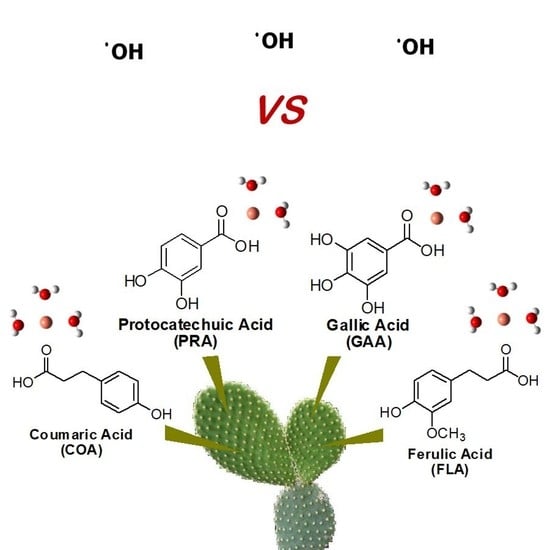
Antioxidants into Nopal (Opuntia ficus-indica), Important Inhibitors of Free Radicals’ Formation
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
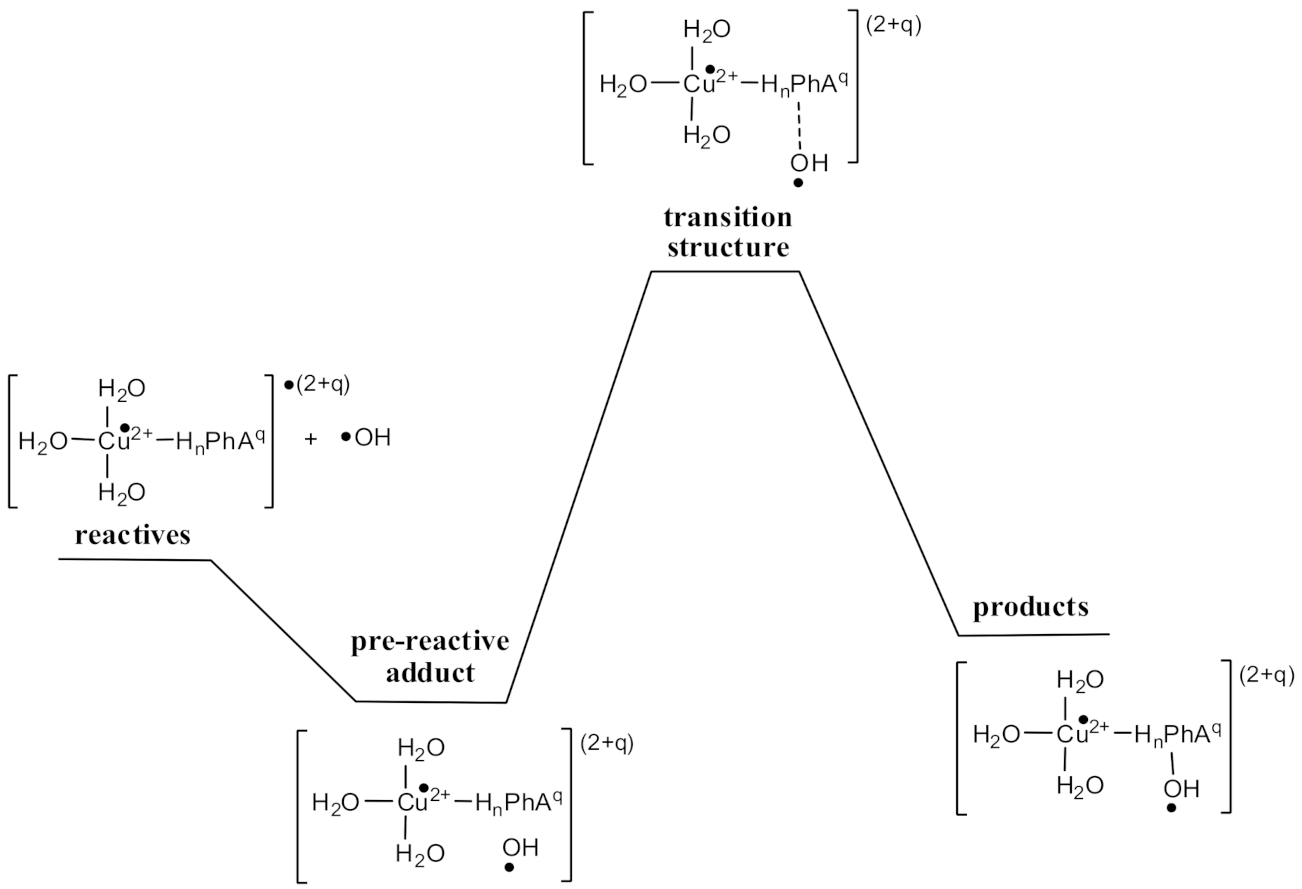
3.1. Pro-Oxidant Effects by Cu(II) Reduction
3.2. •OH-Inactivating Ligand Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butterfield, D.A.; Hensley, K.; Harris, M.; Mattson, M.; Carney, J. β-Amyloid Peptide Free Radical Fragments Initiate Synaptosomal Lipoperoxidation in a Sequence-Specific Fashion: Implications to Alzheimer′s Disease. Biochem. Biophys. Res. Commun. 1994, 200, 710–715. [Google Scholar] [CrossRef]
- Boyd, N.F.; McGuire, V. The possible role of lipid peroxidation in breast cancer risk. Free Radic. Biol. Med. 1991, 10, 185–190. [Google Scholar] [CrossRef]
- Nelson, R.L. Dietary iron and colorectal cancer risk. Free Radic. Biol. Med. 1992, 12, 161–168. [Google Scholar] [CrossRef]
- Knekt, P.; Reunanen, A.; Takkunen, H.; Aromaa, A.; Heliovarara, M.; Hakulinen, T. Body iron stores and risk of cancer. Int. J. Cancer 1994, 56, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a Combination of Beta Carotene and Vitamin A on Lung Cancer and Cardiovascular Disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panasenko, O.M.; Vol’nova, T.V.; Azizova, O.A.; Vladimirov, Y.A. Free radical modification of lipoproteins and cholesterol accumulation in cells upon atherosclerosis. Free Radic. Biol. Med. 1991, 10, 137–148. [Google Scholar] [CrossRef]
- Steinberg, D. Antioxidants and atherosclerosis. A current assessment. Circulation 1991, 84, 1420–1425. [Google Scholar] [CrossRef] [Green Version]
- Riemmersma, R.A.; Wood, D.A.; Macityre, C.C.; Elton, R.A.; Gey, K.F.; Oliver, M.F. Risk of angina pectoris and plasma concentrations of vitamins A, C, and E and carotene. Lancet 1991, 337, 1–5. [Google Scholar] [CrossRef]
- Salonen, J.T.; Nyyssoner, K.; Korpela, H.; Tuomilehto, J.; Seppanen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Street, D.A.; Comstock, G.; Salkeldy, R.; Klag, M. Serum antioxidants and myocardial infarction. Are low levels of carotenoids and alpha-tocopherol risk factors for myocardial infarction? Circulation 1994, 90, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense system, and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and Medicinal Use of Cactus Pear (Opuntia spp.) Cladodes and Fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef]
