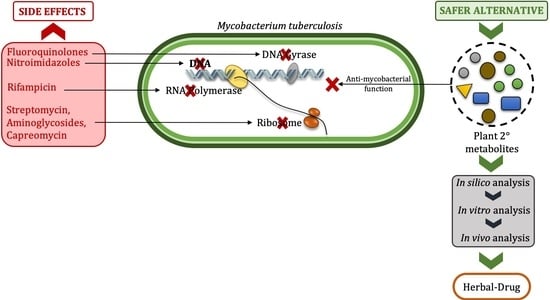
Potential Anti-Mycobacterium tuberculosis Activity of Plant Secondary Metabolites: Insight with Molecular Docking Interactions
Abstract
1. Introduction
2. Plant Secondary Metabolites as Antioxidant and Antimycobacterial Agents
3. Current Status and Severity of Tuberculosis
4. Management of MDR-Mtb: A Herbal Approach
5. Computational Analysis
5.1. Selection and Retrieval of Receptor Proteins
5.2. Selection and Retrieval of Ligand and Molecules
5.3. Docking Algorithm
5.4. Toxicity Assay of Test Ligands
6. Bioinformatics Opportunities for Medicinal Plant Studies
7. Concluding Remarks and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDR | multi-drug resistance |
| SMs | secondary metabolites |
| TB | tuberculosis |
| XDR | extensively drug resistance |
| TDR | total drug resistance |
| Mtb | Mycobacterium tuberculosis |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| ISN | isoniazid |
| EMB | ethambutol |
| INH | isonicotinic acid hydrazide |
| ATCC | American Type Culture Collection |
| PDB | Protein Data Bank |
| MOA | mechanism of action |
| RMSD | root mean square deviation |
References
- Chin, Y.-W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Ambrose, P.G.; Chambers, H.F.; Ebright, R.H.; Jezek, A.; Murray, B.E.; Newland, J.G.; Ostrowsky, B.; Rex, J.H.; Infectious Diseases Society of America. White Paper: Developing Antimicrobial Drugs for Resistant Pathogens, Narrow-Spectrum Indications, and Unmet Needs. J. Infect. Dis. 2017, 216, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jacobo-Herrera, N.J.; Altemimi, A.B.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- WHO. Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 26 October 2021).
- Temesgen, E.; Belete, Y.; Haile, K.; Ali, S. Prevalence of active tuberculosis and associated factors among people with chronic psychotic disorders at St. Amanuel Mental Specialized Hospital and Gergesenon Mental Rehabilitation center, Addis Ababa, Ethiopia. BMC Infect. Dis. 2021, 21, 1100. [Google Scholar] [CrossRef]
- Sharma, D.; Yadav, J.P. An Overview of Phytotherapeutic Approaches for the Treatment of Tuberculosis. Mini Rev. Med. Chem. 2016, 17, 167–183. [Google Scholar] [CrossRef]
- Bunalema, L.; Fotso, G.W.; Waako, P.; Tabuti, J.; Yeboah, S.O. Potential of Zanthoxylum leprieurii as a source of active compounds against drug resistant Mycobacterium tuberculosis. BMC Complement. Altern. Med. 2017, 17, 89. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Pathan, S.K. In silico PASS analysis and determination of antimycobacterial, antifungal, and antioxidant efficacies of maslinic acid in an extract rich in pentacyclic triterpenoids. Int. J. Mycobacteriol. 2016, 5, 417–425. [Google Scholar] [CrossRef]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017, 17, 133. [Google Scholar] [CrossRef]
- Robinson, M.M.; Zhang, X. The World Medicines Situation 2011, Traditional Medicines: Global Situation, Issues and Challenges; World Health Organization: Geneva, Switzerland, 2011; pp. 1–2. [Google Scholar]
- Teoh, E.S. Secondary Metabolites of Plants. In Medicinal Orchids of Asia; Springer: Cham, Switzerland, 2016; pp. 59–73. [Google Scholar]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, J.A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiototics 2020, 9, 170. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Venkatachalam, R.; Kalimuthu, K.; Chinnadurai, V.; Saravanan, M.; Radhakrishnan, R.; Shanmuganathan, R.; Pugazhendhi, A. Various solvent effects on phytochemical constituent profiles, analysis of antioxidant and antidiabetic activities of Hopea parviflora. Process. Biochem. 2020, 89, 227–232. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Reis, S.; Segundo, M.A. Kinetic matching approach applied to ABTS assay for high-throughput determination of total antioxidant capacity of food products. J. Food Compos. Anal. 2014, 33, 187–194. [Google Scholar] [CrossRef]
- Pavithra, G.M.; Siddiqua, S.; Naik, A.S.; TR, P.K.; Vinayaka, K.S. Antioxidant and antimicrobial activity of flowers of Wendlandia thyrsoidea, Olea dioica, Lagerstroemia speciosa and Bombax malabaricum. J. Appl. Pharmac. Sci. 2013, 3, 114. [Google Scholar] [CrossRef]
- Yahia, Y.; Benabderrahim, M.A.; Tlili, N.; Bagues, M.; Nagaz, K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS ONE 2020, 15, e0232599. [Google Scholar] [CrossRef]
- Wagay, N.A.; Khan, N.A.; Rothe, S.P. Profiling of secondary metabolites and antimicrobial activity of Crateva religiosa G. Forst. bark-A rare medicinal plant of Maharashtra India. Int. J. Biosci. 2017, 10, 343–354. [Google Scholar] [CrossRef]
- Köksal, E.; Tohma, H.; Kılıç, Ö.; Alan, Y.; Aras, A.; Gülçin, I.; Bursal, E. Assessment of Antimicrobial and Antioxidant Activities of Nepeta trachonitica: Analysis of Its Phenolic Compounds Using HPLC-MS/MS. Sci. Pharm. 2017, 85, 24. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Elvin-Lewis, M.; Lewis, W.H. New Concepts and Medical and Dental Ethnobotany; Dioscorides Press: Portland, OR, USA, 1995; pp. 303–310. [Google Scholar]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Reyburn, H.; Mtove, G.; Hendriksen, I.; Von Seidlein, L. Oral quinine for the treatment of uncomplicated malaria. BMJ 2009, 339, b2066. [Google Scholar] [CrossRef] [PubMed]
