New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Extraction Procedure
2.4. Total Phenol Content (TPC)
2.5. Total Flavonoid Content (TFC)
2.6. High Pressure Liquid Chromatography-Diode-Array Detection-Electrospray Ionization-Quadrupole-Mass Spectroscopy (HPLC-DAD-ESI-Q-MS) Compounds Profiling
2.7. In Vitro Antioxidant Activity
2.8. Cell Cultures
2.9. Reactive Oxygen Species (ROS) Assessment
2.10. NO Production in LPS-Stimulated RAW 264.7 Cells
2.11. Cell Viability Assay
2.12. Immuno-Fluorescence Monitoring Nuclear Factor Kappa B (NF-κB) Translocation
2.13. Quantitative PCR Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Chemical Profile
3.2. Antioxidant Effects
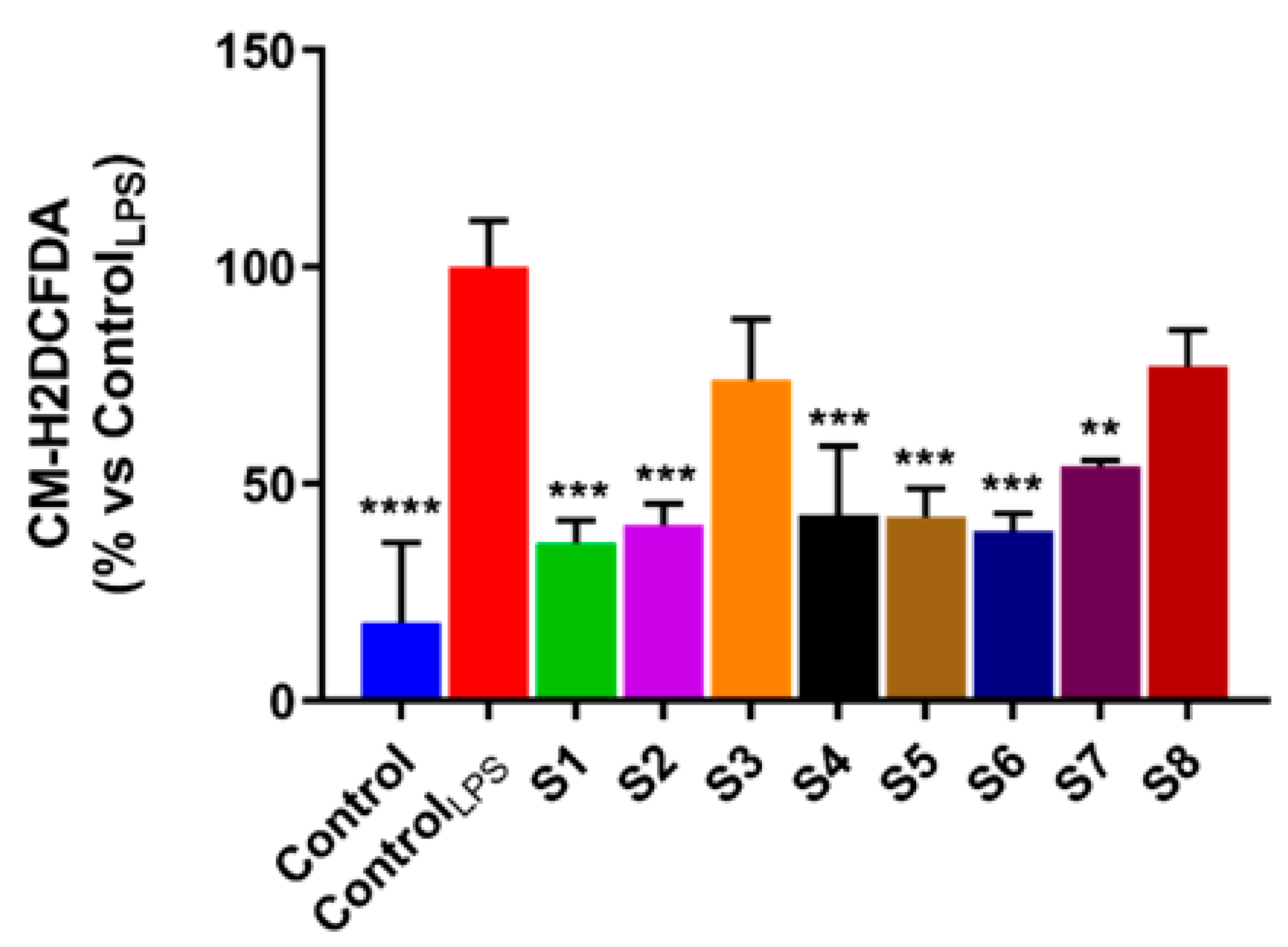
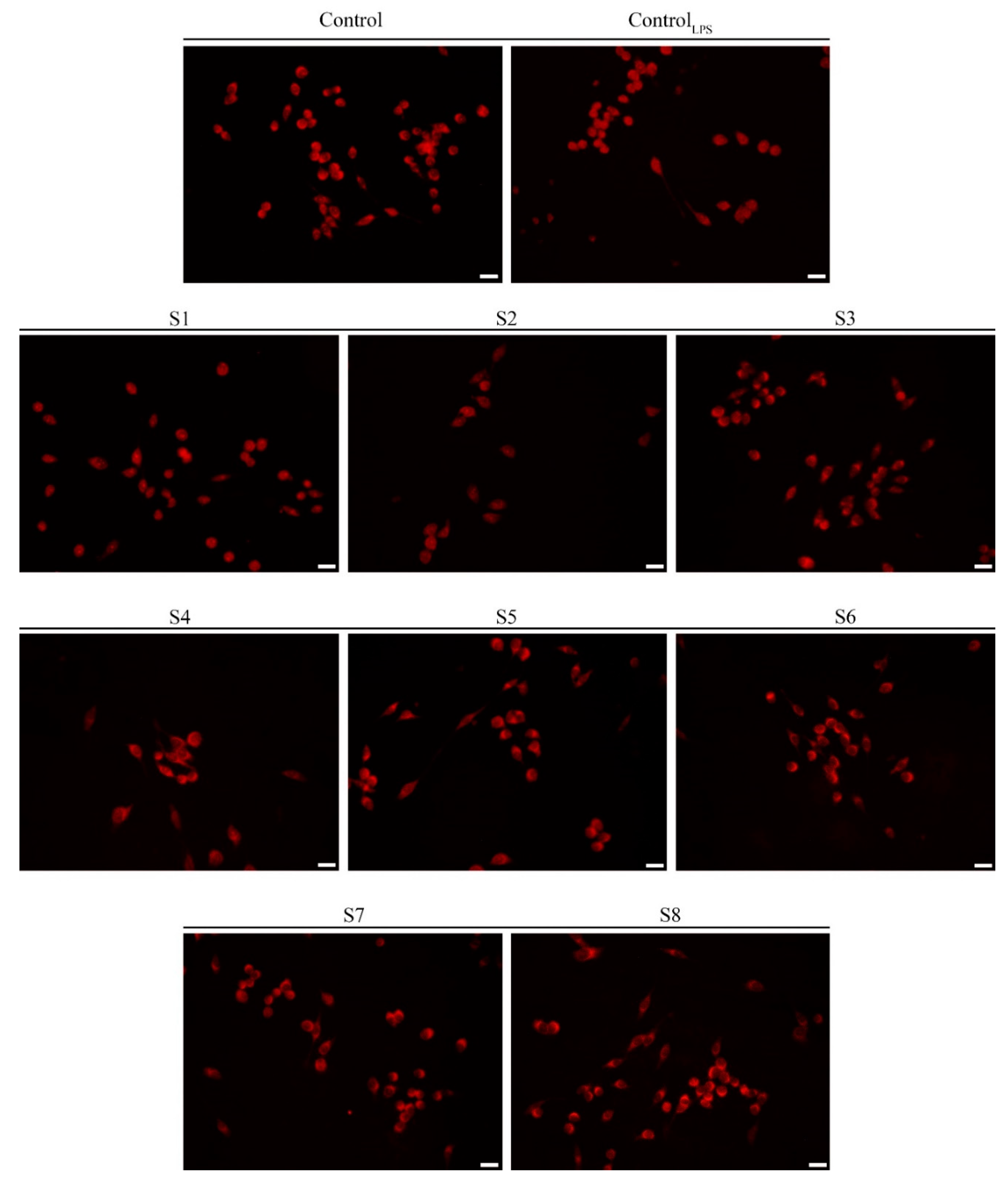
3.3. S. officinalis Extracts Reduce ROS Levels
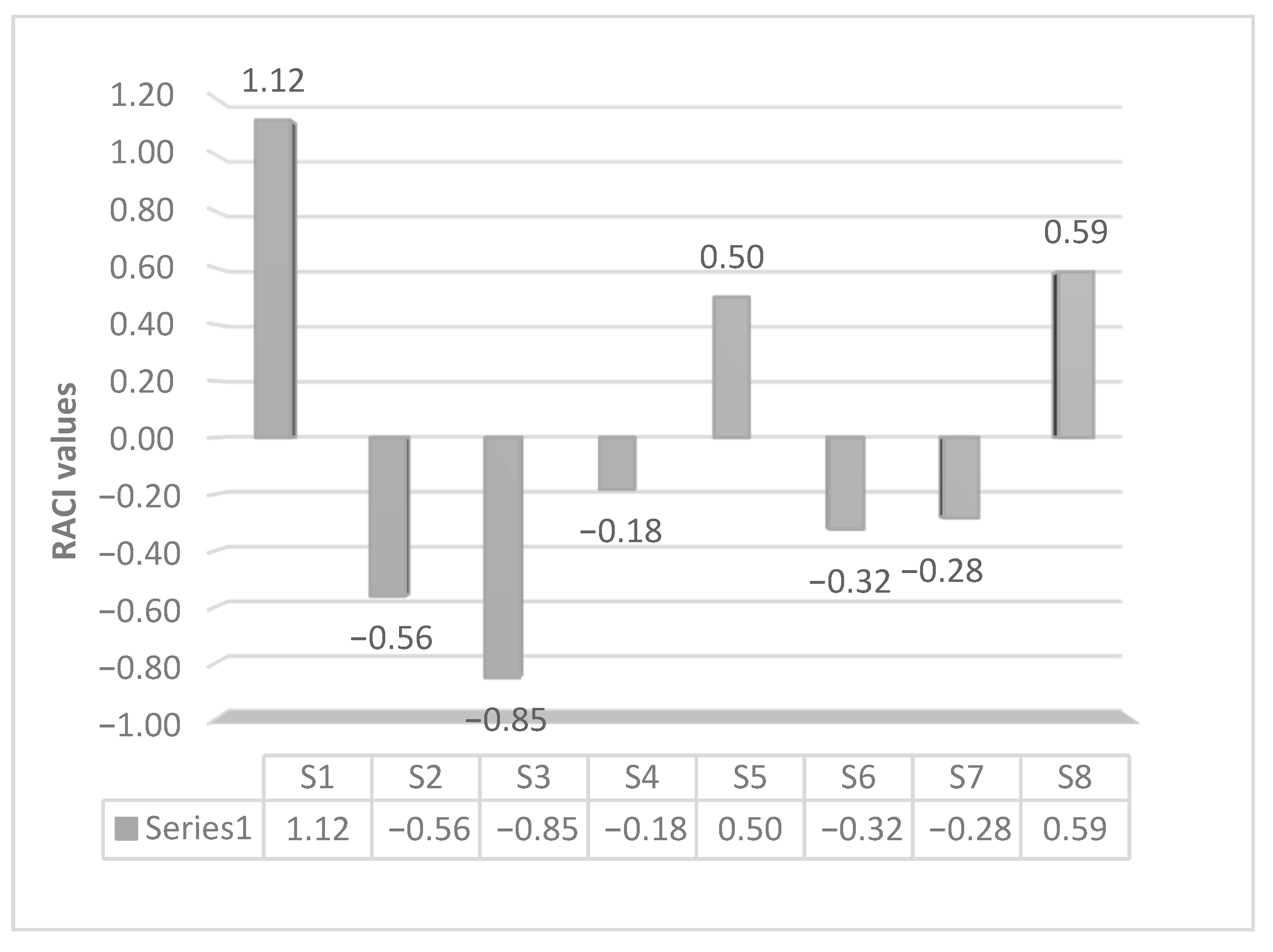
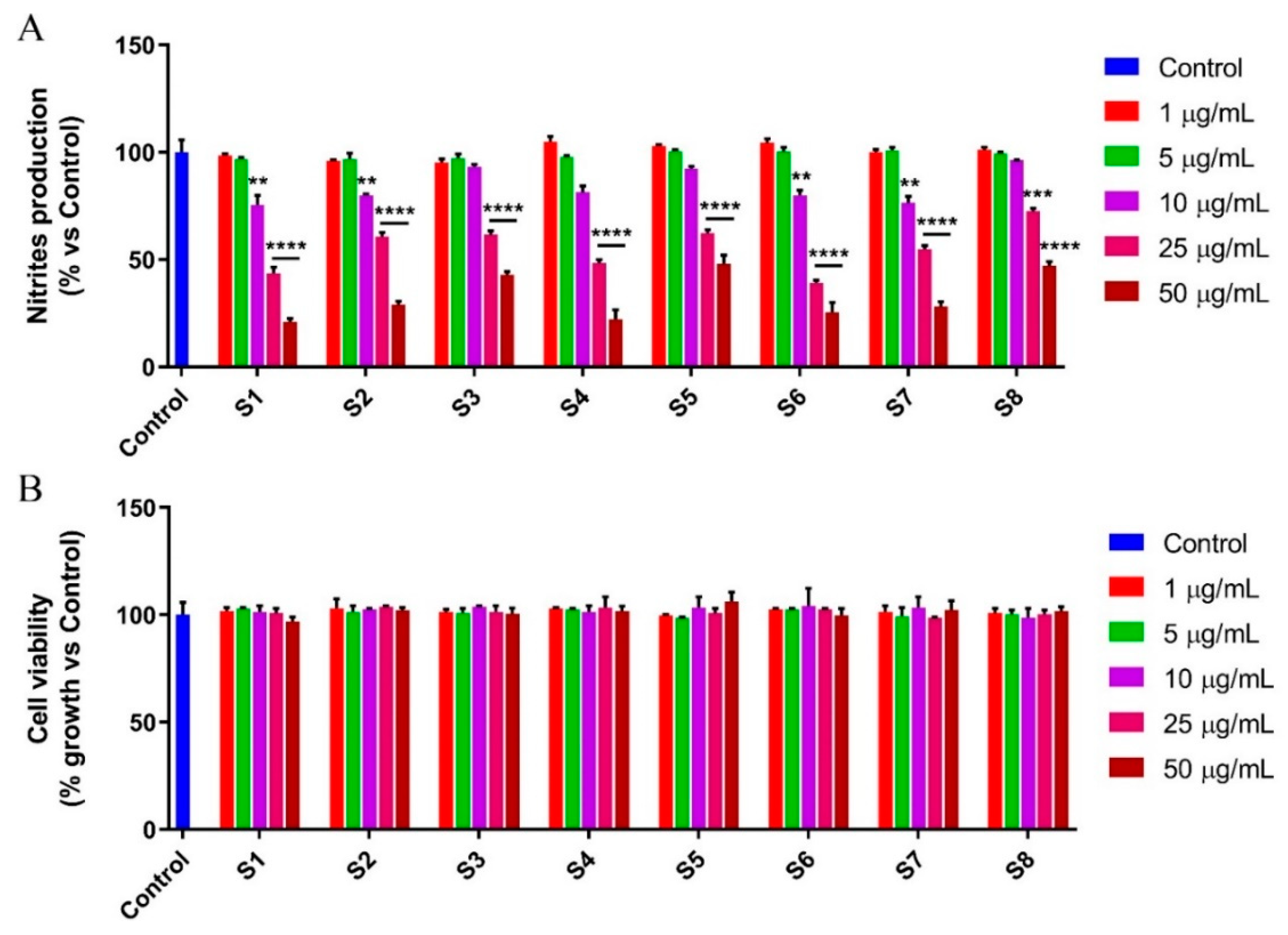
3.4. S. officinalis Extracts Reduce NO Production Inhibiting NF-κB
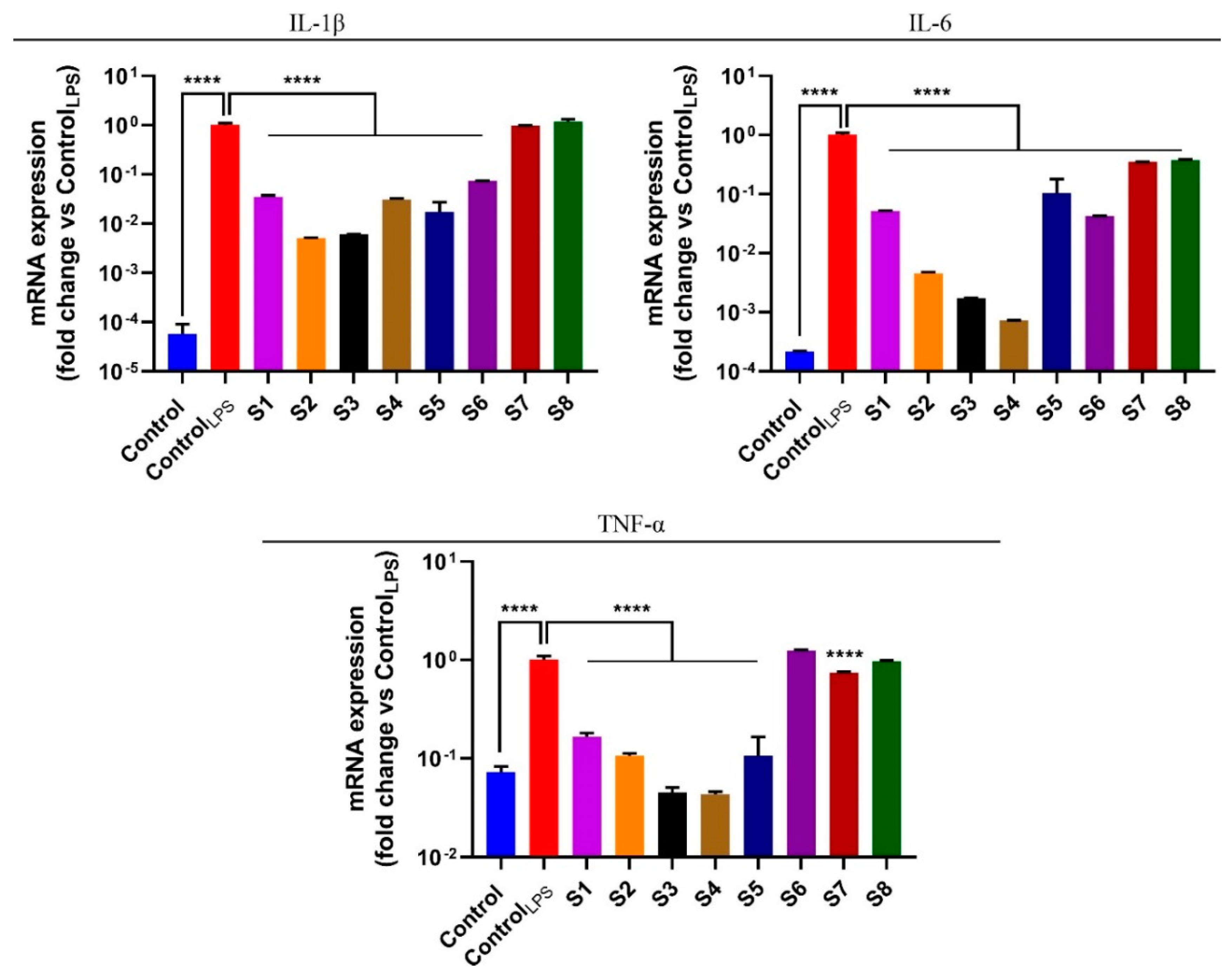
3.5. S. officinalis Extracts Reduce Pro-Inflammatory Cytokines
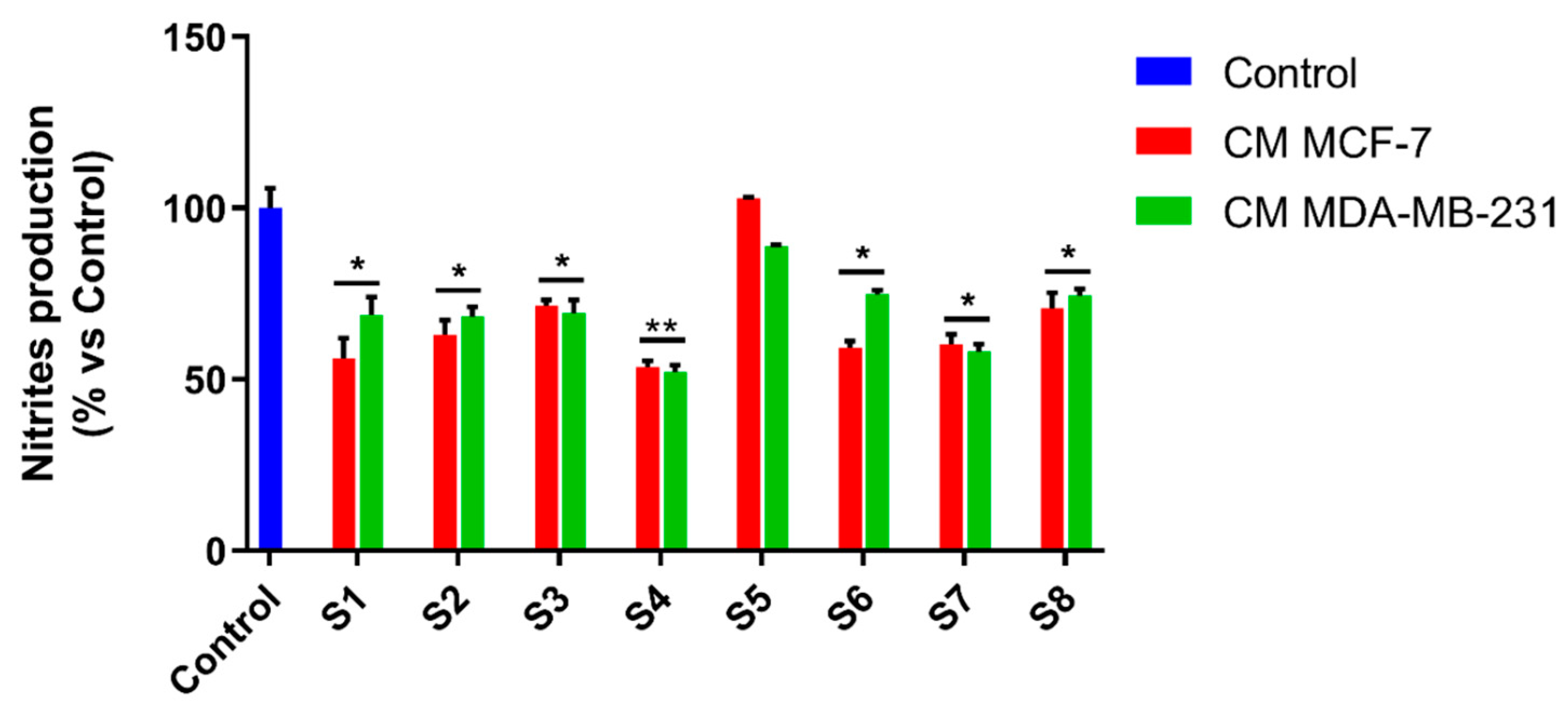
3.6. S. officinalis Extracts Interfere with Inflammation Associated with Neoplastic Progression
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walker, J.B.; Sytsma, K.J. Staminal evolution in the genus Salvia (Lamiaceae): Molecular phylogenetic evidence for multiple origins of the staminal lever. Ann. Bot. 2007, 100, 375–391. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive profile of various Salvia officinalis L. preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Bonesi, M.; Leporini, M.; Menichini, F.; Passalacqua, N. A study of Salvia fruticosa Mill subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi, an endemic Sage of Southern Italy. Plant Biosyst. 2018, 152, 130–141. [Google Scholar]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M.R. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar] [PubMed]
