Powdered Green Tea (Matcha) Attenuates the Cognitive Dysfunction via the Regulation of Systemic Inflammation in Chronic PM2.5-Exposed BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Animals and In Vivo Experimental Design
2.4. Behavioral Test
2.4.1. Y-Maze Test
2.4.2. Passive Avoidance Test
2.4.3. Morris Water Maze Test
2.5. Preparation of Tissue
2.6. Antioxidant System
2.6.1. Ferric-Reducing/Antioxidant Power (FRAP) in Serum
2.6.2. SOD Contents
2.6.3. Reduced GSH Contents
2.6.4. MDA Contents
2.7. Cerebral Cholinergic System
2.7.1. ACh Contents
2.7.2. AChE Activities
2.8. Mitochondrial Activity
2.8.1. Mitochondrial Isolation
2.8.2. Mitochondrial ROS Contents
2.8.3. Mitochondrial Membrane Potential
2.8.4. ATP Contents
2.9. Western Blot
2.10. Statistical Analysis
3. Results
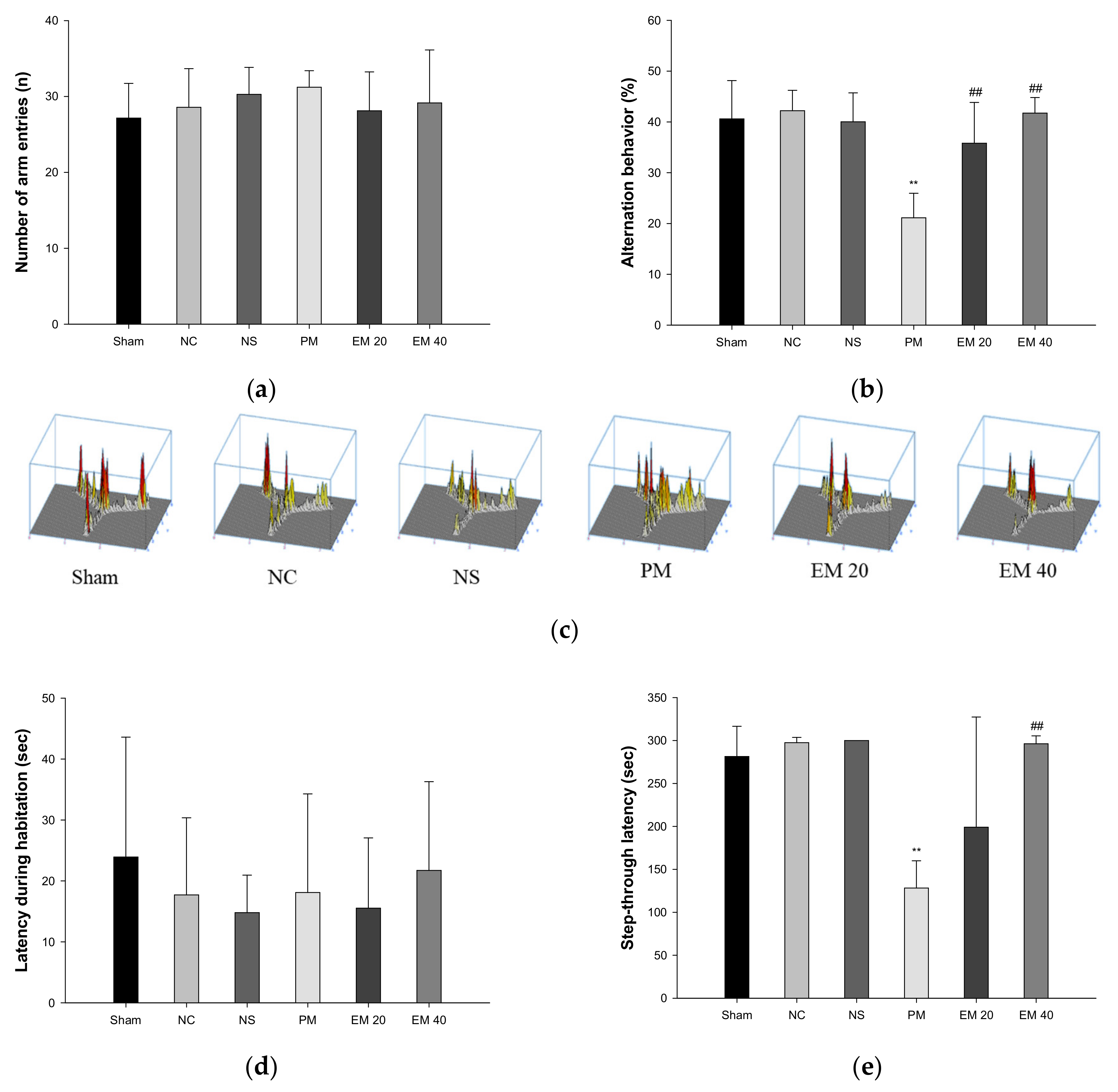
3.1. Behavioral Tests
3.1.1. Y-Maze Test
3.1.2. Passive Avoidance Test
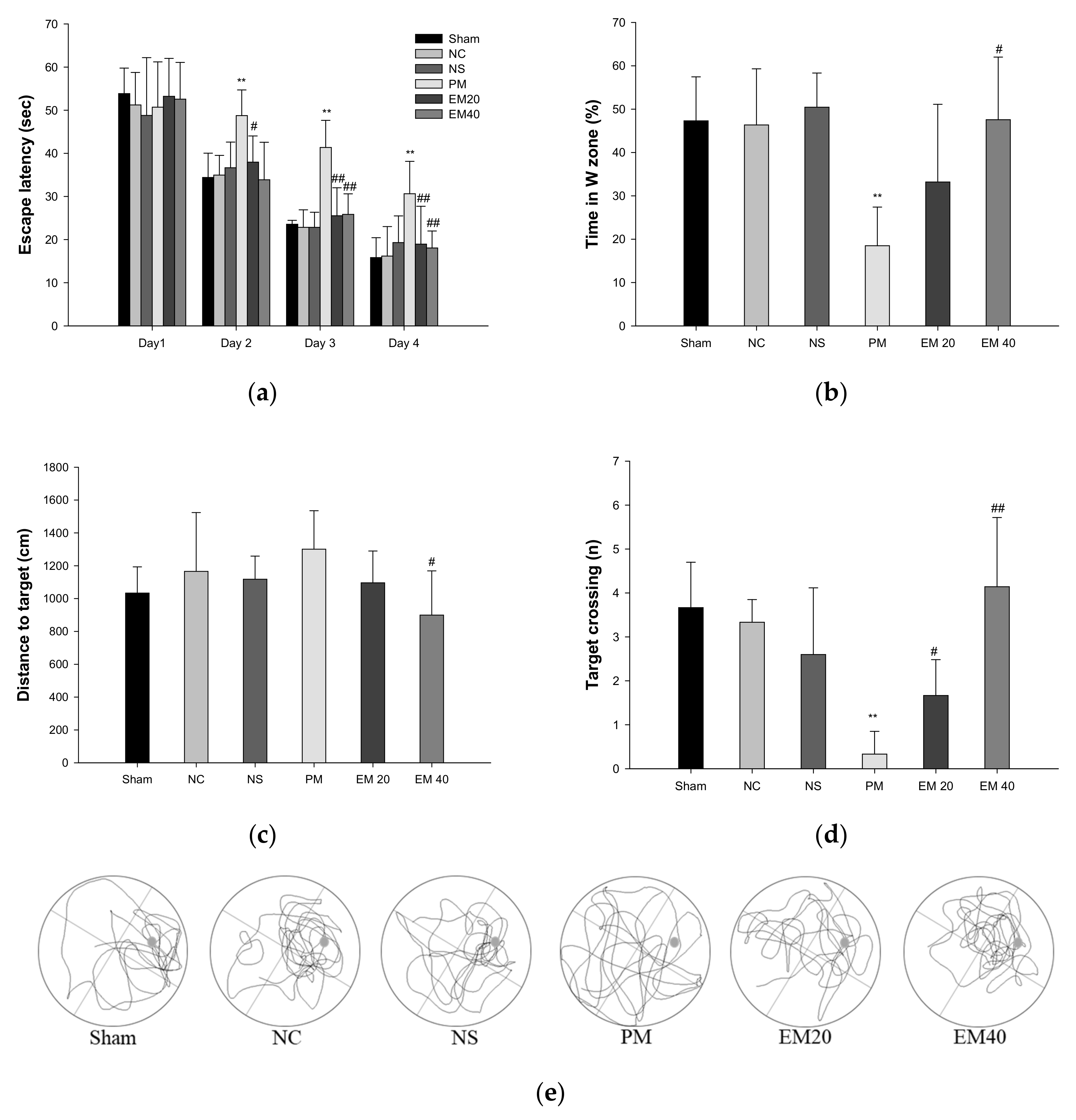
3.1.3. MWM Test
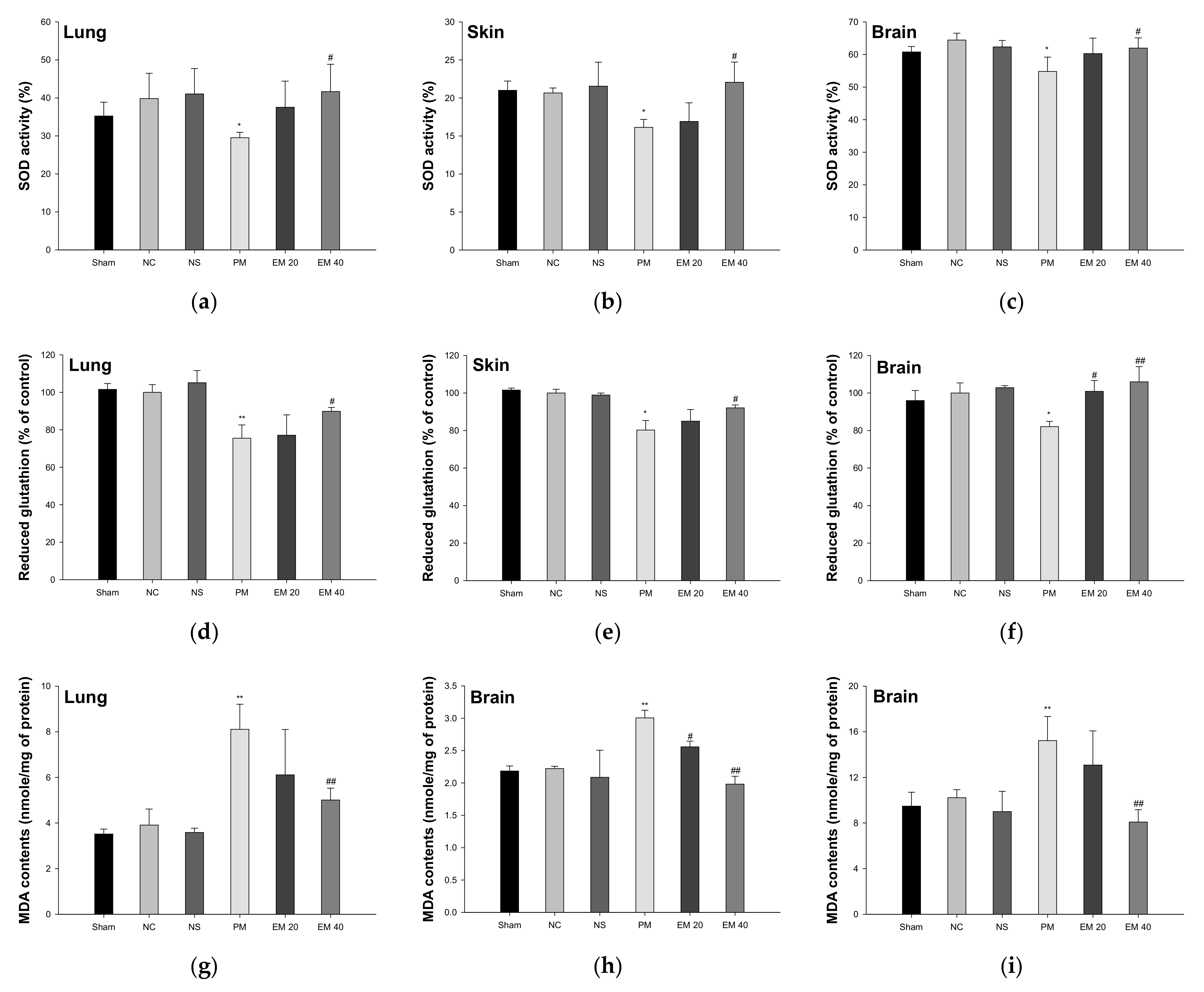
3.2. Antioxidant System