- Rodney, C.; Toni, M.; Kutchan, N.; Lewis, G. Biochemistry and Molecular Biology of Plants. In Natural Products; Buchanan, B., Gruissem, W., Jones, R., Eds.; Wiley: Rockville, MD, USA, 2000; pp. 1253–1348. [Google Scholar]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Perez, D.R.; Lim, W.; Seiler, J.P.; Yi, G.; Peiris, M.; Shortridge, K.F.; Webster, R.G. Role of Quail in the Interspecies Transmission of H9 Influenza A Viruses: Molecular Changes on HA That Correspond to Adaptation from Ducks to Chickens. J. Virol. 2003, 77, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; De Campos, R.O.; Miguel, O.G.; Filho, V.C.; Siani, A.C.; Yunes, R.A.; Calixto, J.B. Antinociceptive properties of extracts of new species of plants of the genus Phyllanthus (Euphorbiaceae). J. Ethnopharmacol. 2000, 72, 229–238. [Google Scholar] [CrossRef]
- Nichenametla, S.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A Review of the Effects and Mechanisms of Polyphenolics in Cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- Prats, E.; Galindo, J.C.; Bazzalo, M.E.; León, A.; Macías, F.A.; Rubiales, D.; Jorrín, J.V. Antifungal Activity of a New Phenolic Compound from Capitulum of a Head Rot-resistant Sunflower Genotype. J. Chem. Ecol. 2007, 33, 2245–2253. [Google Scholar] [CrossRef]
- Okunade, A.L.; Hufford, C.D.; Clark, A.M.; Lentz, D. Antimicrobial properties of the constituents of Piper aduncum. Phytotherapy Research: An International. J. Devoted Med. Sci. Res. Plants Plant Prod. 1997, 11, 142–144. [Google Scholar]
- Kato, T.; Tsuda, H.; Ishitani, Y.; Takemura, Y.; Suzuki, Y. 6-Acetyl-8-hydroxy-2,2-dimethylchromene, an Antioxidant in Sunflower Seeds; Its Isolation and Synthesis and Antioxidant Activity of Its Derivatives. Heterocycles 1997, 44, 139. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of Cancer Cell Proliferation in Vitro by Fruit and Berry Extracts and Correlations with Antioxidant Levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef]
- Swargiary, A.; Verma, A.K.; Singh, S.; Roy, M.K.; Daimari, M. Antioxidant and Antiproliferative Activity of Selected Medicinal Plants of Lower Assam, India: An In Vitro and In Silico Study. Anti-Cancer Agents Med. Chem. 2021, 21, 267–277. [Google Scholar] [CrossRef]
- Meghashree, K.S.; Latha, K.P.; Vagdevi, H.M. Antioxidant and antitubercular activities of leaf extracts of Canthium dicoccum (Gaertn.) and Amischophacelus axillaris(L.). Indian J. Nat. Prod. Resour. 2021, 11, 244–249. [Google Scholar]
- Hussain, K.; Ismail, Z.; Sadikun, A.; Ibrahim, P. Antioxidant, anti-TB activities, phenolic and amide contents of standardised extracts of Piper sarmentosum Roxb. Nat. Prod. Res. 2009, 23, 238–249. [Google Scholar] [CrossRef]
- Trevizan, L.N.F.; do Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; do Carmo Vieira, M.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuber-culosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.). Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar]
- Khlifi, D.; Hamdi, M.; El Hayouni, A.; Cazaux, S.; Souchard, J.P.; Couderc, F.; Bouajila, J. Global Chemical Composition and Antioxidant and Anti-Tuberculosis Activities of Various Extracts of Globularia alypum L. (Globulariaceae) Leaves. Molecules 2011, 16, 10592–10603. [Google Scholar] [CrossRef]
- Tawde, K.; Gacche, R.; Pund, M. Evaluation of selected Indian traditional folk medicinal plants against Mycobacterium tuberculosis with antioxidant and cytotoxicity study. Asian Pac. J. Trop. Dis. 2012, 2, S685–S691. [Google Scholar] [CrossRef]
- Janmanchi, H.; Raju, A.; Degani, M.; Ray, M.; Rajan, M. Antituberculosis, antibacterial and antioxidant activities of Aegiceras corniculatum, a mangrove plant and effect of various extraction processes on its phytoconstituents and bioactivity. S. Afr. J. Bot. 2017, 113, 421–427. [Google Scholar] [CrossRef]
- Qamar, M.T.U.; Maryam, A.; Muneer, I.; Xing, F.; Ashfaq, U.A.; Khan, F.A.; Anwar, F.; Geesi, M.H.; Khalid, R.R.; Rauf, S.A.; et al. Computational screening of medicinal plant phytochemicals to discover potent pan-serotype inhibitors against dengue virus. Sci. Rep. 2019, 9, 1433. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Gemma, S. Structure-Based Design of Drugs and Other Bioactive Molecules: Tools and Strategies; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Sandeep, G.; Nagasree, K.P.; Hanisha, M.; Kumar, M.M.K. AUDocker LE: A GUI for virtual screening with AUTODOCK Vina. BMC Res. Notes 2011, 4, 445. [Google Scholar] [CrossRef]
- Singh, H.; Bharadvaja, N. Treasuring the computational approach in medicinal plant research. Prog. Biophys. Mol. Biol. 2021, 164, 19–32. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Baker, W. Tuberculosis: A search for novel therapy starting with natural products. Med. Res. Rev. 1998, 18, 363–374. [Google Scholar] [CrossRef]
- Gautam, R.; Saklani, A.; Jachak, S.M. Indian medicinal plants as a source of antimycobacterial agents. J. Ethnopharmacol. 2007, 110, 200–234. [Google Scholar] [CrossRef]