- Seyoum, A.; Asres, K.; El-Fiky, F.K. Structure-Radical Scavenging Activity Relationships of Flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, M.; Azmat, A.; Ahmad, S.I.; Faizi, Z.; Abidi, L.; Faizi, S. Hypotensive activity, toxicology and histopathology of opuntioside-I and methanolic extract of Opuntia dilleniid. Biol. Pharm. Bull. 2005, 28, 1844–1851. [Google Scholar] [CrossRef] [Green Version]
- Corrales-Garcia, J.; Pena-Valdivia, C.B.; Razo-Martinez, Y.; Sanchez-Hernandez, M. Acidity changes and pH-buffering capacity of nopalitos (Opuntia spp.). Post. Biol. Tech. 2004, 32, 169–174. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.M.; Ha, H.J.; Moon, C.J.; Shin, T.K.; Kim, J.M.; Lee, N.H.; Kim, H.C.; Jang, K.J.; Wie, M.B. Opuntia ficus-indica attenuates neuronal injury in in vitro and in vivo models of cerebral ischemia. J. Ethnopharmacol. 2006, 104, 257–262. [Google Scholar] [CrossRef]
- Nuñez-López, M.A.; Paredes-López, O.; Reynoso-Camacho, R. Functional and hypoglycemic properties of nopal cladodes (O. ficus-indica) at different maturity stages using in vitro and in vivo tests. J. Agric. Food Chem. 2013, 61, 10981–10986. [Google Scholar] [CrossRef] [PubMed]
- Paiz, R.C.; Juárez-Flores, B.I.; Aguirre-Rivera, R.J.; Cárdenas-Ortega, N.C.; Reyes-Agüero, J.A.; García-Chávez, E.; Álvarez-Fuentes, G. Glucose-lowering effect of xoconostle (Opuntia joconostle A. Web., Cactaceae) in diabetic rats. J. Med. Plants Res. 2010, 4, 2326–2333. [Google Scholar]
- Stintzing, F.C.; Schieber, A.; Carle, R. Phytochemical and nutritional significance of cactus pear. Eur. Food Res. Technol. 2001, 212, 396–407. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical characterization and biological effects of Sicilian Opuntia ficus indica (L.) mill. Fruit juice: Antioxidant and antiulcerogenic activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Monforte, M.T.; Galluzzo, M.; Miceli, N.; Tripodo, M.M. Effect of Opuntia ficus-indica (L.) Mill. Cladodes in the Wound-Healing Process. J. Prof. Assoc. Cactus Dev. 2003, 5, 1–16. [Google Scholar]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, D.M.; Brewer, M.; Garcia, F.; Feugang, J.M.; Wang, J.; Zang, R.; Liu, H.; Zou, C. Cactus pear: A natural product in cancer chemoprevention. Nutr. J. 2005, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Dok-Go, H.; Lee, K.H.; Kim, H.J.; Lee, E.H.; Lee, J.; Song, Y.S.; Lee, Y.H.; Jin, C.; Lee, Y.S.; Cho, J. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 2003, 965, 130–136. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Lauriano, E.R.; Taviano, M.F.; Galluzzo, M.; Miceli, N. Opuntia ficus indica (L.) Mill. fruit juice protects liver from carbon tetrachloride-induced injury. Phytother. Res. 2005, 19, 796–800. [Google Scholar] [CrossRef]
