sci. Dell’Aliment. 1992, 4, 535–543. [Google Scholar]
- Stomfay-Stitz, L.; Kominos, S.D. Über bakteriostatische Wirkung des Honigs. Z. Für Lebensm. Unters. Forsch. 1960, 113, 304–309. [Google Scholar] [CrossRef]
- Lindner, K.E. Ein Beitrag zur Frage der antimikrobiellen Wirkung der Naturhonige. Zent. Bakteriol. Parasitenkd. Lnfektionkrankheiten Hyg. 1962, 115, 720–736. [Google Scholar]
- Alamanni, M.C.; Juliano, C.; Floris, I. Indagine preliminare sull’attività antibatterica in vitro di alcuni mieli reperiti in Sardegna. La Riv. Della Soc. Ital. Sci. Dell’Aliment. 1991, 19, 35. [Google Scholar]
- Ahmed Wadi, M. Antibacterial activity of different global honey samples against standard organism. Asian J. Microbiol. Biotechnol. Environ. Sci. 2019, 21, 924–930. [Google Scholar]
- Allen, K.L.; Molan, P.C.; Reid, G.M. A survey of the antibacterial activity of some New Zealand honeys. J. Pharm. Pharmacol. 1991, 43, 817–822. [Google Scholar] [CrossRef]
- De Santis, E.; Manca, G.; Floris, I.; Marras, P.M. Attività inibente del miele nei confronti di Bacillus subtilis e Bacillus stearothermophilus. Atti Della Soc. Ital. Delle Sci. Vet. 1992, 46, 767–771. [Google Scholar]
- Mullai, V.; Menon, T. Bactericidal Activity of Different Types of Honey Against Clinical and Environmental Isolates of Pseudomonas Aeruginosa. J. Altern. Complementary Med. 2007, 13, 439–441. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2016, 86, 2046–2056. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallosc, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Anbazhakan, K.; Sadasivam, K.; Praveena, R.; Salgado, G.; Cardona, W.; Glossman-Mitnik, D.; Gerli, L. Theoretical assessment of antioxidant property of polyproponoid and its derivatives. Struct. Chem. 2020, 31, 1089–1094. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dess, M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- D˙zugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Bundit, T.; Anothai, T.; Pattaramart, P.; Roongpet, T.; Chuleeporn, S. Comparison of Antioxidant Contents of Thai Honeys to Manuka Honey. Malays. J. Nutr. 2016, 22, 413–420. [Google Scholar]
- Afrin, S.; Forbes-Hernandez, T.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Pistollato, F.; Ansary, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, D.; Simal-Gandara, J.; Quiles, J.L.; et al. Strawberry tree honey as a new potential functional food. Part 1: Strawberry tree honey reduces colon cancer cell proliferation and colony formation ability, inhibits cell cycle and promotes apoptosis by regulating EGFR and MAPKs signaling pathways. J. Funct. Foods 2019, 57, 439–452. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Pistollato, F.; Zhang, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Bompadre, S.; et al. Strawberry tree honey as a new potential functional food. Part 2: Strawberry tree honey increases ROS generation by suppressing Nrf2-ARE and NF-кB signaling pathways and decreases metabolic phenotypes and metastatic activity in colon cancer cells. J. Funct. Foods 2019, 57, 477–487. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Maia, M.; Brigas, A.F. Physicochemical Parameters and Bioactive Compounds of Strawberry Tree (Arbutus unedo L.) Honey. J. Chem. 2015, 2015, 602792. [Google Scholar] [CrossRef]
- Jurič, A.; Gašić, U.; Karačonji, I.B.; Jurica, K.; Milojković-Opsenica, D. The phenolic profile of strawberry tree (Arbutus unedo L.) honey. J. Serb. Chem. Soc. 2020, 85, 1–10. [Google Scholar] [CrossRef]
- Tariba Lovaković, B.; Lazarus, M.; Brčić Karačonji, I.; Juricab, K.; ŽivkovićSemrena, T.; Lušićc, D.; Brajenović, N.; Pelaić, Z.; Pizent, A. Multi-elemental composition and antioxidant properties of strawberry tree (Arbutus unedo L.) honey from the coastal region of Croatia: Risk-benefit analysis. J. Trace Elem. Med. Biol. 2018, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on Manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.A.; Blair, S.E.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E.J. Therapeutic Manuka honey: No longer so alternative. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floris, I.; Pusceddu, M.; Satta, A. The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings. Antioxidants 2021, 10, 506. https://doi.org/10.3390/antiox10040506
Floris I, Pusceddu M, Satta A. The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings. Antioxidants. 2021; 10(4):506. https://doi.org/10.3390/antiox10040506
Chicago/Turabian StyleFloris, Ignazio, Michelina Pusceddu, and Alberto Satta. 2021. "The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings" Antioxidants 10, no. 4: 506. https://doi.org/10.3390/antiox10040506
APA StyleFloris, I., Pusceddu, M., & Satta, A. (2021). The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings. Antioxidants, 10(4), 506. https://doi.org/10.3390/antiox10040506