- Miryala, S.K.; Basu, S.; Naha, A.; Debroy, R.; Ramaiah, S.; Anbarasu, A.; Natarajan, S. Identification of bioactive natural compounds as efficient inhibitors against Mycobacterium tuberculosis protein-targets: A molecular docking and molecular dynamics simulation study. J. Mol. Liq. 2021, 341, 117340. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sarma, G.; Janardhanam, B.; Ramachandran, P.; Santha, T.; Sivasubramanian, S.; Somasundaram, P.; Tripathy, S. Hepatic toxicity in south indian patients during treatment of tuberculosis with short-course regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle 1986, 67, 99–108. [Google Scholar] [CrossRef]
- Mohan, A.; Sharma, S.K. Side effects of antituberculosis drugs. Am. J. Respir. Crit. Care Med. 2004, 169, 882–883. [Google Scholar] [CrossRef]
- Yew, W.W.; Leung, C.C. Antituberculosis drugs and hepatotoxicity. Respirology 2006, 11, 699–707. [Google Scholar] [CrossRef]
- Goldberger, M.J. Antituberculosis Agents. Med. Clin. N. Am. 1988, 72, 661–668. [Google Scholar] [CrossRef]
- Tostmann, A.; Boeree, M.J.; Aarnoutse, R.E.; De Lange, W.C.; van der Ven, A.J.; Dekhuijzen, R. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol. 2008, 23, 192–202. [Google Scholar] [CrossRef]
- Yee, D.; Valiquette, C.; Pelletier, M.; Parisien, I.; Rocher, I.; Menzies, D. Incidence of Serious Side Effects from First-Line Antituberculosis Drugs among Patients Treated for Active Tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Arellanes, A.; Meckes, M.; Ramírez, R.; Torres, J.; Luna-Herrera, J. Activity against multidrug-resistantMycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytotherapy Res. 2003, 17, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Fauziyah, P.N.; Sukandar, E.Y.; Ayuningtyas, D.K. Combination Effect of Antituberculosis Drugs and Ethanolic Extract of Selected Medicinal Plants against Multi-Drug Resistant Mycobacterium tuberculosis Isolates. Sci. Pharm. 2017, 85, 14. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Narayanasamy, M.; Feussner, K.-D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Meckes, M.; Torres, J.; Luna-Herrera, J. Antimycobacterial triterpenoids from Lantana hispida (Verbenaceae). J. Ethnopharmacol. 2007, 111, 202–205. [Google Scholar] [CrossRef]
- Erdemoglu, N.; Sener, B.; Palittapongarnpim, P. Antimycobacterial Activity ofTaxus baccata. Pharm. Biol. 2003, 41, 614–615. [Google Scholar] [CrossRef][Green Version]
- Ignacimuthu, S.; Shanmugam, N. Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica Ness. leaves. J. Biosci. 2010, 35, 565–570. [Google Scholar] [CrossRef]
- Madikizela, B.; Aderogba, M.; Finnie, J.; Van Staden, J. Isolation and characterization of antimicrobial compounds from Terminalia phanerophlebia Engl. & Diels leaf extracts. J. Ethnopharmacol. 2014, 156, 228–234. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Ganesh, M.; Peng, M.M.; Aziz, A.S.; Jang, H.T. Comparative antioxidant and antimycobacterial activities ofOpuntia ficus-indicafruit extracts from summer and rainy seasons. Front. Life Sci. 2015, 8, 182–191. [Google Scholar] [CrossRef]
- Mohamad, S.; Zin, N.M.; Wahab, H.A.; Ibrahim, P.; Sulaiman, S.F.; Zahariluddin, A.S.M.; Noor, S.S.M. Antituberculosis potential of some ethnobotanically selected Malaysian plants. J. Ethnopharmacol. 2011, 133, 1021–1026. [Google Scholar] [CrossRef]
- Luo, X.; Pires, D.; Aínsa, J.A.; Gracia, B.; Duarte, N.; Mulhovo, S.; Anes, E.; Ferreira, M.-J.U. Zanthoxylum capense constituents with antimycobacterial activity against Mycobacterium tuberculosis in vitro and ex vivo within human macrophages. J. Ethnopharmacol. 2013, 146, 417–422. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.C.; Gouveia, C.A.; Carvalho, M.J.; Rodrigues, A.I.P.C.; Nording, M.L.; Castilho, P.C. Antioxidant Capacity, Cytotoxicity and Antimycobacterial Activity of Madeira Archipelago Endemic Helichrysum Dietary and Medicinal Plants. Antioxidants 2014, 3, 713–729. [Google Scholar] [CrossRef]
- Jyoti, A.; Nam, K.-W.; Jang, W.S.; Kim, Y.-H.; Kim, S.-K.; Lee, B.-E.; Song, H.-Y. Antimycobacterial activity of methanolic plant extract of Artemisia capillaris containing ursolic acid and hydroquinone against Mycobacterium tuberculosis. J. Infect. Chemother. 2016, 22, 200–208. [Google Scholar] [CrossRef]
- Fadipe, V.O.; Mongalo, N.I.; Opoku, A.R.; Dikhoba, P.M.; Makhafola, T.J. Isolation of anti-mycobacterial compounds from Curtisia dentata (Burm.f.) C.A.Sm (Curtisiaceae). BMC Complement. Altern. Med. 2017, 17, 306. [Google Scholar] [CrossRef]
- León-Díaz, R.; Meckes, M.; Said-Fernández, S.; Molina-Salinas, G.M.; Vargas-Villarreal, J.; Torres, J.; Luna-Herrera, J.; Jiménez-Arellanes, A. Antimycobacterial neolignans isolated from Aristolochia taliscana. Memórias Inst. Oswaldo Cruz 2010, 105, 45–51. [Google Scholar] [CrossRef]
- Prabu, A.; Seenivasan, P.; Kumar, V. Antimycobacterial activity of certain mangrove plants against multi-drug resistant Mycobacterium tuberculosis. Asian J. Med. Sci. 2014, 5, 54–57. [Google Scholar] [CrossRef]
- Kirimuhuzya, C.; Waako, P.; Joloba, M.; Odyek, O. The anti-mycobacterial activity of Lantana camara a plant traditionally used to treat symptoms of tuberculosis in South-western Uganda. Afr. Health Sci. 2009, 9, 40–45. [Google Scholar]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G.; Otchere, I.; Kissi-Twum, A. Antimycobacterial and cytotoxic activity of selected medicinal plant extracts. J. Ethnopharmacol. 2016, 182, 10–15. [Google Scholar] [CrossRef]