- Sreekanth, D.; Arunasree, M.K.; Roy, K.R.; Chandramohan, T.; Reddy, G.V.; Reddanna, P. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia Cell line-K562. Phytomedicine 2007, 14, 739–746. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.E.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.Y.; Yin, M.C. Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2009, 6, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Huang, C.S.; Huang, C.Y.; Yin, M.C. Anticoagulatory, antiinflammatory and antioxidative effects of protocatechuic acid in diabetic mice. J. Agric. Food Chem. 2009, 57, 6661–6667. [Google Scholar] [CrossRef] [PubMed]
- Kroes, B.H.; van den Berg, A.J.; Quarles van Ufford, H.C.; van Dijk, H.; Labadie, R.P. Anti-Inflammatory Activity of Gallic Acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar]
- Stagos, D.; Kazantzoglou, G.; Theofanidou, D.; Kakalopoulou, G.; Magiatis, P.; Mitaku, S.; Kouretas, D. Activity of Grape Extracts from Greek Varieties of Vitis vinifera against Mutagenicity Induced by Bleomycin and Hydrogen Peroxide in Salmonella typhimurium Strain TA102. Mutat. Res. 2006, 609, 165–175. [Google Scholar] [CrossRef]
- Abdelwahed, A.; Bouhlel, I.; Skandrani, I.; Valenti, K.; Kadri, M.; Guiraud, P.; Steiman, R.; Mariotte, A.M.; Ghedira, K.; Laporte, F.; et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem. Biol. Interact. 2007, 165, 1–13. [Google Scholar] [CrossRef]
- Kratz, J.M.; Andrighetti-Frohner, C.R.; Kolling, D.J.; Leal, P.C.; Cirne-Santos, C.C.; Yunes, R.A.; Nunes, R.J.; Trybala, E.; Bergstrom, T.; Frugulhetti, I.C.P.P.; et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem. Inst. Oswaldo Cruz 2008, 103, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Song, J.H.; Bhatt, L.R.; Baek, S.H. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother. Res. 2010, 24, 1292–1296. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Prince, P.S.M. Cardioprotective Effect of Gallic Acid on Cardiac Troponin-T, Cardiac Marker Enzymes, Lipid Peroxidation Products and Antioxidants in Experimentally Induced Myocardial Infarction in Wistar Rats. Chem. Biol. Interact. 2009, 179, 118–124. [Google Scholar] [CrossRef]
- Zhongbing, L.; Guangjun, N.; Belton, P.S.; Tang, H.; Zhao, B. Structure-Activity Relationship Analysis of Antioxidant Ability and Neuroprotective Effect of Gallic Acid Derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar]