- Kolac, U.K.; Ustuner, M.C.; Tekin, N.; Ustuner, D.; Colak, E.; Entok, E. The anti-inflammatory and antioxidant effects of Salvia officinalis on lipopolysaccharide-induced inflammation in rats. J. Med. Food 2017, 20, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lou, J.; Mou, Y.; Li, P.; Wu, J.; Zhou, L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules 2011, 16, 2259–2267. [Google Scholar] [CrossRef]
- Qnais, E.Y.; Abu-Dieyeh, M.; Abdulla, F.A.; Abdalla, S.S. The antinociceptive and anti-inflammatory effects of Salvia officinalis leaf aqueous and butanol extracts. Pharm. Biol. 2010, 48, 1149–1156. [Google Scholar] [CrossRef]
- Baricevic, D.; Bartol, T.V. Pharmacology 11. The biological/pharmacological activity of the Salvia genus. Genus Salvia 2000, 143–184. [Google Scholar]
- Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. The pharmacological properties of salvia essential oils. J. Appl. Pharm. Sci. 2013, 3, 122. [Google Scholar]
- Mansourabadi, A.H.; Sadeghi, H.M.; Razavi, N.; Rezvani, E. Anti-inflammatory and analgesic properties of salvigenin, Salvia officinalis flavonoid extracted. Adv. Herb. Med. 2016, 2, 31–41. [Google Scholar]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. chemical profile, antioxidant, anti-inflammatory, and anti-cancer effects of Italian Salvia rosmarinus Spenn. methanol leaves Extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef]