- Gupta, P.; Bhatter, P.; D’Souza, D.; Tolani, M.; Daswani, P.; Tetali, P.; Birdi, T. Evaluating the anti Mycobacterium tuberculosis activity of Alpinia galanga (L.) Willd. axenically under reducing oxygen conditions and in intracellular assays. BMC Complement. Altern. Med. 2014, 14, 84. [Google Scholar] [CrossRef]
- Nirmal, C.R.; Ebenezer, R.S.; Kannan, P.; Balasubramanian, M.; Thirunavukkarasu, I.; Mondal, R.; Dusthackeer, A. Anti-tuberculosis activity of bio-active compounds from Lantana camara L., Euphorbia hirta L., Mukia maderaspatana (L.) M. Roem, and Abutilon indicum (L.). Eur. J. Integr. Med. 2020, 35, 101105. [Google Scholar] [CrossRef]
- Martini, M.C.; Zhang, T.; Williams, J.T.; Abramovitch, R.B.; Weathers, P.J.; Shell, S.S. Artemisia annua and Artemisia afra extracts exhibit strong bactericidal activity against Mycobacterium tuberculosis. J. Ethnopharmacol. 2020, 262, 113191. [Google Scholar] [CrossRef] [PubMed]
- Assam, J.P.A.; Tcham, M.F.Y.; Moni, N.E.D.F.; Betote, D.P.H.; Fossi, T.C.; Penlap, B.V. Phytochemical screening, Antimycobacterial activity of three medicinal Cameroonians plants and Acute toxicity of hydroethanolic extract of Vitellaria paradoxa. J. Drug Deliv. Ther. 2020, 10, 96–104. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Uc-Cachón, A.H.; Peña-Rodríguez, L.M.; Beh, A.D.J.D.; Gracía-Medrano, R.M.E.; García-Medrano, R.M.E. Bactericidal Effect of the Leaf Extract from Musa spp. (AAB Group, Silk Subgroup), cv. “Manzano” Against Multidrug-Resistant Mycobacterium tuberculosis. J. Med. Food 2019, 22, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Chavez, A.C.; Salazar, A.; Zepeda-Vallejo, L.G.; Jesús, M.D.L.H.D.; Quintos-Escalante, M.; Vargas-Díaz, M.E.; Luna-Herrera, J. Trixis angustifolia hexanic extract displays synergistic antibacterial activity against M. tuberculosis. Nat. Prod. Res. 2017, 33, 1477–1481. [Google Scholar] [CrossRef]
- Hernández-García, E.; García, A.; Garza-González, E.; Alanís, F.G.A.; Rivas-Galindo, V.M.; Rodríguez-Rodríguez, J.; Alcantar-Rosales, V.M.; Delgadillo-Puga, C.; Camacho-Corona, M.D.R. Chemical composition of Acacia farnesiana (L) wild fruits and its activity against Mycobacterium tuberculosis and dysentery bacteria. J. Ethnopharmacol. 2019, 230, 74–80. [Google Scholar] [CrossRef]
- Kahaliw, W.; Aseffa, A.; Abebe, M.; Teferi, M.; Engidawork, E. Evaluation of the antimycobacterial activity of crude extracts and solvent fractions of selected Ethiopian medicinal plants. BMC Complement. Altern. Med. 2017, 17, 143. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, H. Antitubercular activity and phytochemical screening of selected medicinal plants. Orient. J. Chem. 2015, 31, 597–600. [Google Scholar] [CrossRef]
- Rahgozar, N.; Khaniki, G.B.; Sardari, S. Evaluation of Antimycobacterial and Synergistic Activity of Plants Selected Based on Cheminformatic Parameters. Iran. Biomed. J. 2018, 22, 401–407. [Google Scholar] [CrossRef]
- Sudjarwo, S.A.; Wardani, G.; Eraiko, K. The potency of Pinus merkusii extract nanoparticles as anti Mycobacterium tuber-culosis: An in vitro study. Int. J. Nutr. Pharmacol. Neurol. Dis. 2019, 9, 48. [Google Scholar] [CrossRef]
- Bhagat, V.C.; Kondawar, M.S. Antitubercular Potential of Dendrophthoe Falcate (L.) and Tridax Procumbens (L.) Plants Extracts Against H37rv Stain of Mycobacteria Tuberculosis. Int. J. Pharmaceut. Sci. Res. 2019, 10, 51–259. [Google Scholar]
- Tiam, E.R.; Bikobo, D.S.N.; Zintchem, A.A.A.; Ii, N.M.N.; Ndedi, E.D.F.M.; Diboué, P.H.B.; Nyegue, M.A.; Atchadé, A.D.T.; Pegnyemb, D.E.; Bochet, C.G.; et al. Secondary metabolites from Triclisia gilletii (De Wild) Staner (Menispermaceae) with antimycobacterial activity against Mycobacterium tuberculosis. Nat. Prod. Res. 2017, 33, 642–650. [Google Scholar] [CrossRef]
- Komape, N.P.M.; Bagla, V.P.; Kabongo-Kayoka, P.; Masoko, P. Anti-mycobacteria potential and synergistic effects of combined crude extracts of selected medicinal plants used by Bapedi traditional healers to treat tuberculosis related symptoms in Limpopo Province, South Africa. BMC Complement. Altern. Med. 2017, 17, 128. [Google Scholar] [CrossRef]
- Vaidya, S.; Sharma, J.; Maniar, J.; Prabhu, N.; Mamawala, M.; Joshi-Pundit, S.; Chowdhary, A. Assessment of anti-tuberculosis activity of extracts of cinnamomum verum and solanun surattense along with isoniazid. BMC Complement. Alt. Med. 2018, 18, 5. [Google Scholar] [CrossRef]
- Mohamad, S.; Ismail, N.N.; Parumasivam, T.; Ibrahim, P.; Osman, H.; Wahab, H.A. Antituberculosis activity, phytochemical identification of Costus speciosus (J. Koenig) Sm., Cymbopogon citratus (DC. Ex Nees) Stapf. and Tabernaemontana coronaria (L.) Willd. and their effects on the growth kinetics and cellular integrity of Mycobacterium tuberculosis H37Rv. BMC Complement. Altern. Med. 2018, 18, 5. [Google Scholar] [CrossRef]
- Jang, W.S.; Jyoti, A.; Kim, S.; Nam, K.-W.; Ha, T.K.Q.; Oh, W.K.; Song, H.-Y. In vitro antituberculosis activity of diterpenoids from the Vietnamese medicinal plant Croton tonkinensis. J. Nat. Med. 2015, 70, 127–132. [Google Scholar] [CrossRef]
- Choi, W.H.; Lee, I.A. The anti-tubercular activity of Melia azedarach L. and Lobelia chinensis Lour. and their potential as effective anti-Mycobacterium tuberculosis candidate agents. Asian Pac. J. Trop. Biomed. 2016, 6, 830–835. [Google Scholar] [CrossRef]