- Rodriguez-Garcia, M.E.; de Lira, C.; Hernandez-Becerra, E.; Cornejo-Villegas, M.A.; Palacios-Fonseca, A.J.; Rojas-Molina, I.; Reynoso, R.; Quintero, L.C.; Del-Real, A.; Zepeda, T.A.; et al. Physicochemical characterization of nopal pads (Opuntia ficus indica) and dry vacuum nopal powders as a function of the maturation. Plant. Foods Hum. Nutr. 2007, 62, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, T.; Carrillo-López, A.; Guevara-Lara, F.; Cruz-Hernández, A.; Paredes-López, O. Biochemical and nutritional characterization of three prickly pear species with different ripening behavior. Plant. Foods Hum. Nutr. 2005, 60, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Fazzari, M.; Allegra, M.; Livrea, M.A. Biothiols, taurine, and lipid-soluble antioxidants in the edible pulp of Sicilian cactus pear (Opuntia ficus-indica) fruits and changes of bioactive juice components upon industrial processing. J. Agric. Food Chem. 2005, 53, 7851–7855. [Google Scholar] [CrossRef]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan prickly pear fruits (Opuntia ficus indica). Arab. J. Chem. 2016, 9, S278–S281. [Google Scholar] [CrossRef] [Green Version]
- De Leo, M.; De Abreu, M.B.; Pawlowska, A.M.; Cioni, P.L.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Ennouri, M.; Evelyne, B.; Laurence, M.; Hamadi, A. Fatty acid composition and rheological behaviour of prickly pear seed oils. Food Chem. 2005, 93, 431–437. [Google Scholar] [CrossRef]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-products of Opuntia ficus-indica as a source of antioxidant dietary fiber. Plant. Food Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Infante, J.A.; Rocha-Guzman, N.E.; González-Laredo, R.F.; Reynoso-Camacho, R.; Medina-Torres, L.; Cervantes-Cardozo, V. Effect of air flow rate on the polyphenols content and antioxidant capacity of convective dried cactus pear cladodes (Opuntia ficus indica). Int. J. Food Sci. Nutr. 2009, 60, 80–87. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, Chemical, and Biochemical Characterization of Cladodes from Prickly Pear (Opuntia ficus-indica (L.) Mill.). J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Terpinc, P.; Polak, T.; Segatin, N.; Hanzlowsky, A.; Ulrih, N.P.; Abramovic, H. Antioxidant properties of 4-vinyl derivatives of hydroxycinnamic acids. Food Chem. 2011, 128, 62–69. [Google Scholar] [CrossRef]
- Pino, E.; Campos, A.M.; Lopez-Alarcon, C.; Aspee, A.; Lissi, E. Free radical scavenging capacity of hydroxycinnamic acids and related compounds. J. Phys. Org. Chem. 2006, 19, 759–764. [Google Scholar] [CrossRef]