- Lehbili, M.; Alabdul Magid, A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Abedini, A.; Morjani, H.; Gangloff, S.C.; Kabouche, Z. Antibacterial, antioxidant and cytotoxic activities of triterpenes and flavonoids from the aerial parts of Salvia barrelieri Etl. Nat. Prod. Res. 2018, 32, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Koutsoulas, A.; Čarnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and Salvia fruticosa extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef]
- Brindisi, M.; Fiorillo, M.; Frattaruolo, L.; Sotgia, F.; Lisanti, M.P.; Cappello, A.R. Cholesterol and mevalonate: Two metabolites involved in breast cancer progression and drug resistance through the ERRalpha pathway. Cells 2020, 9, 1819. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Fiorillo, M.; Tóth, F.; Brindisi, M.; Sotgia, F.; Lisanti, M.P. Deferiprone (DFP) targets cancer stem cell (CSC) propagation by inhibiting mitochondrial metabolism and inducing ROS production. Cells 2020, 9, 1529. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Frattaruolo, L.; Haworth, I.; Brindisi, M.; El-magboub, A.; Ferrario, A.; Gomer, C.; Aiello, F.; Adams, J.D. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, e01366. [Google Scholar] [CrossRef]
- Bonesi, M.; Brindisi, M.; Armentano, B.; Curcio, R.; Sicari, V.; Loizzo, M.R.; Cappello, M.S.; Bedini, G.; Peruzzi, L.; Tundis, R. Exploring the anti-proliferative, pro-apoptotic, and antioxidant properties of Santolina corsica Jord. & Fourr. (Asteraceae). Biomed. Pharmacother. 2018, 107, 967–978. [Google Scholar]
- Armentano, B.; Curcio, R.; Brindisi, M.; Mancuso, R.; Rago, V.; Ziccarelli, I.; Frattaruolo, L.; Fiorillo, M.; Dolce, V.; Gabriele, B. 5-(Carbamoylmethylene)-oxazolidin-2-ones as a promising class of heterocycles inducing apoptosis triggered by increased ros levels and mitochondrial dysfunction in breast and cervical cancer. Biomedicines 2020, 8, 35. [Google Scholar] [CrossRef]