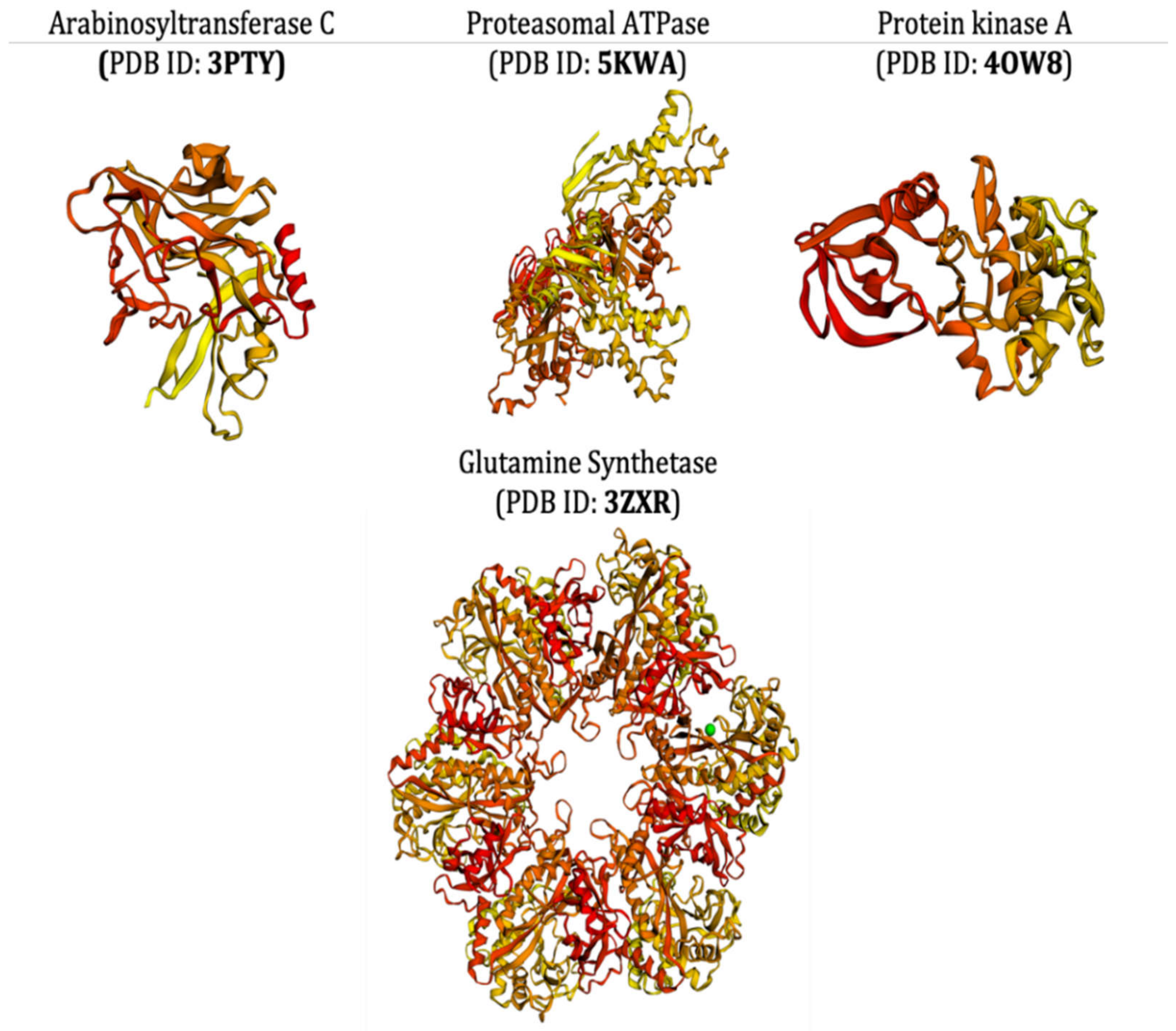
- Das, N.; Jena, P.K.; Pradhan, S.K. Arabinosyltransferase C enzyme of Mycobacterium tuberculosis, a potential drug target: An insight from molecular docking study. Heliyon 2020, 6, e02693. [Google Scholar] [CrossRef]
- Sundar, S.; Thangamani, L.; Manivel, G.; Kumar, P.; Piramanayagam, S. Molecular docking, molecular dynamics and MM/PBSA studies of FDA approved drugs for protein kinase a of Mycobacterium tuberculosis; application insights of drug repurposing. Inform. Med. Unlocked 2019, 16, 100210. [Google Scholar] [CrossRef]
- Gising, J.; Nilsson, M.T.; Odell, L.R.; Yahiaoui, S.; Lindh, M.; Iyer, H.; Sinha, A.M.; Srinivasa, B.R.; Larhed, M.; Mowbray, S.L.; et al. Trisubstituted Imidazoles as Mycobacterium tuberculosis Glutamine Synthetase Inhibitors. J. Med. Chem. 2012, 55, 2894–2898. [Google Scholar] [CrossRef]
- Pearce, M.J.; Arora, P.; Festa, R.A.; Butler-Wu, S.M.; Gokhale, R.S.; Darwin, K.H. Identification of substrates of the Mycobacterium tuberculosis proteasome. EMBO J. 2006, 25, 5423–5432. [Google Scholar] [CrossRef]
- Kumar, M.; Chung, S.-M.; Enkhtaivan, G.; Patel, R.; Shin, H.-S.; Mistry, B. Molecular Docking Studies and Biological Evaluation of Berberine–Benzothiazole Derivatives as an Anti-Influenza Agent via Blocking of Neuraminidase. Int. J. Mol. Sci. 2021, 22, 2368. [Google Scholar] [CrossRef]
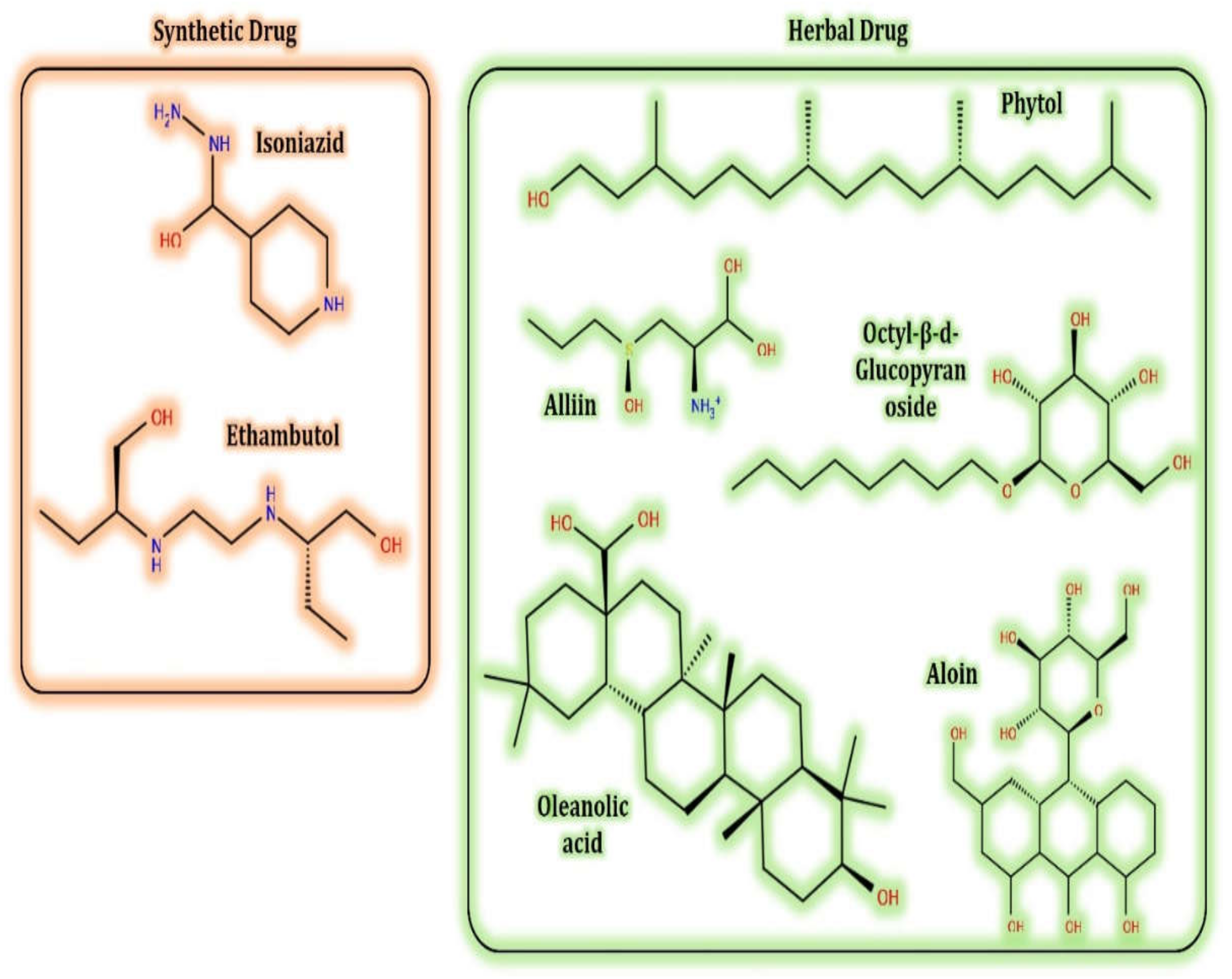
- Quintero-Fabian, S.; Ortuño-Sahagún, D.; Vázquez-Carrera, M.; López-Roa, R.I. Alliin, a Garlic (Allium sativum) Compound, Prevents LPS-Induced Inflammation in 3T3-L1 Adipocytes. Mediat. Inflamm. 2013, 2013, 381815. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evidence-Based Complement. Altern. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef]
- Bergfield, W.F. Final report of the cosmetic ingredient review. Int. J. Toxicol. 2007, 26, 1–50. [Google Scholar]
- Groom, Q.J.; Reynolds, T. Barbaloin inAloeSpecies. Planta Med. 1987, 53, 345–348. [Google Scholar] [CrossRef]
- Dinesh, M.; Roopan, S.M.; Selvaraj, C.I. Photocatalytic degradation of nitrophenol using biologically active Phyllanthus emblica seed extract. J. Photochem. Photobiol. B Biol. 2016, 161, 273–278. [Google Scholar] [CrossRef]
- Phillips, O.A.; Udo, E.E.; Varghese, R. Antimycobacterial Activities of Novel 5-(1H-1,2,3-Triazolyl)Methyl Oxazolidinones. Tuberc. Res. Treat. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Ramos-Guerra, M.C.; Vargas-Villarreal, J.; Mata-Cárdenas, B.D.; Becerril-Montes, P.; Said-Fernández, S. Bactericidal Activity of Organic Extracts from Flourensia cernua DC against Strains of Mycobacterium tuberculosis. Arch. Med. Res. 2006, 37, 45–49. [Google Scholar] [CrossRef]
- Prakash, P.; Kumari, N.; Gayathiri, E.; Selvam, K.; Ragunathan, M.G.; Chandrasekaran, M.; Al-Dosary, M.A.; Hatamleh, A.A.; Nadda, A.K.; Kumar, M. In Vitro and In Silico Toxicological Properties of Natural Antioxidant Therapeutic Agent Azima tetracantha. LAM. Antioxidants 2021, 10, 1307. [Google Scholar] [CrossRef]
- Romano, J.D.; Tatonetti, N.P. Informatics and Computational Methods in Natural Product Drug Discovery: A Review and Perspectives. Front. Genet. 2019, 10, 368. [Google Scholar] [CrossRef]
- Sharma, V.; Sarkar, I.N. Bioinformatics opportunities for identification and study of medicinal plants. Brief. Bioinform. 2013, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Babar, M.; Zaidi, N.-U.-S.S.; Pothineni, V.R.; Ali, Z.; Faisal, S.; Hakeem, K.; Gul, A. Application of Bioinformatics and System Biology in Medicinal Plant Studies. In Springer Protocols Handbooks; Springer: Cham, Switzerland, 2017; pp. 375–393. [Google Scholar]
| Generic Names | Medicinal Compound | Side Effects | Mode of Action | References |
|---|---|---|---|---|
| Isoniazid, isoniazide, azuren, INH, L 1945, Mybasan, neumadin, RP 5015, tubomel, vazadrine, isoniazidum | Isonicotinic acid hydrazide (isoniazid) | Hepatotoxic (hepatitis, nausea, vomiting, and decreased appetite) | Suppresses the multiplication of mycobacteria | [54] |
| Streptomicina, streptomycin, streptomycine, strepidin-4-α-streptobiosaminosid, streptomycin sulfate, streptomycini sulfas, streptomycinsulfat | Streptomycin | Ototoxicity | Inhibition of protein synthesis of mycobacteria in the ribosome | [55] |
| Ethionamide, TH 1314, aethionamidum, Bayer 5312, etionizina, ETP, ethionamidum | Ethionamide | Hepatitis, depression, hypersensitivity | A prodrug that is activated by the enzyme ethA, a mono-oxygenase in Mycobacterium tuberculosis; binds NAD+ to form an adduct that inhibits InhA in the same way as isoniazid | |
| Cycloserine, lilly 106-7, MK 65, PA 94, Ro 1-9213, SC 49088, cicloserina, cycloserinum | Cycloserine | Psychosis, rashes | Cycloserine is a broad-spectrum antibiotic with only moderate anti-TB activity. It inhibits cell wall synthesis. The MIC of cycloserine in the Bactec 460-TB system is 25–75 μg/mL | |
| Capreomycin sulfate, capreomycin, CAM, capromycin, L 29275 | Capreomycin | Deafness, vestibular toxicity | Inhibit protein synthesis by binding to the 70S ribosomal unit | |