- Kadoma, Y.; Fujisawa, S. A Comparative Study of the Radical-scavenging Activity of the Phenolcarboxylic Acids Caffeic Acid, p-Coumaric Acid, Chlorogenic Acid and Ferulic Acid, With or Without 2-Mercaptoethanol, a Thiol, Using the Induction Period Method. Molecules 2008, 13, 2488–2499. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.F.; An, L.J.; Jiang, B.; Guan, S.; Bao, Y.M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006, 403, 206–210. [Google Scholar] [CrossRef]
- Hyogo, A.; Kobayashi, T.; del Saz, E.G.; Seguchi, H. Antioxidant Effects of Protocatechuic Acid, Ferulic Acid, and Caffeic Acid in Human Neutrophils Using a Fluorescent Substance. Int. J. Morphol. 2010, 28, 911–920. [Google Scholar] [CrossRef]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giftson, J.S.; Jayanthi, S.; Nalini, N. Chemopreventive Efficacy of Gallic Acid, an Antioxidant and Anticarcinogenic Polyphenol, against 1, 2-Dimethyl Hydrazine Induced Rat Colon Carcinogenesis. Invest. New Drugs 2010, 28, 251–259. [Google Scholar] [CrossRef]
- Erdemgil, F.Z.; Sanli, S.; Sanli, N.; Ozkan, G.; Barbosa, J.; Guiteras, J.; Beltran, J.L. Determination of pK(a) values of some hydroxylated benzoic acids in methanol-water binary mixtures by LC methodology and potentiometry. Talanta 2007, 72, 489–496. [Google Scholar] [CrossRef]
- Galano, A.; Pérez-González, A. On the free radical scavenging mechanism of protocatechuic acid, regeneration of the catechol group in aqueous solution. Theor. Chem. Acc. 2012, 131, 1265–1277. [Google Scholar] [CrossRef]
- Castillo, J.; Benavente-Garcia, O.; Lorente, J.; Alcaraz, M.; Redondo, A.; Ortuno, A.; Del-Rio, J.A. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (Procyanidins) from grape seeds (Vitis vinifera): Comparative study versus other phenolic and organic compounds. J. Agric. Food Chem. 2000, 48, 1738–1745. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Fox, D.J. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Galano, A.; Alvarez-Idaboy, J.R. A Computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Hase, W.L.; Hynes, J.T. Erratum: Current status of transition-state theory. J. Phys. Chem. 1983, 87, 2667–2682. [Google Scholar] [CrossRef]
- Kuppermann, A.; Truhlar, D.G. Exact tunneling calculations. J. Am. Chem. Soc. 1971, 93, 1840–1851. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Pure Appl. Chem. 1997, 69, 13–29. [Google Scholar] [CrossRef]
- Collins, F.C.; Kimball, G.E. Diffusion-Controlled Reaction Rates. J. Colloid Sci. 1949, 4, 425–437. [Google Scholar] [CrossRef]
- Von Smoluchowski, M. Versucheiner Mathematischen Theorie der Koagulations Kinetic Kolloider Lousungen. Z. Phys. Chem. 1917, 92, 129–168. [Google Scholar]