- Casaburi, I.; Avena, P.; De Luca, A.; Chimento, A.; Sirianni, R.; Malivindi, R.; Rago, V.; Fiorillo, M.; Domanico, F.; Campana, C.; et al. Estrogen related receptor alpha (ERRalpha) a promising target for the therapy of adrenocortical carcinoma (ACC). Oncotarget 2015, 6, 25135–25148. [Google Scholar] [CrossRef]
- Mazzotta, S.; Frattaruolo, L.; Brindisi, M.; Ulivieri, C.; Vanni, F.; Brizzi, A.; Carullo, G.; Cappello, A.R.; Aiello, F. 3-Amino-alkylated indoles: Unexplored green products acting as anti-inflammatory agents. Future Med. Chem. 2020, 12, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tanumihardjo, S. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Hu, X.; Deng, Y.; Hou, J.; Lei, M.; Ji, H.; Zhou, J.; Qu, H.; Wu, W.; Guo, D. Four New Depsides Isolated from Salvia miltiorrhiza and their significant nerve-protective activities. Molecules 2018, 23, 3274. [Google Scholar] [CrossRef]
- Kasimu, R.; Tanaka, K.; Tezuka, Y.; Gong, Z.-N.; Li, J.-X.; Basnet, P.; Namba, T.; Kadota, S. Comparative study of seventeen Salvia plants: Aldose reductase inhibitory activity of water and MeOH extracts and liquid chromatography-mass spectrometry (LC-MS) analysis of water extracts. Chem. Pharm. Bull. 1998, 46, 500–504. [Google Scholar] [CrossRef]
- Moharram, F.A.E.; Marzouk, M.S.; El-Shenawy, S.M.; Gaara, A.H.; El Kady, W.M. Polyphenolic profile and biological activity of Salvia splendens leaves. J. Pharm. Pharmacol. 2012, 64, 1678–1687. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 2000, 55, 263–267. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Berset, C.; Richard, H. Antioxidant constituents in sage (Salvia officinalis). J. Agric. Food Chem. 1994, 42, 665–669. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.S.; Tsatsakis, A.M.; Kouretas, D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 2012, 17, 11–21. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants–A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Yurtseven, S.; Cetin, M.; Şengül, T.; Sögüt, B. Effect of sage extract (Salvia officinalis) on growth performance, blood parameters, oxidative stress and DNA damage in partridges. S. Afr. J. Anim. Sci. 2008, 38, 145–152. [Google Scholar]
- Castillo-Morales, R.M.; Otero, A.L.C.; Mendez-Sanchez, S.C.; Da Silva, M.A.N.; Stashenko, E.E.; Duque, J.E. Mitochondrial affectation, DNA damage and AChE inhibition induced by Salvia officinalis essential oil on Aedes aegypti larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Seo, E.-J.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef]
- Sousa, L.P.; Alessandri, A.L.; Pinho, V.; Teixeira, M.M. Pharmacological strategies to resolve acute inflammation. Curr. Opin. Pharmacol. 2013, 13, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, E.; Ko, E.; Ham, M.; Lee, H.M.; Kim, E.-S.; Koh, M.; Lim, H.K.; Jung, J.; Park, S.Y. Tumor-associated macrophages secrete CCL2 and induce the invasive phenotype of human breast epithelial cells through upregulation of ERO1-α and MMP-9. Cancer Lett. 2018, 437, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell Biochem. 2004, 265, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]

| Primer Name | Sequence (5′-3′) |
|---|---|
| TNFα Fw | CAGGCGGTGCCTATGTCTC |
| TNFα Rv | CGATCACCCCGAAGTTCAGTAG |
| IL-1β Fw | GAAATGCCACCTTTTGACAGTG |
| IL-1β Rv | TGGATGCTCTCATCAGGACAG |
| IL-6 Fw | CTGCAAGAGACTTCCATCCAG |
| IL-6 Rv | AGTGGTATAGACAGGTCTGTTGG |
| GAPDH Fw | ACCACAGTCCATGCCATCAC |
| GAPDH Rv | TCCACCACCCTGTTGCTGTA |