| Kanamicina, kanamycin, kanamycine, kanamycin monosulfate, kanamycin sulfate, kanamycin acid sulfate, kanamycin monosulfate, kanamycinmonosulfat | Kanamycin | Deafness, nephrotoxic | Inhibits protein synthesis by tightly binding to the conserved A site of 16S rRNA in the 30S ribosomal subunit | |
| Rifampicin | Rifampicin | Hepatotoxic, interaction with other drugs, a potent inducer of microsomal enzymes | Inhibits bacterial DNA-dependent RNA synthesis by inhibiting bacterial DNA-dependent RNA polymerase | [56] |
| Pirazinamide, pyrazinamide, pyrazinecarboxamide, pyrazinoic acid amide, pyrizinamide, pyrazinamidum | Pyrazinamide | Hepatitis, Hyperuricemia, arthralgia, arthritis | It diffuses into the granuloma of M. tuberculosis, where the tuberculosis enzyme pyrazinamidase converts pyrazinamide to the active form of pyrazinoic acid | |
| Ethambutol, ethambutolo, ethambutol hydrochloride, CL 40881, ethambutol hydrochloride, ethambutoldihydrochlorid, ethambutoli hydrochloridum | Ethambutol | Optic neuritis | It works by obstructing the formation of the cell wall. Mycolic acids attach to the 5’-hydroxyl groups of D-arabinose residues of arabinogalactan and form mycolylarabinogalactan-peptidoglycan complex in the cell wall | [57] |
| Protionamide, PTH, PTP, RP, protionamidum, prothionamide | Prothionamide | Hepatotoxic, hypersensitivity, idiosyncrasy | It is activated by mono-oxygenase (EthA), forms covalent adducts with nicotinamide adenine dinucleotide (NAD), and inhibits InhA, leading to blocking of the mycolic acid pathway | |
| P.A.S., Para-aminosalicylic acid, pasalicylum, aminosalicylic acid, aminosalicylate sodium, para-aminosalicylsaures natrium-2-wasser, parasal sodium, sodium para-aminosalicylate, natrii aminosalicylas dihydricus, sodium aminosalicylate dihydrate | Para-aminosalicylic acid | Hepatotoxic, hypersensitivity, idiosyncrasy | It targets dihydrofolate reductase (DHFR); it is incorporated into the folate pathway by two enzymes, dihydropteroate synthase (DHPS) and dihydrofolate synthase (DHFS) to produce a hydroxyl dihydrofolate compound that inhibits DHFR, and subsequently blocks the folate pathway |
| Plant (Bioactive Compound) | Extract | Mtb | MIC | References |
|---|---|---|---|---|
| Lantana hispida (-acetoxy-22-(2′-methyl-2Z-butenyloxy)-12-oleanen-28-oic acid, hydroxy-22β-(2′-methyl-2Z-butenoyloxy)-12-oleanen-28-oic acid and oleanolic acid) | Hexane extract | Mycobacterium tuberculosis strain H37Rv | 50, 50 and 25 μg/mL respectively | [61] |
| Taxus baccata | Chloroform extract of heartwood and ethanolic extract of leaves | M. tuberculosis strain H37Ra | 200 μg/mL | [62] |
| Adhatoda vasica (2-acetyl benzylamine and vasicine acetate) | Hexane extract | Mtb | 200 and 50 μg/mL, respectively | [63] |
| Terminalia phanerophlebia | Ethanolic extract of leaves | M. tuberculosis H37Ra | 390 μg/mL | [64] |
| Opuntia ficus-indica | Methanolic extract of the plant (summer season) | M. tuberculosis strain H37Rv (ATCC 27294) | 50 μg/mL | [65] |
| Angiopteris evecta | Methanolic extract of leaves | M. tuberculosis H37Rv | 400 μg/mL | [66] |
| Costus speciosus, Piper sarmentosum, Pluchea indica, Pluchea indica, and Tabernaemontana coronaria | Methanolic extract | 800 μg/mL | ||
| Zanthoxylum capense (Decarine) | Methanolic extract of roots | M. tuberculosis H37Ra (ATCC 25177) and M. tuberculosis H37Rv (ATCC 27294) | 1.6 μg/mL | [67] |
| Helichrysum devium | Methanolic extract | M. tuberculosis H37Rv | 50 μg/mL | [68] |
| H. melaleucum | 100 μg/mL | |||
| H. obconicum | 200 μg/mL | |||
| Artemisia capillaris (hydroquinone and ursolic acid) | Methanolic extract | M. tuberculosis strain H37Rv and two clinical isolates (resistant and sensitive) | 12.5 μg/mL against sensitive strains of Mtb while a range of 12.5 to 25 μg/mL against the resistant strains | [69] |
| Curtisia dentata | Methanolic extract of leaves | M. tuberculosis H37RV (ATCC 27294) | 22.2 μg/mL | [70] |
| Curtisia dentata (ursolic acid acetate) | Ethanolic extract | 3.4 µg/mL | ||
| Aristolochia taliscana (Licarin A) | Hexane extract | M. tuberculosis strains: H37Rv, four mono-resistant H37Rv variants and 12 clinical MDR isolates | 3.12–12.5 μg/mL | [71] |
| Excoecaria agallocha | Methanolic extract | M. tuberculosis H37Rv and two clinical isolates of Mtb | 88.95% of antimycobacterial activity against M. tuberculosis H37Rv while 70.02% and 82.54% for other two isolates at 500 µg/mL concentration | [72] |
| Lantana camara | Chloroform and methanol extracts of leaves | Mycobacterium tuberculosis H37Rv, rifampicin-resistant TMC-331 and a non-resistant wildstrain (28–25271 | 5.0 mg/mL to 50.0 mg/mL | [73] |
| Solanum torvum Sw. | hydro-ethanolic extracts | Mycobacterium tuberculosis H37Ra | 156.3 µg/mL | [74] |
| Alpinia galanga L. Willd. | Acetone, aqueous and ethanolic extracts of rhizomes | Mycobacterium tuberculosis (M.tb) H37Rv | 50–100 μg/mL | [75] |
| Lantana camara L., Euphorbia hirta L., Mukia maderaspatana (L.) M. Roem, and Abutilon indicum L. | Methanolic crude extracts | Mycobacterium tuberculosis (Mtb) and Mtb H37Rv | 400–1600 μg/mL | [76] |
| Artemisia annua | Dichloromethane extracts | Mycobacterium tuberculosis (Mtb), Mycobacterium abscessus | 37.5 µg/mL | [77] |
| and A. afra | <1.3 µg/mL | |||
| Zingiber officinale | Hydroethanolic extract of rhizomes | M. tuberculosis H37Rv (ATCC 27294) | 1250 μg/mL | [78] |