- Einstein, A. Investigations on the theory of the brownian movement. Ann. Phys. 1905, 17, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A. Computational study of copper (II) complexation and hydrolysis in aqueous solutions using mixed cluster/continuum models. J. Phys. Chem. A 2009, 113, 9559–9567. [Google Scholar] [CrossRef] [Green Version]
- Beltran, J.L.; Sanli, N.; Fonrodona, G.; Barren, D.; Ozkan, G.; Barbosa, J. Spectrophotometric, Potentiometric and Chromatographic pKa Values of Polyphenolic Acids in Water and Acetonitrile-Water Media. Anal. Chim. Acta 2003, 484, 253–264. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Alvarez-Idaboy, J.R.; Galano, A. On the peroxyl scavenging activity of hydroxycinnamic acid derivatives: Mechanisms, kinetics, and importance of the acid–base equilibrium. Phys. Chem. Chem. Phys. 2012, 14, 12534–12543. [Google Scholar] [CrossRef]
- Marino, T.; Galano, A.; Russo, N. Radical scavenging ability of gallic acid toward OH and OOH radicals. Reaction mechanism and rate constants from the density functional theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B.J. Reactivity of HO2/O−2 Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Gaubert, S.; Bouchaut, M.; Brumas, V.; Berthon, G. Copper—Ligand interactions and the physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic. Res. 2000, 32, 451–461. [Google Scholar] [CrossRef]
- Miche, H.; Brumas, V.; Berthon, G. Copper (II) interactions with nonsteroidal anti-inflammatory agents. II. Anthranilic acid as a potential OH-inactivating ligand. J. Inorg. Biochem. 1997, 68, 27–38. [Google Scholar] [CrossRef]
- Berthon, G. Is copper pro- or anti-inflammatory? A reconciling view and a novel approach for the use of copper in the control of inflammation. Agents Actions 1993, 39, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation of the Antioxidant and Pro-oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef]


| PhAs | pKa1 | pKa2 | pKa3 | pKa4 | Mf (HnA) | Mf (Hn−1A−) | Mf (Hn−2A−2) | Mf (Hn−3A−3) | Mf (Hn−4A−4) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| COA | 4.38 | 8.68 | 0.001 | 0.949 | 0.050 | [56,72,73] | ||||
| FLA | 4.56 | 8.65 | 0.001 | 0.945 | 0.053 | [56,73] | ||||
| PRA | 4.38 | 8.74 | 10.67 | 0.001 | 0.955 | 0.044 | <0.001 | [56,57] | ||
| GAA | 4.32 | 8.24 | 9.97 | 13.1 | 0.001 | 0.871 | 0.127 | <0.001 | <0.001 | [58,74] |
| ∆G | λ | ∆G≠ | k | |
|---|---|---|---|---|
| O2•− | −24.01 | 51.87 | 3.74 | 4.66 × 109 |
| Asc− | −4.67 | 34.14 | 6.36 | 1.33 × 108 |