| S. officinalis | Part | Extraction Procedure | Yield (%) a | |
|---|---|---|---|---|
| Orsomarso | S1 | Leaves | Maceration | 7.8 |
| S2 | Leaves | Ultrasound-assisted extraction | 6.2 | |
| S3 | Flowers | Maceration | 8.3 | |
| S4 | Flowers | Ultrasound-assisted extraction | 3.8 | |
| Civita | S5 | Leaves | Maceration | 9.3 |
| S6 | Leaves | Ultrasound-assisted extraction | 8.4 | |
| S7 | Flowers | Maceration | 8.9 | |
| S8 | Flowers | Ultrasound-assisted extraction | 5.2 |
| Peak | Rt (min) | UV λ (nm) | Molecular ion [M − H]− (m/z) | Molecular Ion [M + H]+ (m/z) | Identification | Ref |
|---|---|---|---|---|---|---|
| 1 | 11.5 | 220/240/295/325 | 179 | Caffeic acid | [2] | |
| 2 | 18.4 | 205/253/270/350 | 447 | 449 | Luteolin-7-glucoside2 | [2] |
| 3 | 19.6 | 208/253/270/345 | 461 | Luteolin-O-glucuronide | [2] | |
| 4 | 20.8 | 220/251/290/330 | 359 | 361 | Rosmarinic acid (cis,trans) 2 | [1] |
| 5 | 21.5 | 250/290/330 | 445 | Apigenin-O-glucuronide | [2] | |
| 6 | 22.6 | 290/323 | 555 | 557 | Salvianolic acid K | [3,4] |
| 7 | 28.2 | 263/274/360 | 315 | Nepetin | [2] | |
| 8 | 31.0 | 286/340 | 345 | 347 | Rosmanol isomer 1 | [1,2] |
| 9 | 31.2 | 253/240/350 | 285 | Luteolin | [2] | |
| 10 | 33.3 | 286/340 | 345 | 347 | Rosmanol isomer 1 | [1,2] |
| 11 | 34.3 | 220/270/340 | 299 | Hispidulin | [2] | |
| 12 | 34.8 | 286/340 | 345 | 347 | Rosmanol isomer 1 | [1,2] |
| 13 | 36.3 | 279/340 | 313 | Cirsimaritin | [2] | |
| 14 | 37.5 | 250/300/400 | 269 | Apigenin | [2] | |
| 15 | 42.6 | 265/331 | 383 | Genkwanin | [2] | |
| 16 | 42.8 | 205/290 | 359 | 361 | 7-O-methylrosmanol | [6] |
| 17 | 45.7 | 205/250/305 | 342 | Rosmadial | [2] | |
| 18 | 46.7 | 205/286 | 329 | 331 | Carnosol 2 | [1] |
| 19 | 48.5 | 204/260/280/330 | 315 | 317 | Pedalitin | [5] |