| Vitellaria paradoxa | Hydroethanolic extract of bark | 78.13 μg/mL | ||
| Alstonia boonei | 156 μg/mL | |||
| Musa spp. AAB, cv. ‘‘Manzano’’ | n-hexane extract and ethyl acetate extract | Mycobacterium tuberculosis | 12.5 and 6.25 l g/mL | [79] |
| Trixis angustifolia | Hexane extract | Mycobacterium tuberculosis H37Rv | 12.5- 25.0 μg/mL | [80] |
| Acacia farnesiana | hexane, chloroform and methanolic extracts | Mycobacterium tuberculosis H37Rv and G122 | 100–200 µg/mL | [81] |
| Pterolobium stellatum (Forssk) | Chloroform extracts | M. tuberculosis strain H37RV | 0.312 mg/mL | [82] |
| Persea americana Mill L. | 2.5 mg/mL | |||
| Otostegia integrifolia Benth L. | 0.312 mg/mL | |||
| Aegle marmelos L, Glycyrrhiza glabra L, Lawsonia inermis L, Piper nigrum L, and Syzygium aromaticum L. | Methanolic extract | M. tuberculosis strain H37RV | 0.8 to 100 μg/mL | [83] |
| Boswellia serrata Roxb. ex, Datura stramonium L and Lavandula stoechas L. | Ethanolic extracts | M. tuberculosis strain H37RV | 125 to 250 μg/mL | [84] |
| Pinus merkusii | Ethanolic extract | Mycobacterium tuberculosis H37Rv | 1000 µg/mL | [85] |
| Dendrophthoe falcata L. | Ethanol water and methanol: water extracts | Mycobacterium tuberculosis (H37Rv strain) | 6.25 μg/mL | [86] |
| Tridax procumbens L. | 0.8 μg/mL | |||
| Triclisia gilletii | Methanol extract | Mycobacterium tuberculosis | 3.90 to 62.5 μg/mL | [87] |
| Combretum hereroense | Hexane, dichloromethane, methanol, and acetone | M. smegmatis (ATCC 1441), M. tuberculosis (ATCC H37Rv) | 1.6 mg/mL and 1.3 mg/mL | [88] |
| Citrus lemon | 0.3 mg/mL | |||
| Apodytes dimidiata | 1.3 mg/mL | |||
| Cinnamomum verum | Aqueous & methanolic extracts | Mycobacterium tuberculosis H37Rv | 10 mcg/mL | [89] |
| Solanum surattense | ||||
| Costus speciosus, Cymbopogon citratus, and Tabernaemontana coronaria | Methanol extracts | Mycobacterium tuberculosis H37Rv | 100–200 μg/mL | [90] |
| Croton tonkinensis | Methylene chloride extracts | M. tuberculosis H37Ra, H37Rv | 6.25 and 12.5 µg/mL | [91] |
| Melia azedarach L. and Lobelia chinensis Lour. | Methanol and n-hexane extract | M. tuberculosis | 100 µg/mL | [92] |
| Interaction | Ligand | Interaction | |
|---|---|---|---|
| 3pty | |||
| Alliin |  | Aloin |  |
| EMB | 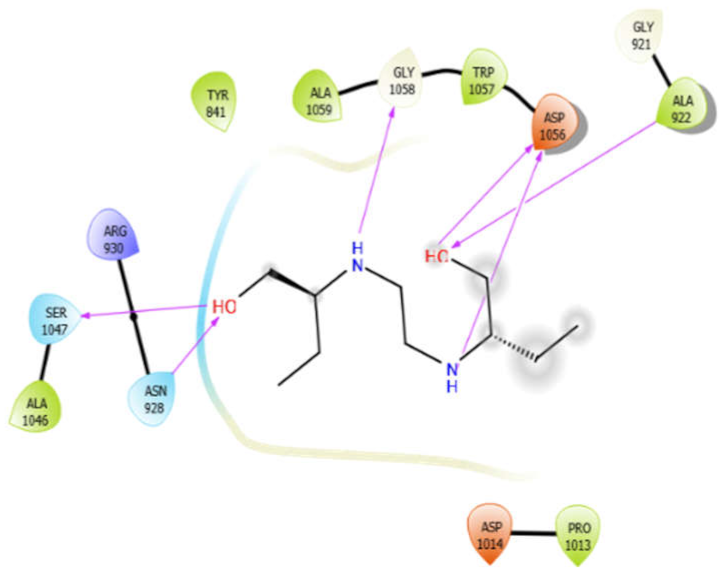 | ISN | 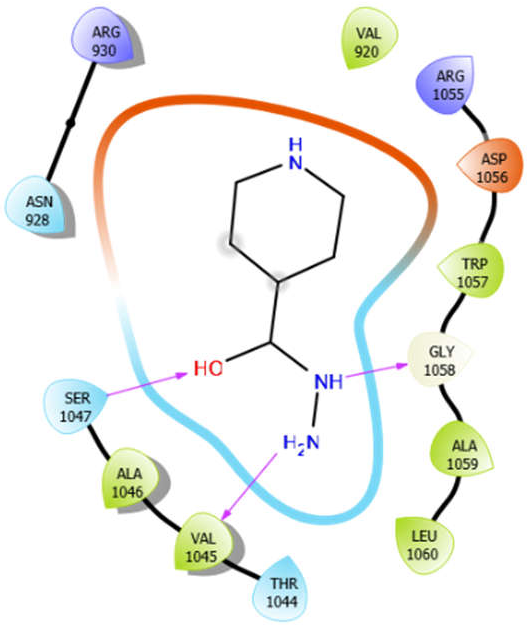 |
| Octyl-β-d-Glucopyranoside | 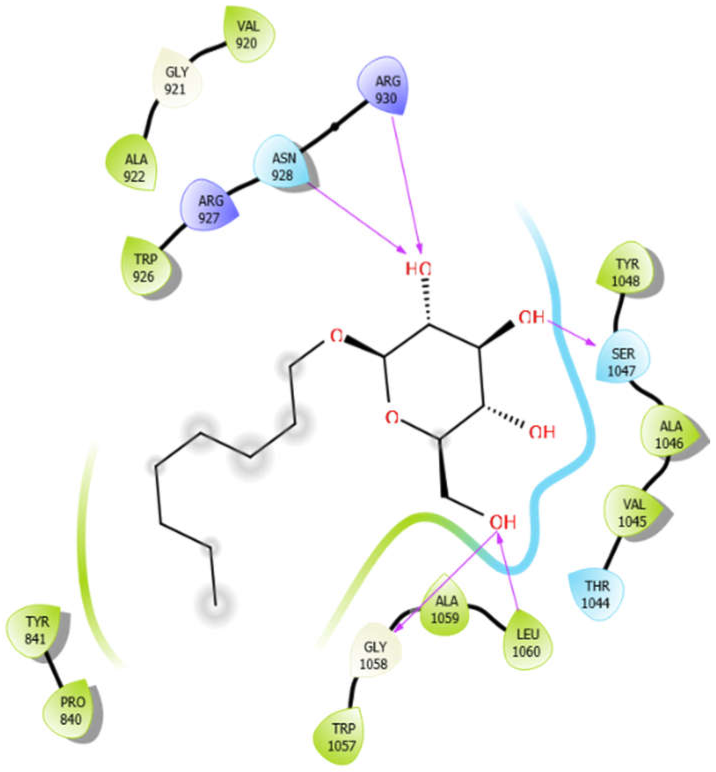 | Oleanolic acid | 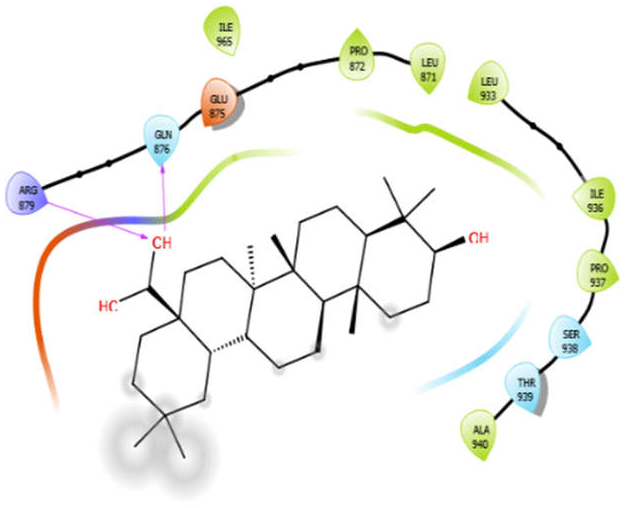 |
| Phytol | 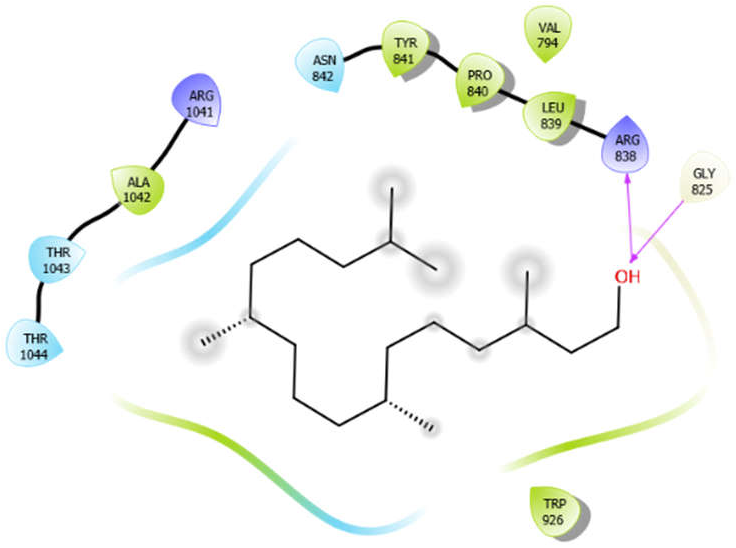 | ||
| 3zxr | |||
| Alliin | 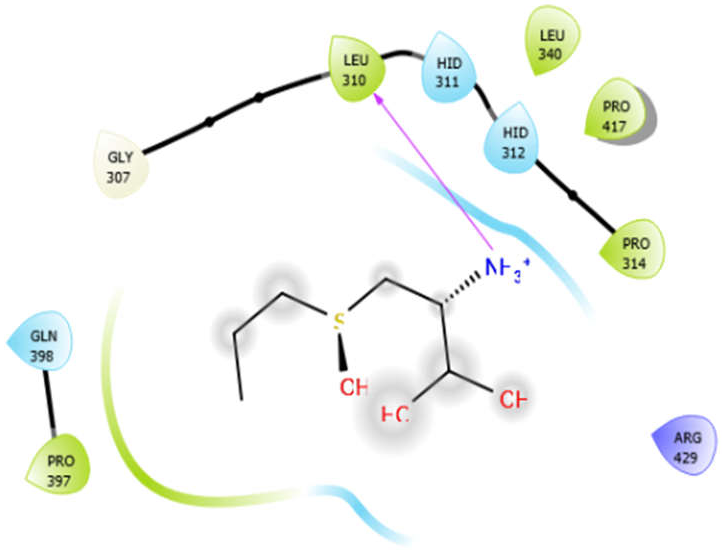 | Aloin | 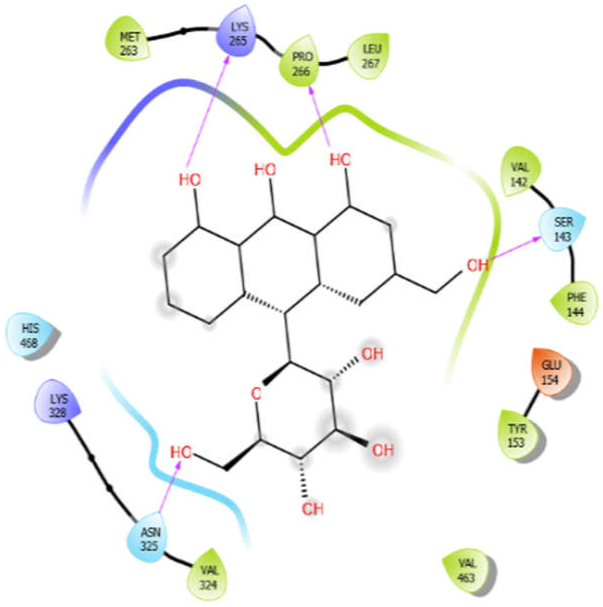 |
| EMB | 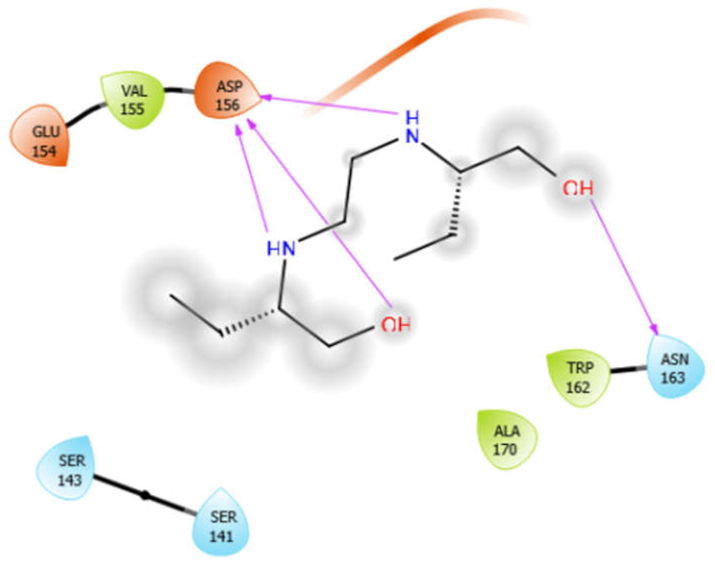 | ISN |  |
| Octyl-β-d-Glucopyranoside | 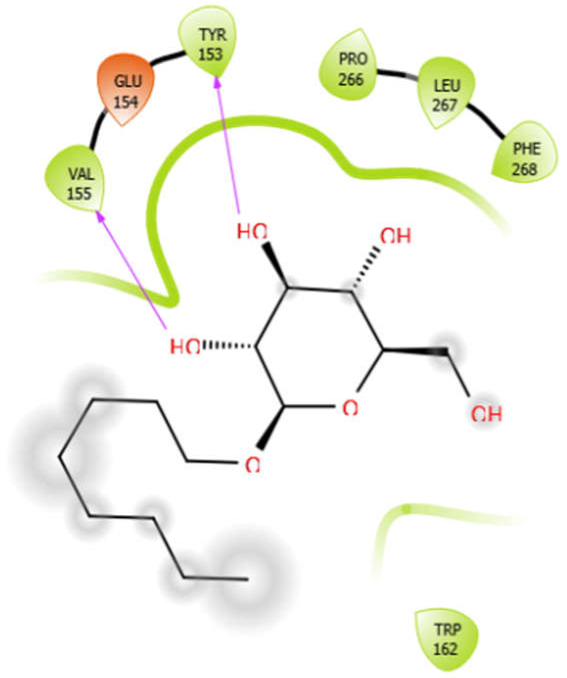 | Oleanolic acid | 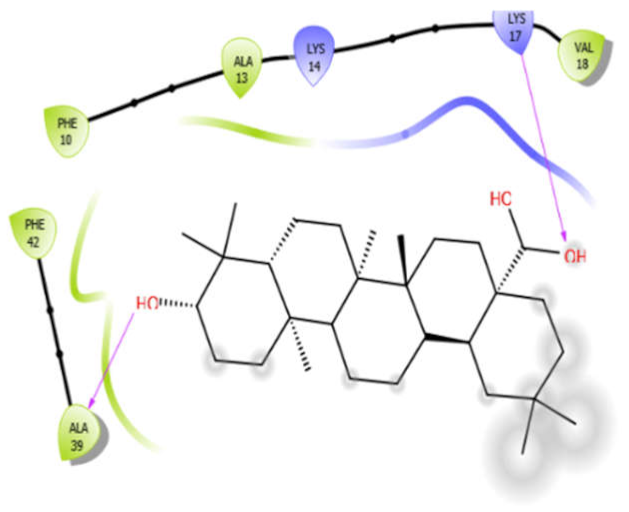 |
| Phytol | 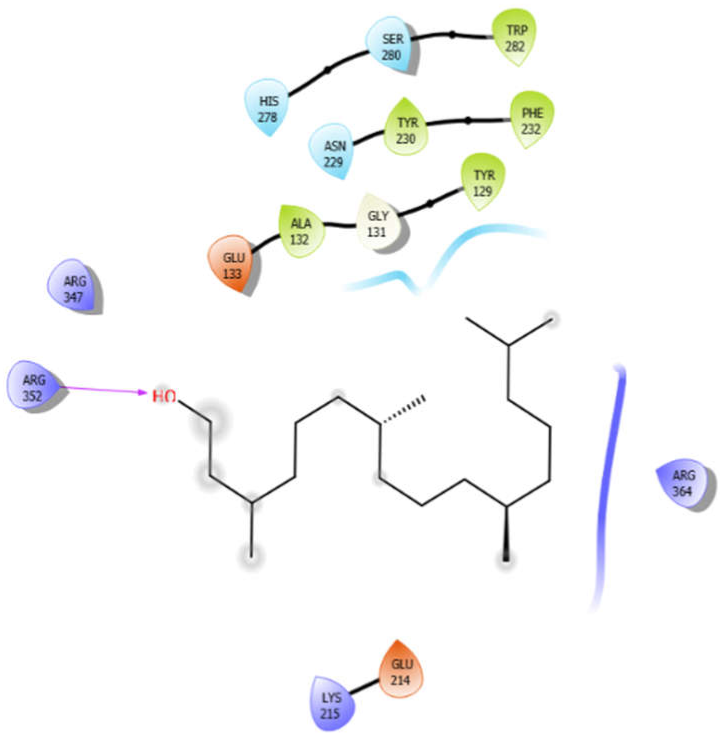 | ||
| 4ow8 | |||
| Alliin |  | Aloin | 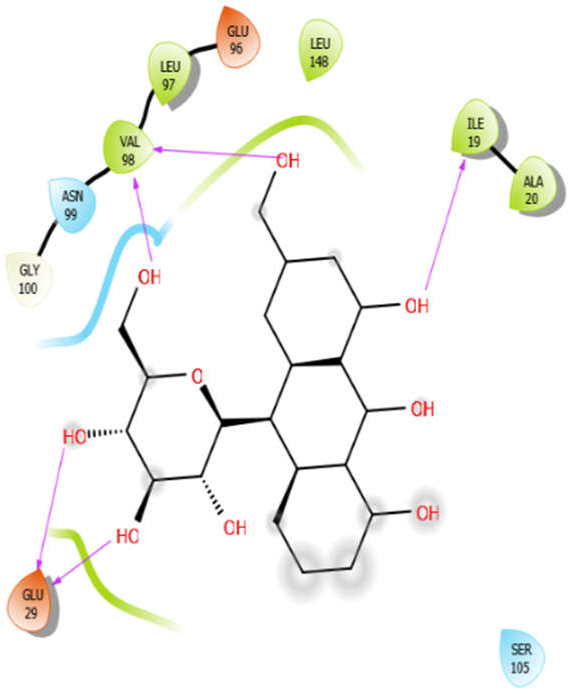 |
| EMB | 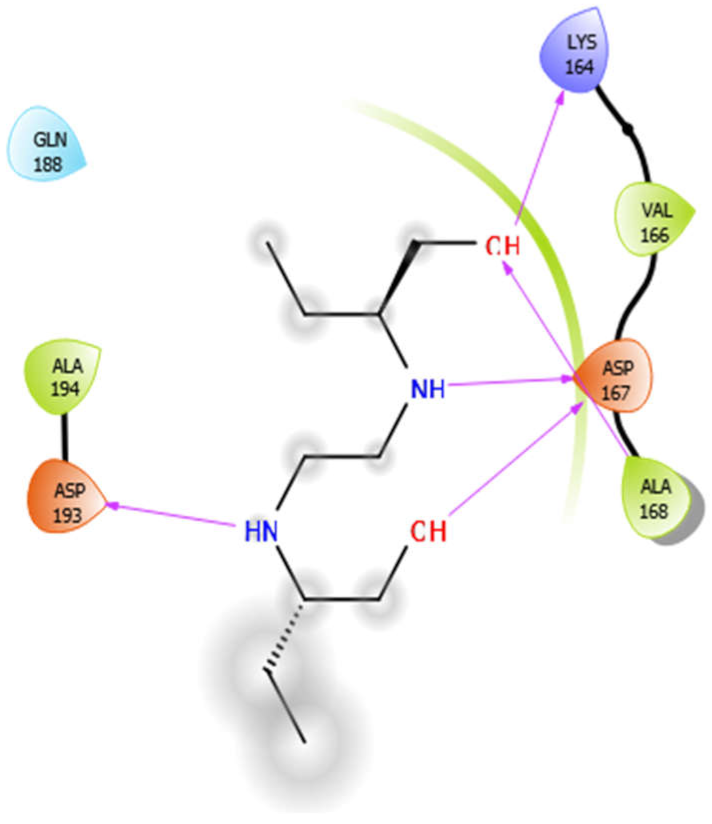 | ISN | 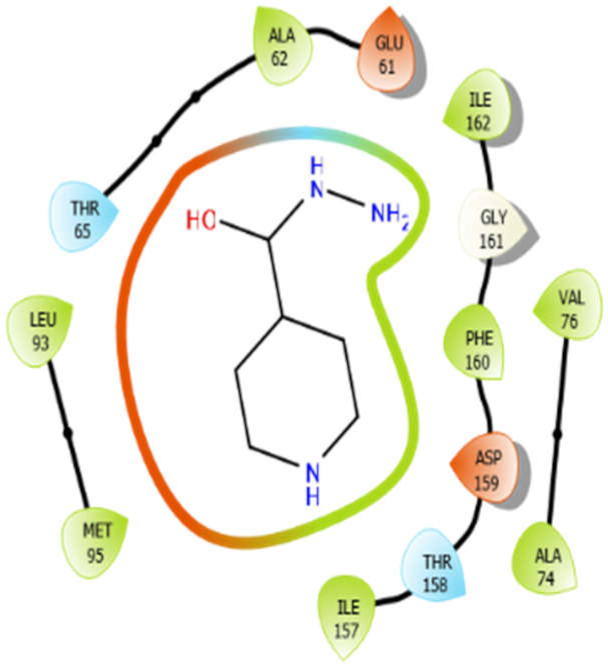 |
| Octyl-β-d-Glucopyranoside | 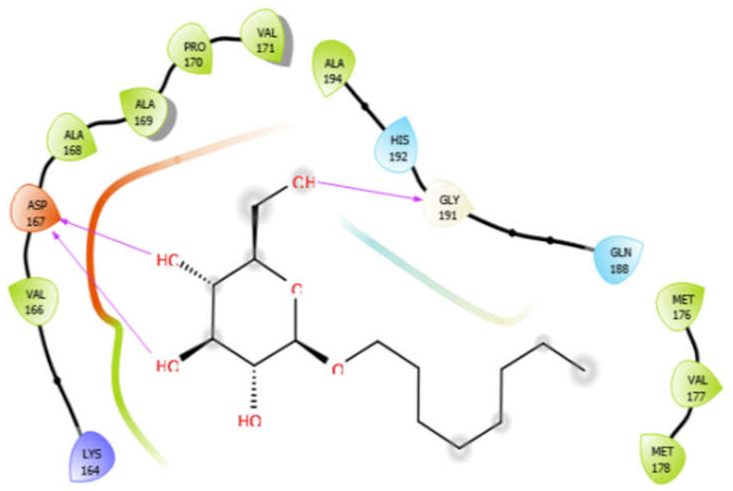 | Oleanolic acid | 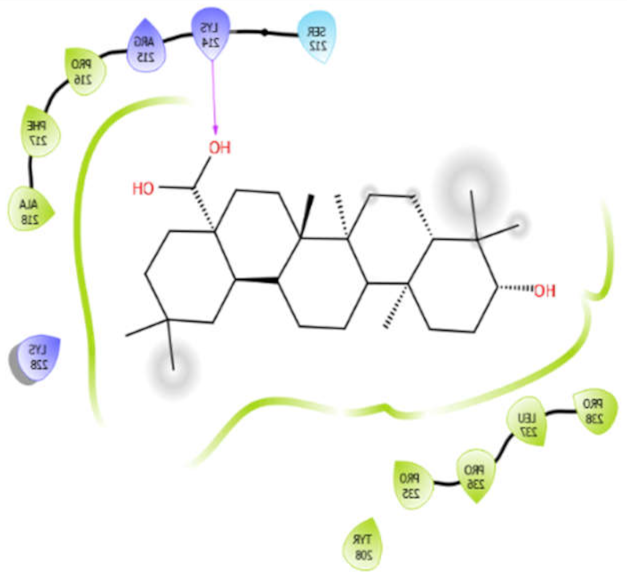 |
| Phytol | 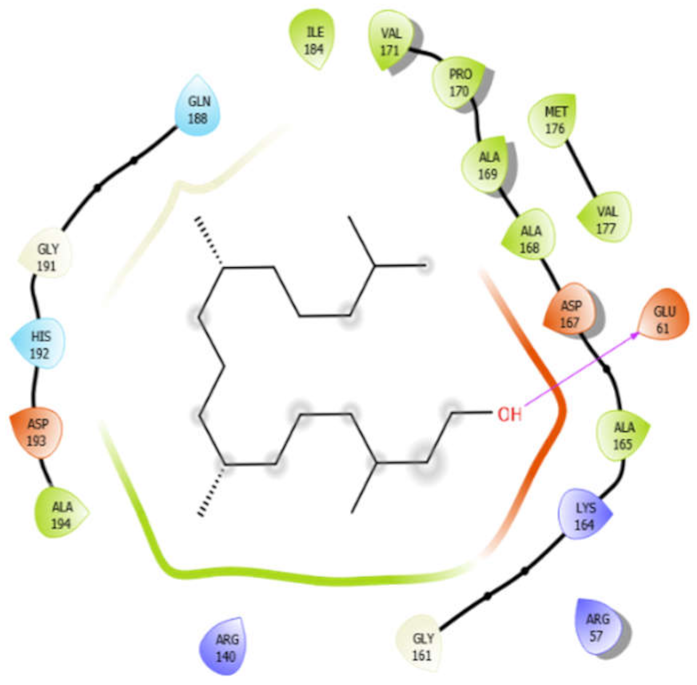 | ||
| 5kwa | |||
| Alliin | 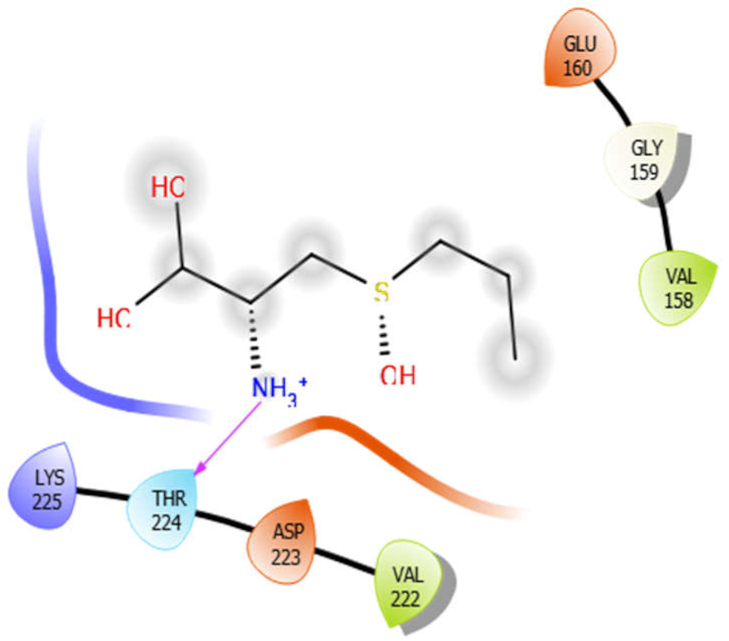 | Aloin | 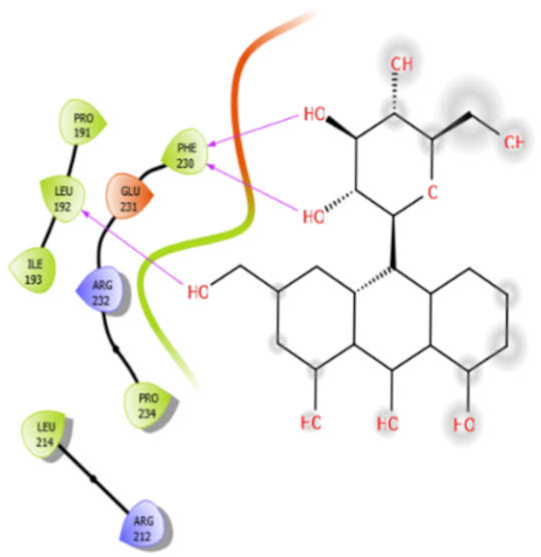 |
| EMB |  | ISN | 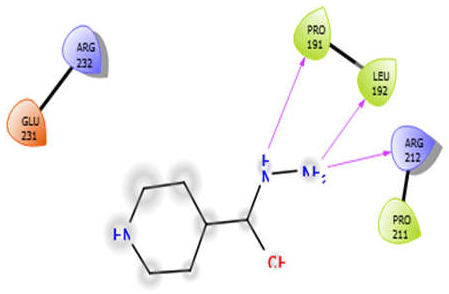 |
| Octyl-β-d-Glucopyranoside |  | Oleanolic acid | 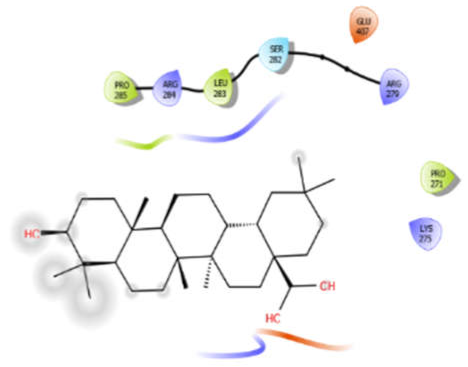 |
| Phytol | 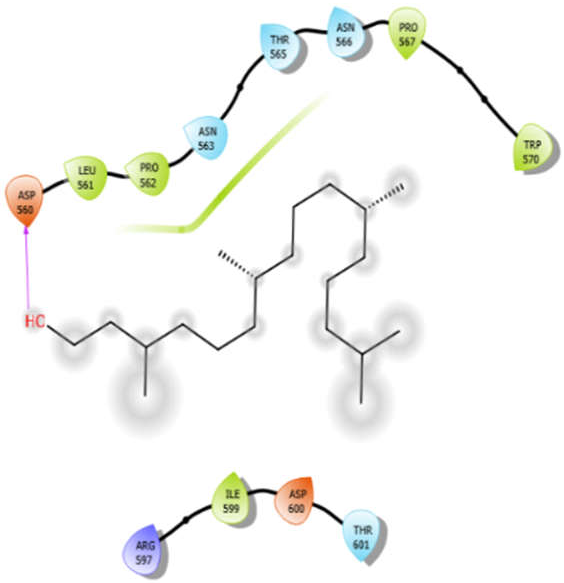 | ||
| Interactions | Bond Type | Resides and Their Legends | Binding Energy (kcal/mol) |
|---|---|---|---|
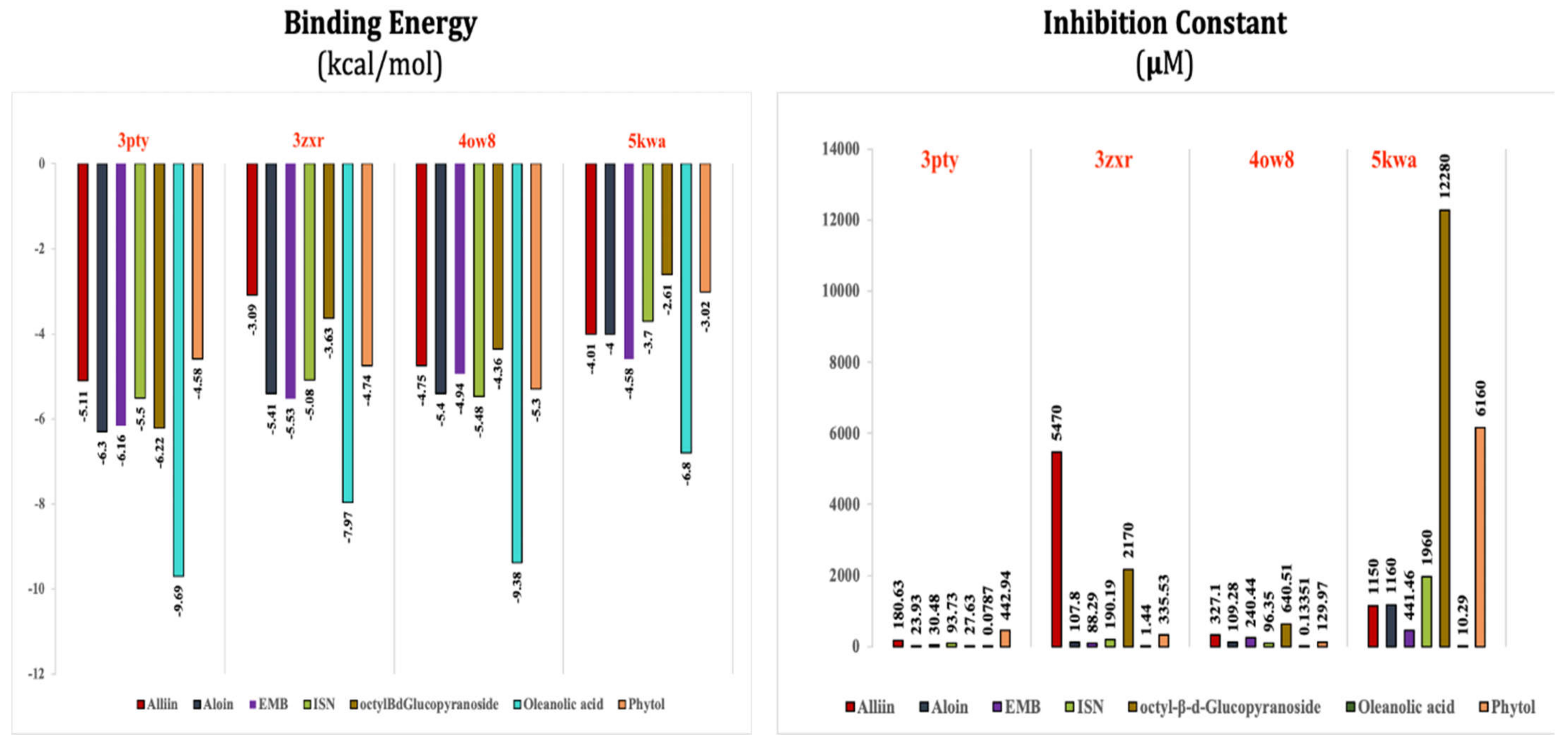
| Alliin with 3pty | Hydrogen bond | GLY921, GLY1058 | −5.11 |
| Hydrophobic bond | VAL920, TRP1057 | ||
| Polar bond | ASN928, SER1047 | ||
| Charged bond | ARG927, ARG930, ARG1055, ASP1056 | ||
| Aloin with 3pty | Hydrophobic bond | LEU871, PRO872, LEU933, ILE936, ALA940 | −6.3 |
| Polar bond | THR873, GUN876, SER934, SER938 | ||
| Charged bond | GLU875, ARG879 | ||
| EMB with 3pty | Hydrogen bond | GLY921, GLY1058 | −6.16 |
| Hydrophobic bond | TYR841, ALA922, PRO1013, ALA1046, TRP1057, ALA1059, | ||
| Polar bond | ASN928, SER1047 | ||
| Charged bond | ARG930, ASP1014, ASP1056 | ||
| ISN with 3pty | Hydrogen bond | GLY1058 | −5.5 |
| Hydrophobic bond | VAL920, VAL1045, ALA1046, TRP1057, ALA1059, LEU1060 | ||
| Polar bond | ASN928, THR1044, SER1047 | ||
| Charged bond | ARG930, ARG1055, ASP1056 | ||
| Octyl-β-d-Glucopyranoside with 3pty | Hydrogen bond | GLY921, GLY1058 | −6.22 |
| Hydrophobic bond | PRO840, TYR841, VAL920, ALA922, TRP926, VAL1045, ALA1046, TYR1048, TRP1057, ALA1059, LEU1060 | ||
| Polar bond | ASN928, THR1044, SER1047 | ||
| Charged bond | ARG927, ARG930 | ||
| Oleanolic acid with 3pty | Hydrophobic bond | LEU871, PRO872, LEU933, ILE936, PRO937, ALA940, ILE965 | −9.69 |
| Polar bond | GLN876, SER938, THR939 | ||
| Charged bond | GLU875, ARG879 | ||
| Phytol with 3pty | Hydrogen bond | GLY825 | −4.58 |
| Hydrophobic bond | ALA1042, TYR841, PRO840, LEU839, TRP926 | ||
| Polar bond | THR1043, THR1044, ASN842 | ||