| HnCOA | 12.90 | 29.40 | 15.22 | 4.34 × 10−2 |
| Hn−1COA– | 5.49 | 30.79 | 10.69 | 8.59 × 104 |
| Hn−2COA2– | −16.64 | 28.53 | 1.24 | 3.96 × 108 |
| HnFLA | 8.46 | 29.45 | 12.20 | 7.11 × 100 |
| Hn−1FLA– | 2.94 | 29.86 | 9.00 | 1.47 × 106 |
| Hn−2FLA2– | −19.39 | 28.45 | 0.72 | 4.22 × 108 |
| HnPRA | 15.69 | 30.97 | 17.57 | 8.12 × 10−4 |
| Hn−1PRA– | 8.14 | 31.35 | 12.43 | 4.57 × 103 |
| Hn−2PRA2– | −16.15 | 29.96 | 1.59 | 3.45 × 108 |
| HnGAA | 15.19 | 31.05 | 17.22 | 1.48 × 10−3 |
| Hn−1GAA– | 8.50 | 31.20 | 12.63 | 2.97 × 103 |
| Hn−2GAA2– | −17.80 | 30.11 | 1.26 | 1.01 × 109 |
| Complex | ΔG’ | %MB | ||
|---|---|---|---|---|
| Hn−2COA2– (2)-C2 | 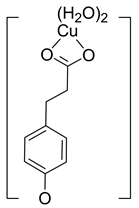 | 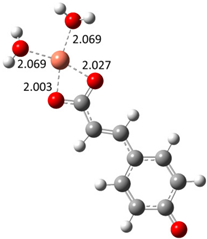 | −22.87 | ~100 |
| Hn−2FLA2– (2)-C5 | 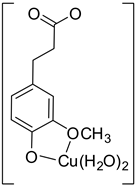 |  | −19.19 | 81.61 |
| Hn−2FLA2– (2)-C2 |  | 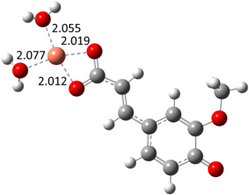 | −18.30 | 18.21 |
| Hn−2PRA2– (2)-C7 | 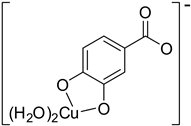 | 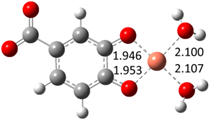 | −22.83 | 99.99 |
| Hn−2GAA2– (2)-C7 | 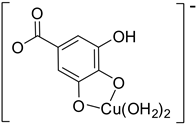 | 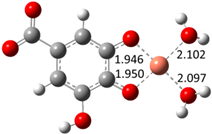 | −20.83 | 98.05 |
| O2•− | Asc− | |||
|---|---|---|---|---|
| ΔG | k | ΔG | k | |
| CuII (H2O)4 | −24.01 | 4.67 × 109 | −4.67 | 1.33 × 108 |
| Hn−2COA2– (2)-C2 | −15.06 | 4.61 × 107 | 4.28 | 3.19 × 104 |
| Hn−2FLA2– (2)-C5 | −13.92 | 1.29 × 108 | 5.41 | 6.62 × 104 |
| Hn−2FLA2– (2)-C2 | −14.59 | 3.53 × 107 | 4.74 | 2.09 × 104 |
| Hn−2PRA2– (2)-C7 | 4.13 | 1.39 × 102 | 23.46 | 1.57 × 10−5 |
| Hn−2GAA2– (2)-C7 | 1.35 | 4.75 × 102 | 20.69 | 3.87 × 10−4 |
| PhAs-Cu(II) | ∆G | λ | ∆G≠ | k |
|---|---|---|---|---|
| Hn−2COA–2-(2)-C2 | −31.72 | 14.01 | 5.59 | 4.64 × 108 |
| Hn−2FLA–2-(2)-C5 | −28.01 | 17.36 | 1.63 | 7.36 × 109 |
| Hn−2FLA–2 -(2)-C2 | −35.61 | 14.76 | 7.37 | 2.46 × 107 |
| Hn−2PRA–2-(2)-C7 | −38.90 | 17.24 | 6.81 | 6.29 × 107 |
| Hn−2GAA–2-(2)-C7 | −39.36 | 17.41 | 6.92 | 5.21 × 107 |
| PhAs-Cu(II) | ∆G | k |
|---|---|---|
| Hn−2COA–2-(2)-C2 from H2O | −42.21 | 2.81 × 109 |
| Hn−2FLA–2-(2)-C5 from H2O | −41.90 | 8.07 × 109 |
| Hn−2FLA–2-(2)-C5 from FLA (site OCH3) | −23.63 | 8.06 × 109 |
| Hn−2FLA–2-(2)-C2 from H2O | −46.28 | 8.08 × 109 |
| Hn−2FLA–2-(2)-C2 from FLA (site OCH3) | −21.83 | 8.08 × 109 |
| Hn−2PRA–2-(2)-C7 from H2O | −46.80 | 7.96 × 109 |
| Hn−2GAA–2-(2)-C7 from GAA (site OH) | −51.76 | 8.03 × 109 |
| Hn−2GAA–2-(2)-C7 from H2O | −47.55 | 8.03 × 109 |
| PhAs-Cu(II) | ΔG | ΔG≠ | k | |
|---|---|---|---|---|
| Hn−2COA2–-(2)-C2 | RAF-1 | 11.18 | 17.53 | 8.76 × 10−1 |