| 20 | 49.9 | 205/230/285 | 345 | 12-O-methylcarnosic acid | [2] | |
| 21 | 50.4 | 204/235/287 | 331 | Carnosic acid 2 | [2] | |
| 22 | 54.5 | 457 | Triterpenic acids (Ursolic acid) 2 | [2] |
| Luteolin-7-Glucoside (Rt = 18.4 min) | Rosmarinic Acid (Rt = 20.8 min) | Triterpene Acids (Rt = 54.5 min) | ||||
|---|---|---|---|---|---|---|
| (*) | (%) ** | (*) | (%) ** | (*) | (%) ** | |
| Orsomarso | ||||||
| S1 | 0.0160 ± 0.0049 | 0.86 | 0.0848 ± 0.0128 | 4.56 | 0.2166 ± 0.0275 | 11.65 |
| S2 | 0.0088 ± 0.0014 | 0.60 | 0.0514 ± 0.0093 | 3.50 | 0.3932 ± 0.0539 | 25.75 |
| S3 | 0 | 0 | 0.0915 ± 0.0193 | 4.08 | 0.4851 ± 0.0511 | 21.66 |
| S4 | 0.0024 ± 0.0005 | 0.12 | 0.1018 ± 0.0144 | 4.99 | 0.1574 ± 0.0017 | 7.72 |
| Civita | ||||||
| S5 | 0.0119 ± 0.0001 | 0.60 | 0.0654 ± 0.0077 | 3.30 | 0.1249 ± 0.0043 | 6.31 |
| S6 | 0.0054 ± 0.0007 | 0.37 | 0.0559 ± 0.0005 | 3.83 | 0.1655 ± 0.0109 | 11.34 |
| S7 | 0.0077 ± 0.0011 | 0.37 | 0.1127 ± 0.0106 | 5.44 | 0.0756 ± 0.0054 | 3.65 |
| S8 | 0.0160 ± 0.0048 | 0.71 | 0.1750 ± 0.0440 | 7.81 | 0.1425 ± 0.0232 | 6.36 |
| Sample | TPC 1 | TFC 2 | DPPH Test 3 | ABTS Test 3 | β-Carotene Bleaching Test 3 | FRAP Test 4 | |
|---|---|---|---|---|---|---|---|
| 30 min | 60 min | ||||||
| S1 | 40.3 ± 1.5 b | 25.2 ± 1.4 b | 12.4 ± 1.2 **** | 0.9 ± 0.08 | 7.1 ± 0.7 **** | 11.6 ± 1.2 **** | 98.4 ± 6.3 |
| S2 | 18.8 ± 1.0 f | 12.6 ± 0.6 f | 10.4 ± 1.0 **** | 0.9 ± 0.04 | 3.5 ± 0.3 | 3.2 ± 0.3 | 65.2 ± 4.2 |
| S3 | 41.9 ± 1.6 a | 15.4 ± 0.9 d | 9.8 ± 0.9 **** | 0.9 ± 0.09 | 2.9 ± 0.2 | 2.5 ± 0.2 | 95.5 ± 6.1 |
| S4 | 25.7 ± 0.8 e | 14.5 ± 0.8 e | 10.8 ± 1.3 **** | 1.1 ± 0.1 | 3.8 ± 0.4 | 4.3 ± 0.4 ** | 72.1 ± 4.4 |
| S5 | 39.8 ± 1.5 c | 17.7 ± 0.9 c | 10.3 ± 1.0 **** | 1.9 ± 0.2 | 5.1 ± 0.5 ** | 4.8 ± 0.4 ** | 69.1 ± 4.4 |