| Charged bond | ARG1041, ARG838 | ||
| Alliin with 3zxr | Hydrogen bond | GLY307 | −3.09 |
| Hydrophobic bond | LEU310, PRO314, LEU340, PRO417, PRO397 | ||
| Polar bond | HID311, HID312, GLN398 | ||
| Charged bond | ARG429 | ||
| Aloin with 3zxr | Hydrophobic bond | MET263, PRO266, LEU267, VAL142, PHE144, TYR153, VAL463, VAL324 | −5.41 |
| Polar bond | SER143, HIS468, HIS468, ASN325 | ||
| Charged bond | LYS265, LYS328, GLU154 | ||
| EMB with 3zxr | Hydrophobic bond | VAL155, TRP162, ALA170 | −5.53 |
| Polar bond | ASN163 | ||
| Charged bond | GLU154, ASP156 | ||
| ISN with 3zxr | Hydrogen bond | GLY177 | −5.08 |
| Hydrophobic bond | TYR178, PRO174, PRO191 | ||
| Polar bond | ASN175, GLN194 | ||
| Charged bond | LSY179, ARG176, GLU169 | ||
| Octyl-β-d-glucopyranoside with 3zxr | Hydrophobic bond | VAL155, TYR153, PRO266, LEU267, PHE268, TRP162 | −3.63 |
| Polar bond | HIS182 | ||
| Charged bond | GLU154 | ||
| Oleanolic acid with 3zxr | Hydrophobic bond | PHE10, ALA13, VAL18, PHE42, ALA39 | −7.97 |
| Charged bond | LYS14, LYS17 | ||
| Phytol with 3zxr | Hydrogen bond | GLY131 | −4.74 |
| Hydrophobic bond | TRP282, TYR230, PHE232, ALA132, TYR129 | ||
| Polar bond | HIS278, SER280, ASN229 | ||
| Charged bond | GLU133, ARG347, ARG352, ARG364, GLU214, LYS215 | ||
| Alliin with 4ow8 | Hydrogen bond | GLY191 | −4.75 |
| Hydrophobic bond | ALA194 | ||
| Polar bond | GLN188, HID192 | ||
| Charged bond | ASP193, LYS164, ARG140 | ||
| Aloin with 4ow8 | Hydrogen bond | GLY100 | −5.4 |
| Hydrophobic bond | VAL98, LEU97, LEU148, ILE19, ALA20 | ||
| Polar bond | ASN99 | ||
| Charged bond | GLU96, GLU29 | ||
| EMB with 4ow8 | Hydrophobic bond | ALA194, VAL166, ALA168 | −4.94 |
| Polar bond | GLN188 | ||
| Charged bond | ASP193, LYS164, ASP167 | ||
| ISN with 4ow8 | Hydrogen bond | GLY161 | −5.48 |
| Hydrophobic bond | MET95, LEU93, ALA62, ILE162, PHE160, ILE157, VAL76, ALA74 | ||
| Polar bond | THR65, THR158 | ||
| Charged bond | GLU61, ASP159 | ||
| Octyl-β-d-glucopyranoside with 4ow8 | Hydrogen bond | GLY191 | −4.36 |
| Hydrophobic bond | VAL166, ALA168, ALA169, PRO170, VAL171, ALA194, MET176, VAL177, MET178 | ||
| Polar bond | HIS192, GLN188 | ||
| Charged bond | LYS164, ASP167 | ||
| Oleanolic acid with 4ow8 | Hydrophobic bond | PRO216, PHE217, ALA218, PRO238, LEU237, PRO236, PRO235, TYR208 | −9.38 |
| SER212 | |||
| Polar bond | LYS214, ARG215, LYS228 | ||
| Phytol with 4ow8 | Hydrogen bond | GLY191, GLY161 | −5.3 |
| Hydrophobic bond | ALA194, ILE184, VAL171, PRO170, ALA169, ALA168, ALA165, MET176, VAL177 | ||
| Polar bond | GLN188, HIS192 | ||
| Charged bond | ASP193, ASP167, LYS164, GLU61, ARG57, ARG140 | ||
| Alliin with 5kwa | Hydrogen bond | GLY159 | −4.01 |
| Hydrophobic bond | VAL158, VAL222 | ||
| Polar bond | THR224 | ||
| Charged bond | GLU160, LYS225, ASP223 | ||
| Aloin with 5kwa | Hydrophobic bond | PRO191, LEU192, ILE193, PHE230, PRO234, LEU214 | −4.0 |
| Charged bond | GLU231, ARG232, ARG212 | ||
| EMB with 5kwa | Hydrophobic bond | LEU241, LEU243 | −4.58 |
| Polar bond | ASN331 | ||
| Charged bond | ASP240, GLU244, GLU245, GLU336, LYS333 | ||
| ISN with 5kwa | Hydrophobic bond | PRO191, LEU192, PRO211 | −3.7 |
| Charged bond | ARG232, GLU231, ARG212 | ||
| Octyl-β-d-glucopyranoside with 5kwa | Hydrogen bond | GLY296, GLY298, GLY516 | −2.61 |
| Hydrophobic bond | CYS297, LEU301, ALA517 | ||
| Polar bond | THR300, ASN416 | ||
| Charged bond | LYS299, ASP371, GLU372 | ||
| Oleanolic acid with 5kwa | Hydrophobic bond | PRO285, LEU283, PRO271 | −6.8 |
| Polar bond | SER282 | ||
| Charged bond | ARG284, ARG279, GLU407, LYS275 | ||
| Phytol with 5kwa | Hydrophobic bond | LEU561, PRO562, PRO567, TRP570, ILE599 | −3.02 |
| Polar bond | ASN563, THR565, ASN566, THR601 | ||
| Charged bond | ASP560, ARG597, ASP600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Singh, S.K.; Singh, P.P.; Singh, V.K.; Rai, A.C.; Srivastava, A.K.; Shukla, L.; Kesawat, M.S.; Kumar Jaiswal, A.; Chung, S.-M.; et al. Potential Anti-Mycobacterium tuberculosis Activity of Plant Secondary Metabolites: Insight with Molecular Docking Interactions. Antioxidants 2021, 10, 1990. https://doi.org/10.3390/antiox10121990
Kumar M, Singh SK, Singh PP, Singh VK, Rai AC, Srivastava AK, Shukla L, Kesawat MS, Kumar Jaiswal A, Chung S-M, et al. Potential Anti-Mycobacterium tuberculosis Activity of Plant Secondary Metabolites: Insight with Molecular Docking Interactions. Antioxidants. 2021; 10(12):1990. https://doi.org/10.3390/antiox10121990
Chicago/Turabian StyleKumar, Manu, Sandeep Kumar Singh, Prem Pratap Singh, Vipin Kumar Singh, Avinash Chandra Rai, Akhileshwar Kumar Srivastava, Livleen Shukla, Mahipal Singh Kesawat, Atul Kumar Jaiswal, Sang-Min Chung, and et al. 2021. "Potential Anti-Mycobacterium tuberculosis Activity of Plant Secondary Metabolites: Insight with Molecular Docking Interactions" Antioxidants 10, no. 12: 1990. https://doi.org/10.3390/antiox10121990
APA StyleKumar, M., Singh, S. K., Singh, P. P., Singh, V. K., Rai, A. C., Srivastava, A. K., Shukla, L., Kesawat, M. S., Kumar Jaiswal, A., Chung, S.-M., & Kumar, A. (2021). Potential Anti-Mycobacterium tuberculosis Activity of Plant Secondary Metabolites: Insight with Molecular Docking Interactions. Antioxidants, 10(12), 1990. https://doi.org/10.3390/antiox10121990