| Hn−2COA2–-(2)-C2 | RAF-2 | 6.35 | 17.90 | 4.72 × 10−1 |
| Hn−2COA2–-(2)-C2 | RAF-3 | 2.19 | 12.46 | 4.57 × 103 |
| Hn−2COA2–-(2)-C2 | RAF-4 | 5.64 | 12.99 | 1.85 × 103 |
| Hn−2FLA2–-(2)-C5 | RAF-1 | 12.43 | 20.11 | 1.13 × 10−2 |
| Hn−2FLA2–-(2)-C5 | RAF-2 | NF | - | - |
| Hn−2FLA2–-(2)-C5 | RAF-3 | 4.18 | 17.81 | 5.44 × 10−1 |
| Hn−2FLA2–-(2)-C5 | RAF-4 | 1.80 | 14.54 | 1.37 × 102 |
| Hn−2FLA2–-(2)-C5 | RAF-5 | 7.38 | 18.15 | 3.06 × 10−1 |
| Hn−2FLA2–-(2)-C5 | RAF-6 | 5.07 | 16.21 | 8.17 × 100 |
| Hn−2FLA2–-(2)-C2 | RAF-1 | 13.57 | 18.67 | 1.28 × 10−1 |
| Hn−2FLA2–-(2)-C2 | RAF-2 | 6.29 | 18.36 | 2.14 × 10−1 |
| Hn−2FLA2–-(2)-C2 | RAF-3 | 0.84 | 14.43 | 1.63 × 102 |
| Hn−2FLA2–-(2)-C2 | RAF-4 | 5.90 | 11.70 | 1.64 × 104 |
| Hn−2FLA2–-(2)-C2 | RAF-5 | 6.72 | 15.12 | 5.09 × 101 |
| Hn−2FLA2–-(2)-C2 | RAF-6 | 5.60 | 15.80 | 1.61 × 101 |
| Hn−2PRA2–-(2)-C7 | RAF-1 | 9.83 | 18.93 | 8.24 × 10−2 |
| Hn−2PRA2–-(2)-C7 | RAF-2 | 8.12 | 16.83 | 2.88 × 100 |
| Hn−2PRA2–-(2)-C7 | RAF-3 | 7.65 | 13.14 | 1.44 × 103 |
| Hn−2PRA2–-(2)-C7 | RAF-4 | 6.18 | 12.53 | 4.05 × 103 |
| Hn−2PRA2–-(2)-C7 | RAF-5 | 10.26 | 17.98 | 4.12 × 10−1 |
| Hn−2PRA2–-(2)-C7 | RAF-6 | 5.52 | 14.53 | 1.38 × 102 |
| Hn−2GAA2–-(2)-C7 | RAF-1 | 11.60 | 20.12 | 1.10 × 10−2 |
| Hn−2GAA2–-(2)-C7 | RAF-2 | 8.25 | 18.85 | 9.40 × 10−2 |
| Hn−2GAA2–-(2)-C7 | RAF-3 | 10.66 | 15.74 | 1.79 × 101 |
| Hn−2GAA2–-(2)-C7 | RAF-4 | 6.53 | 13.42 | 9.05 × 102 |
| Hn−2GAA2–-(2)-C7 | RAF-5 | 7.30 | 17.40 | 1.09 × 100 |
| Hn−2GAA2–-(2)-C7 | RAF-6 | 8.74 | 17.03 | 2.05 × 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Arriaga, R.; Perez-Gonzalez, A.; Marino, T.; Russo, N.; Galano, A. Antioxidants into Nopal (Opuntia ficus-indica), Important Inhibitors of Free Radicals’ Formation. Antioxidants 2021, 10, 2006. https://doi.org/10.3390/antiox10122006
Castañeda-Arriaga R, Perez-Gonzalez A, Marino T, Russo N, Galano A. Antioxidants into Nopal (Opuntia ficus-indica), Important Inhibitors of Free Radicals’ Formation. Antioxidants. 2021; 10(12):2006. https://doi.org/10.3390/antiox10122006
Chicago/Turabian StyleCastañeda-Arriaga, Romina, Adriana Perez-Gonzalez, Tiziana Marino, Nino Russo, and Annia Galano. 2021. "Antioxidants into Nopal (Opuntia ficus-indica), Important Inhibitors of Free Radicals’ Formation" Antioxidants 10, no. 12: 2006. https://doi.org/10.3390/antiox10122006
APA StyleCastañeda-Arriaga, R., Perez-Gonzalez, A., Marino, T., Russo, N., & Galano, A. (2021). Antioxidants into Nopal (Opuntia ficus-indica), Important Inhibitors of Free Radicals’ Formation. Antioxidants, 10(12), 2006. https://doi.org/10.3390/antiox10122006