| S6 | 35.3 ± 1.1 d | 14.9 ± 1.1 e | 11.8 ± 1.1 **** | 1.1 ± 0.1 | 2.0 ± 0.2 | 2.5 ± 0.2 | 84.5 ± 5.1 |
| S7 | 41.6 ± 1.2 a | 26.6 ± 0.7 a | 9.8 ± 0.9 **** | 1.2 ± 0.1 | 4.0 ± 0.4 * | 4.9 ± 0.5 ** | 87.1 ± 6.3 |
| S8 | 18.7 ± 0.1 f | 12.4 ± 0.4 f | 11.8 ± 1.1 **** | 0.9 ± 0.09 | 7.0 ± 0.7 **** | 7.1 ± 0.7 **** | 94.4 ± 6.3 |
| Positive control | |||||||
| Ascorbic acid | 5.0 ± 0.8 | 1.7 ± 0.06 | |||||
| Propyl gallate | 1.0 ± 0.03 | 0.09 ± 0.004 | |||||
| BHT | 63.2 ± 4.3 | ||||||
| Extract | IC50 ± SD (µg/mL) |
|---|---|
| S1 | 21.74 ± 3.019 |
| S2 | 31.92 ± 3.695 |
| S3 | 44.43 ± 4.698 |
| S4 | 25.02 ± 3.901 |
| S5 | 49.83 ± 5.463 |
| S6 | 22.95 ± 3.846 |
| S7 | 28.87 ± 3.638 |
| S8 | 58.83 ± 6.890 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models. Antioxidants 2021, 10, 311. https://doi.org/10.3390/antiox10020311
Brindisi M, Bouzidi C, Frattaruolo L, Loizzo MR, Cappello MS, Dugay A, Deguin B, Lauria G, Cappello AR, Tundis R. New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models. Antioxidants. 2021; 10(2):311. https://doi.org/10.3390/antiox10020311
Chicago/Turabian StyleBrindisi, Matteo, Chouaha Bouzidi, Luca Frattaruolo, Monica R. Loizzo, Maria Stella Cappello, Annabelle Dugay, Brigitte Deguin, Graziantonio Lauria, Anna Rita Cappello, and Rosa Tundis. 2021. "New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models" Antioxidants 10, no. 2: 311. https://doi.org/10.3390/antiox10020311
APA StyleBrindisi, M., Bouzidi, C., Frattaruolo, L., Loizzo, M. R., Cappello, M. S., Dugay, A., Deguin, B., Lauria, G., Cappello, A. R., & Tundis, R. (2021). New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models. Antioxidants, 10(2), 311. https://doi.org/10.3390/antiox10020311