3.2.1. FRAP
3.2.2. SOD Activity
3.2.3. Reduced GSH Contents
3.2.4. MDA Contents
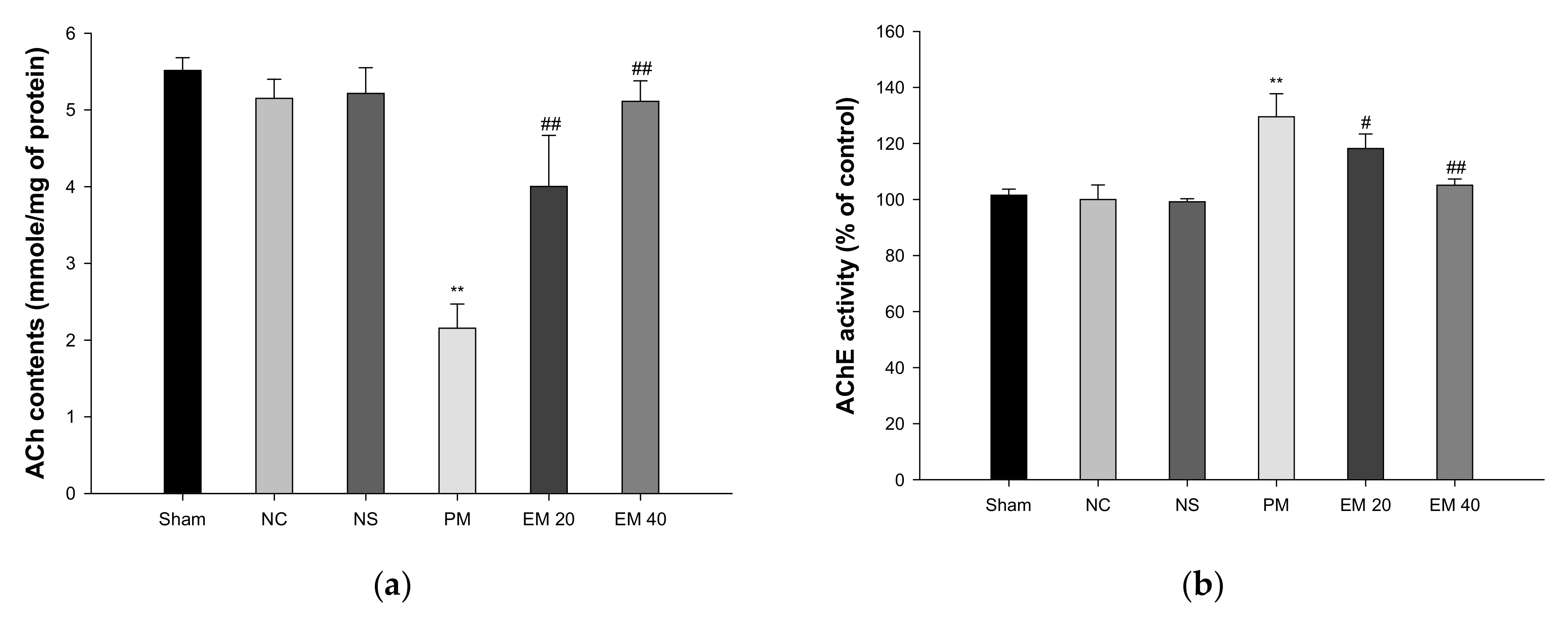
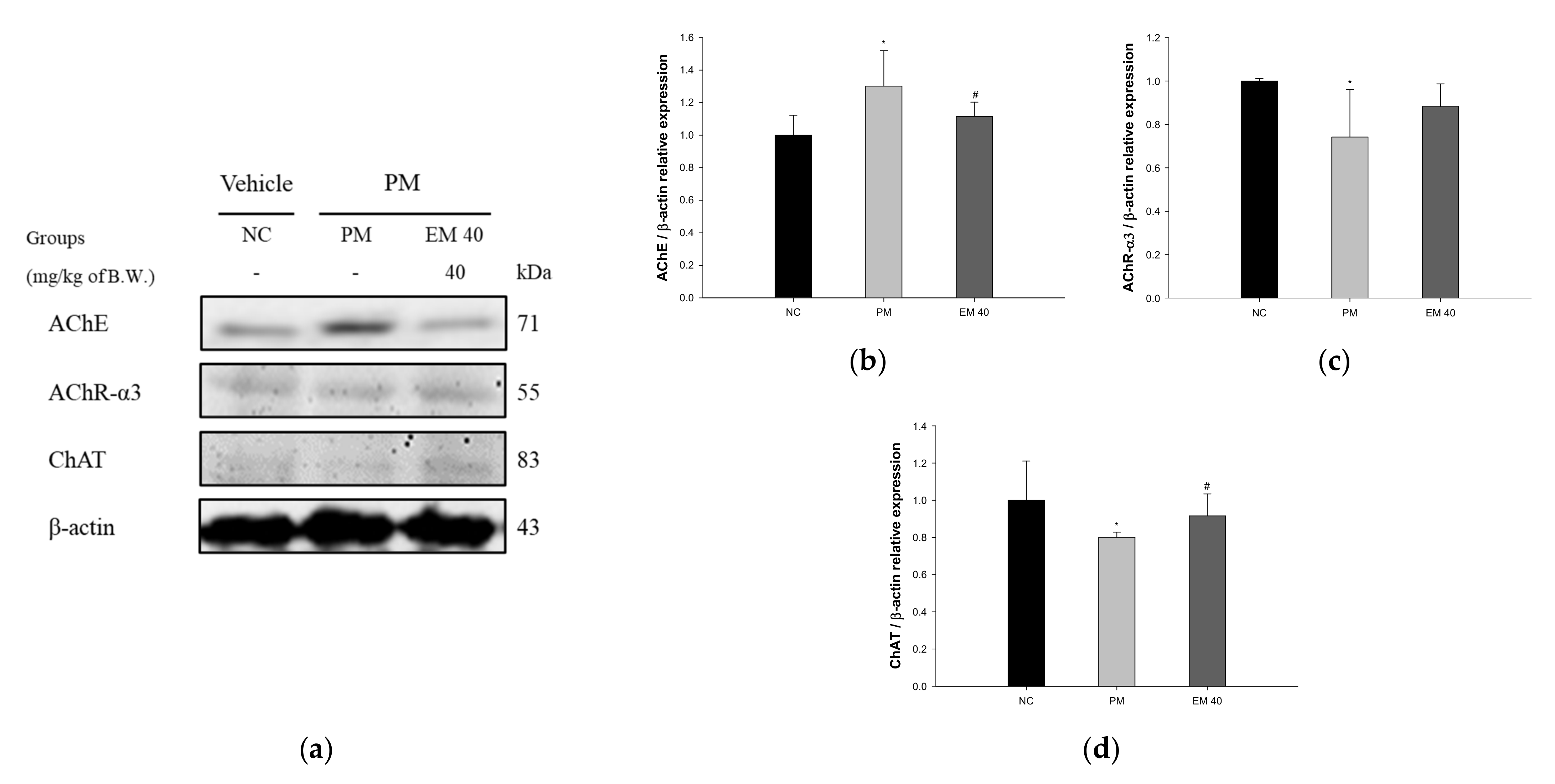
3.3. Cholinergic System
3.3.1. ACh Contents
3.3.2. AChE Activities
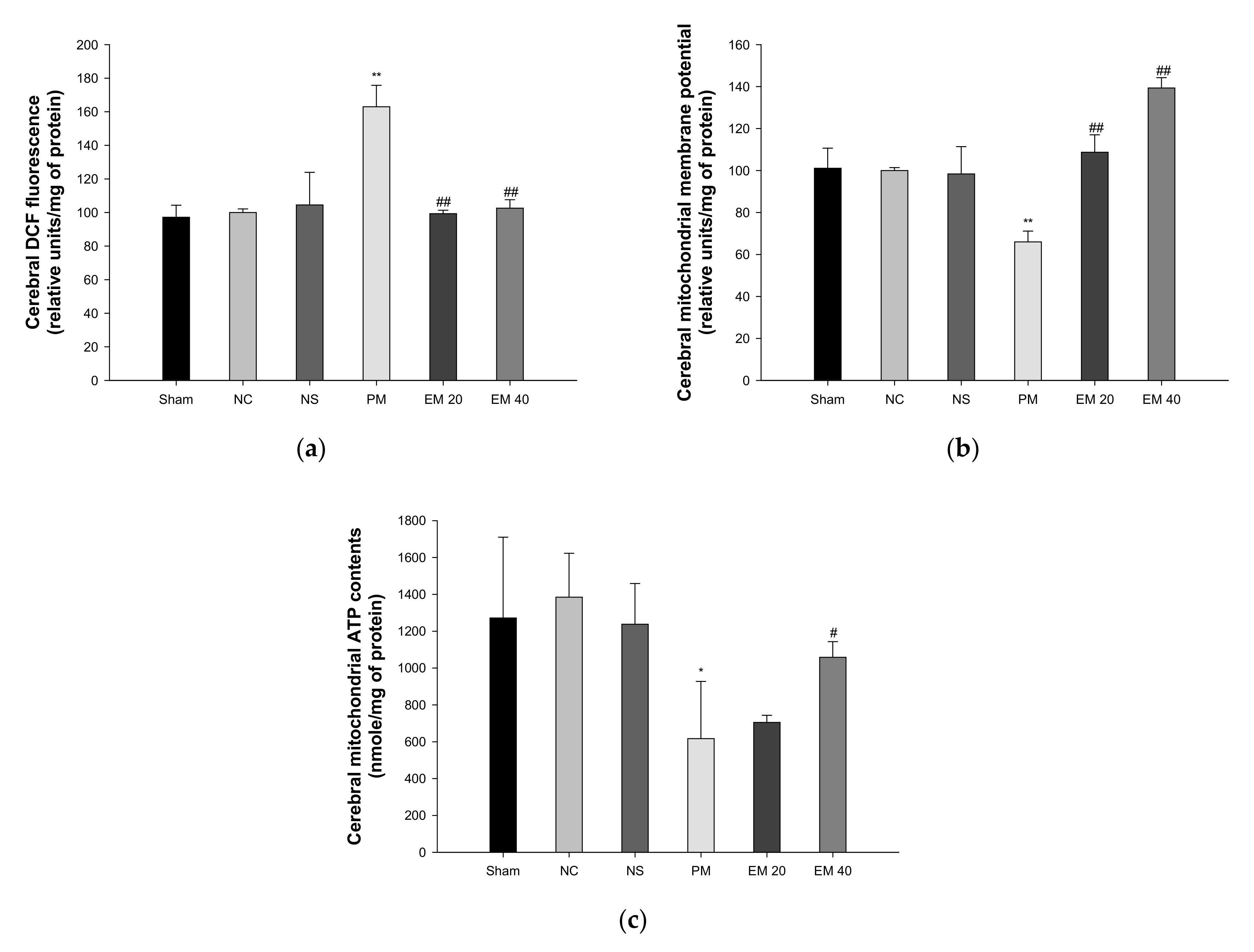
3.4. Mitochondrial Activity
3.4.1. Mitochondrial ROS Contents
3.4.2. MMP
3.4.3. ATP Contents
3.5. Protein Expression in Pulmonary Tissue
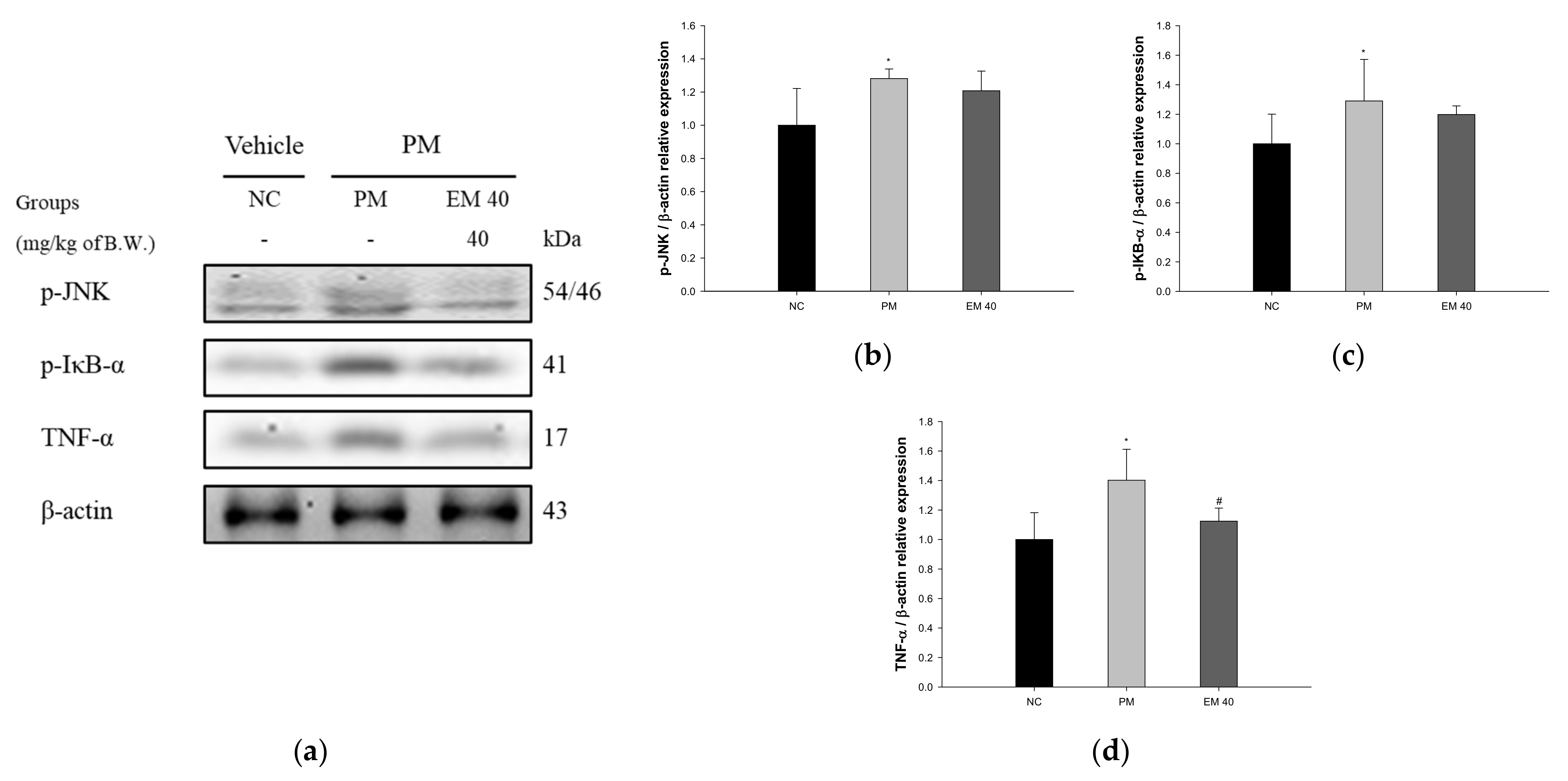
3.6. Protein Expression in Dermal Tissue
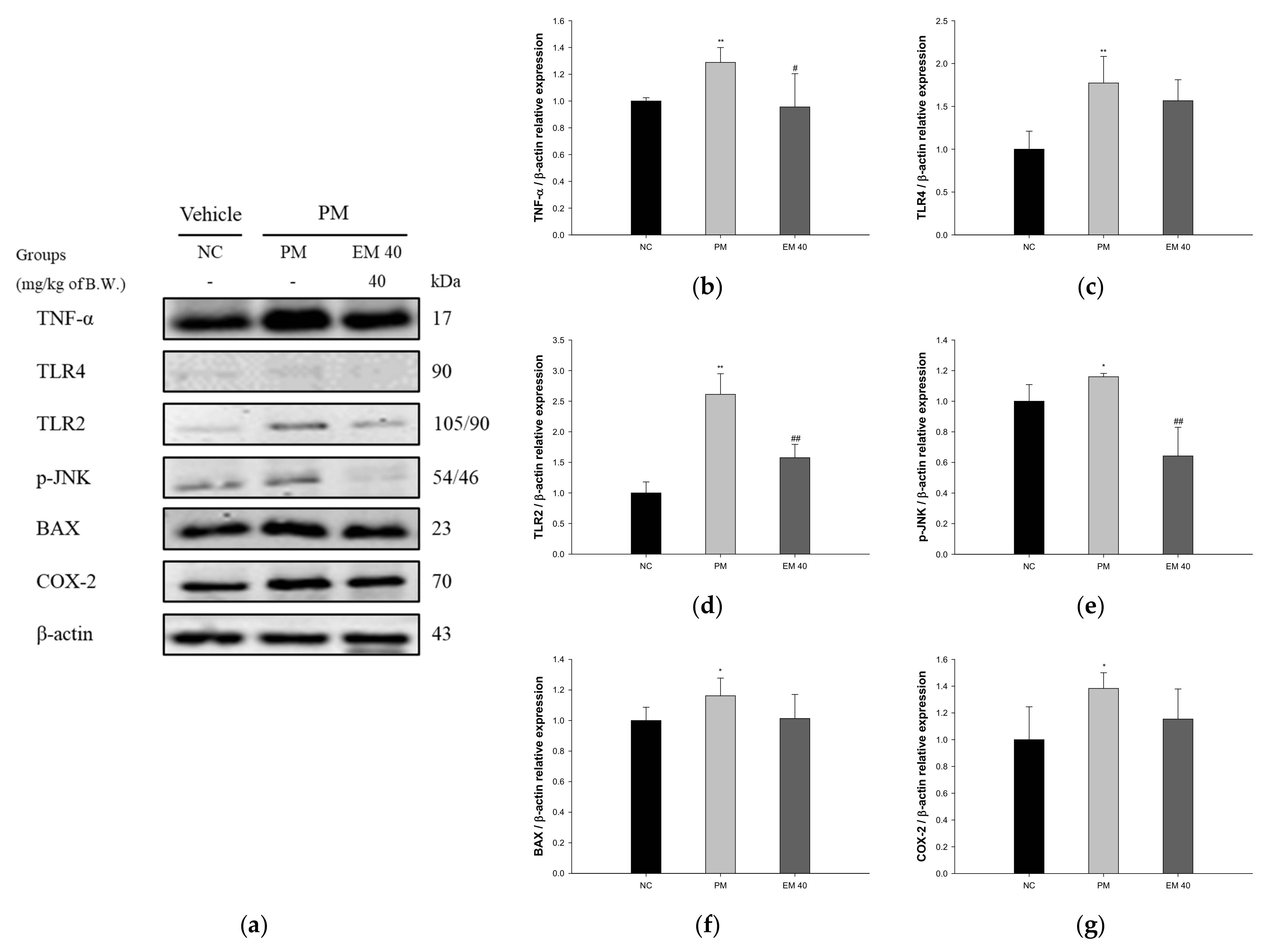
3.7. Protein Expression in Olfactory Bulb Tissue
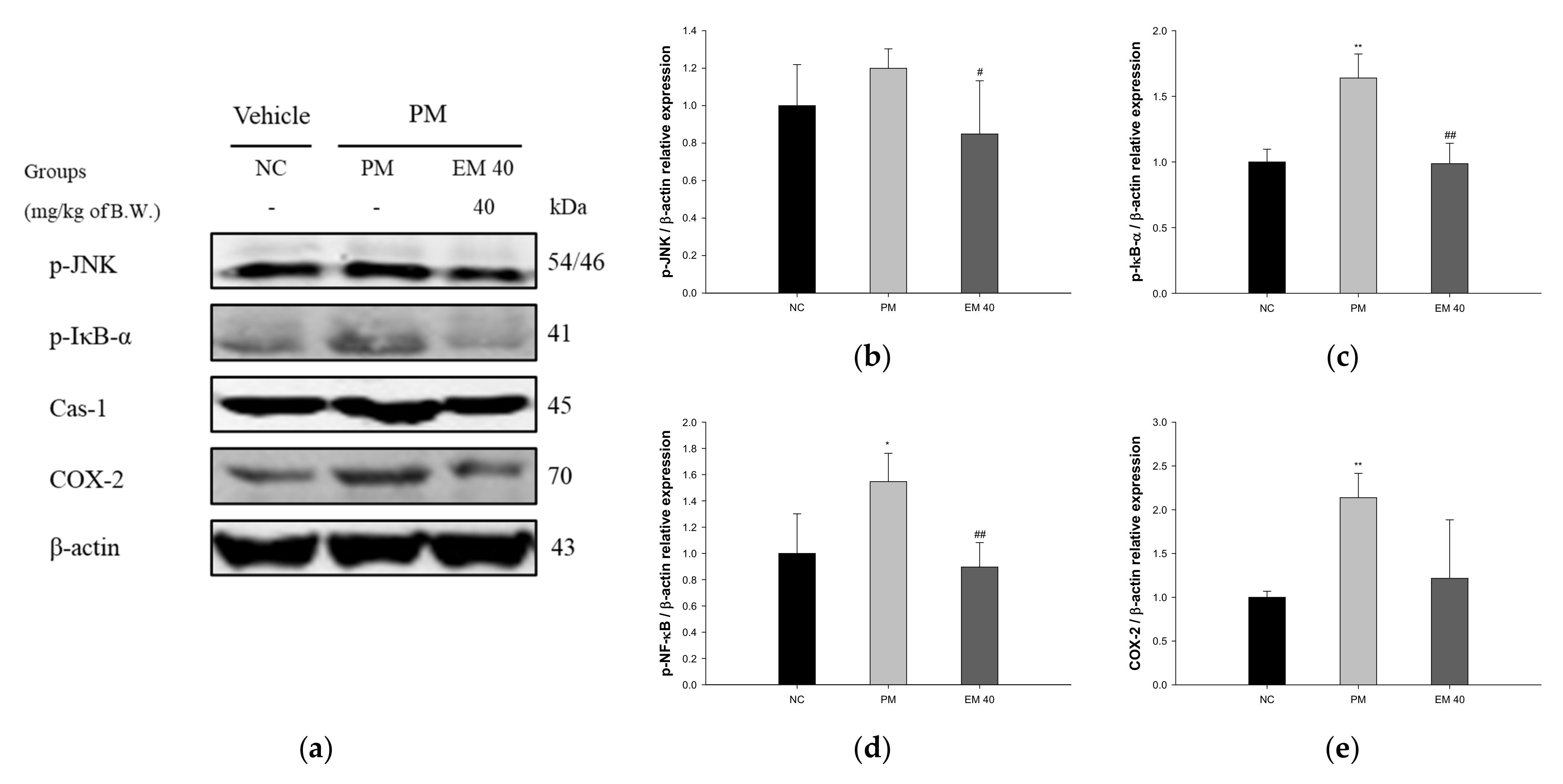
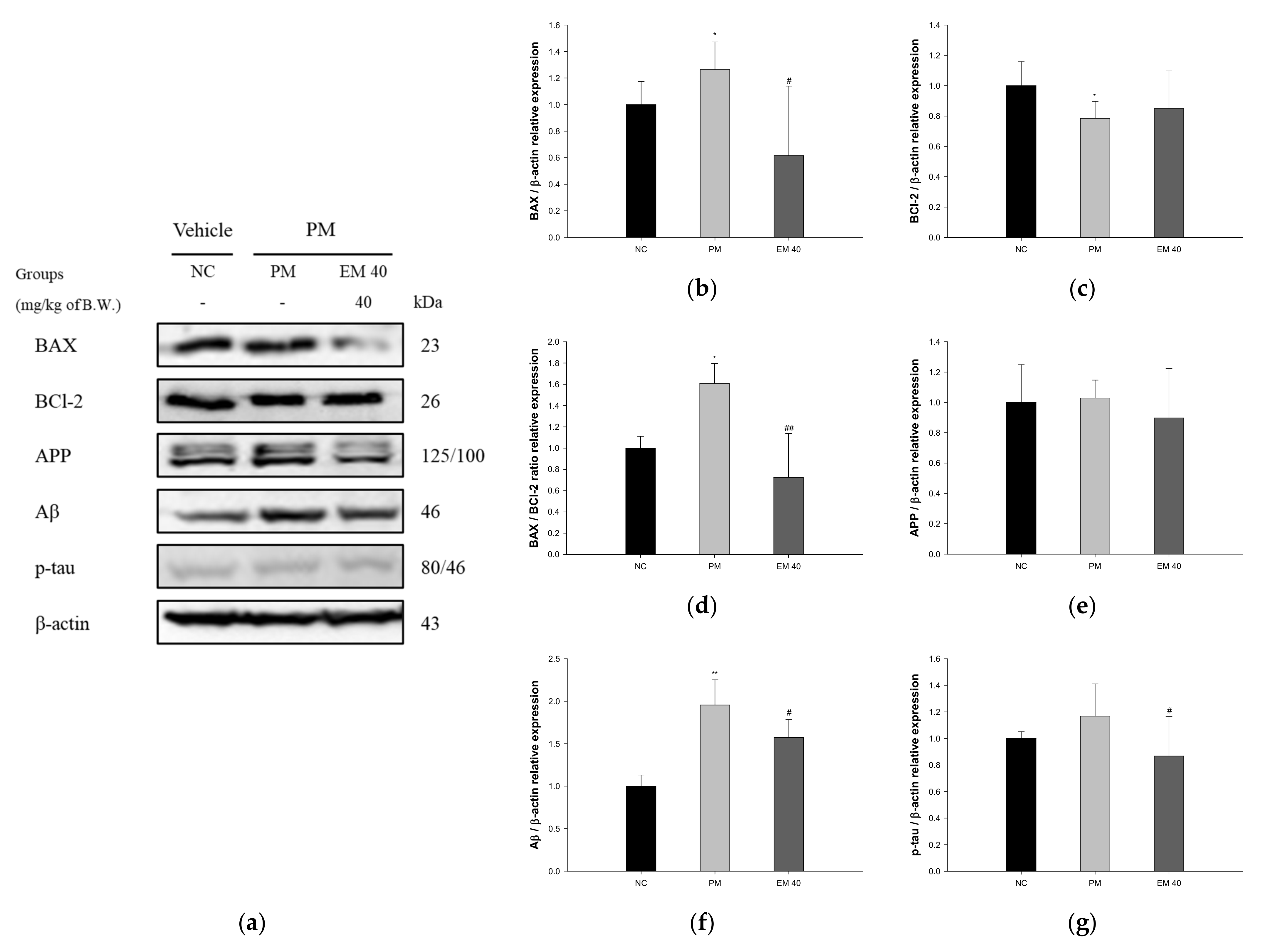
3.8. Protein Expression in Hippocampal Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotox. Environ. Safe. 2019, 174, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Jiang, N.; Yin, S.; Guo, Y.; Li, J.; Kang, P.; Zhang, R.; Tang, X. Characteristics of mass concentration, chemical composition, source apportionment of PM2.5 and PM10 and health risk assessment in the emerging megacity in China. Atmos. Pollut. Res. 2018, 9, 309–321. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.Y.; Choi, I.; Kim, J.; Cho, K.H. Effects of the particulate matter2.5 (PM2.5) on lipoprotein metabolism, uptake and degradation, and embryo toxicity. Mol. Cells 2015, 38, 1096. [Google Scholar]
- Sun, B.; Shi, Y.; Li, Y.; Jiang, J.; Liang, S.; Duan, J.; Sun, Z. Short-term PM2.5 exposure induces sustained pulmonary fibrosis development during post-exposure period in rats. J. Hazard. Mater. 2020, 385, 121566. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Ma, J.; Zhao, Y. PM2.5 impairs neurobehavior by oxidative stress and myelin sheaths injury of brain in the rat. Environ. Pollut. 2018, 242, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—a review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Sato, D. Inhibition of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rats by green tea. Int. J. Urol. 1999, 6, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Han, Y.; Xi, S.; Lu, Y. Catechins reduce inflammation in lipopolysaccharide-stimulated dental pulp cells by inhibiting activation of the NF-κB pathway. Oral Dis. 2020, 26, 815–821. [Google Scholar] [CrossRef]
- Tang, W.; Li, S.; Liu, Y.; Huang, M.T.; Ho, C.T. Anti-diabetic activity of chemically profiled green tea and black tea extracts in a type 2 diabetes mice model via different mechanisms. J. Funct. Food. 2016, 5, 1784–1793. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, U.; Kang, J.Y.; Park, S.K.; Kim, J.C.; Heo, H.J. Matcha improves metabolic imbalance-induced cognitive dysfunction. Oxid. Med. Cell. Longev. 2020, 2020, 8882763. [Google Scholar] [CrossRef] [PubMed]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Yin, H.M.; Zhang, Y.; Zhang, D.J.; Su, X.; Kuang, H.X. Cocrystals of kaempferol, quercetin and myricetin with 4, 4′-bipyridine: Crystal structures, analyses of intermolecular interactions and antibacterial properties. J. Mol. Struct. 2017, 1130, 199–207. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, U.; Kang, J.Y.; Park, S.K.; Shin, E.J.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Kim, G.H.; Jeong, H.R.; et al. Protective effect of matcha green tea (Camellia sinensis) extract on high glucose-and oleic acid-induced hepatic inflammatory effect. Korean J. Food Sci. Technol. 2021, 53, 267–277. [Google Scholar]
- Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 2 May 2018).
- Van der Borght, K.; Havekes, R.; Bos, T.; Eggen, B.J.; Van der Zee, E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav. Neurosci. 2007, 121, 324. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.P.; Kosson, D.S. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J. Abnorm. Psychol. 1986, 95, 252. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Vincent, D.; Segonzac, G.; Vincent, M.C. Colorimetric determination of acetylcholine by the Hestrin hydroxylamine reaction and its application in pharmacy. Ann. Pharm. Françaises 1958, 16, 179–185. [Google Scholar]
- Brown, M.R.; Geddes, J.W.; Sullivan, P.G. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J. Bioenerg. Biomembr. 2004, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Geng, X.; Stone, C.; Cosky, E.E.; Ji, Y.; Du, H.; Zhang, K.; Sun, Q.; Ding, Y. PM2.5 exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ. Toxicol. 2019, 34, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Vojdani, A.; Blaurock-Busch, E.; Busch, Y.; Friedle, A.; Franco-Lira, M.; Sarathi-Mukherjee, P.; Martínez-Aguirre, X.; Park, S.B.; Torres-Jardón, R.; et al. Air pollution and children: Neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J. Alzheimers Dis. 2015, 43, 1039–1058. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, H.M.; Sarkaki, A.; Farbood, Y.; Dianat, M.; Goudarzi, G. Gallic acid affects blood-brain barrier permeability, behaviors, hippocampus local EEG, and brain oxidative stress in ischemic rats exposed to dusty particulate matter. Environ. Sci. Pollut. Res. 2020, 27, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Gong, X.; Zhao, X.; Ma, Z.; Xia, T.; Gu, X. Green tea polyphenols improve isoflurane-induced cognitive impairment via modulating oxidative stress. J. Nutr. Biochem. 2019, 73, 108213. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotox. Environ. Safe. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Jiang, R.; Song, W. Rat lung response to ozone and fine particulate matter (PM2.5) exposures. Environ. Toxicol. 2015, 30, 343–356. [Google Scholar] [CrossRef]
- Zhu, X.; Ji, X.; Shou, Y.; Huang, Y.; Hu, Y.; Wang, H. Recent advances in understanding the mechanisms of PM2.5-mediated neurodegenerative diseases. Toxicol. Lett. 2020, 329, 31–37. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, F.; Cai, Y.; Chen, J. Long short-term memory-Fully connected (LSTM-FC) neural network for PM2.5 concentration prediction. Chemosphere 2019, 220, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, V.L.; Sehgal, A.; Jain, M. Protective effects of green and white tea against benzo (a) pyrene induced oxidative stress and DNA damage in murine model. Nutr. Cancer 2012, 64, 300–306. [Google Scholar] [CrossRef]
- Ben, P.; Zhang, Z.; Zhu, Y.; Xiong, A.; Gao, Y.; Mu, J.; Yin, Z.; Luo, L. l-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Neurotoxicology 2016, 57, 95–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Darland, D.; He, Y.; Yang, L.; Dong, X.; Chang, Y. Reduction of PM2.5 toxicity on human alveolar epithelial cells A549 by tea polyphenols. J. Food Biochem. 2018, 42, 12496. [Google Scholar] [CrossRef]
- Fodale, V.; Quattrone, D.; Trecroci, C.; Caminiti, V.; Santamaria, L.B. Alzheimer’s disease and anaesthesia: Implications for the central cholinergic system. Br. J. Anaesth. 2006, 97, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Hultberg, B.; Andersson, A.; Isaksson, A. Interaction of metals and thiols in cell damage and glutathione distribution: Potentiation of mercury toxicity by dithiothreitol. Toxicology 2001, 156, 93–100. [Google Scholar] [CrossRef]
- Sutherland, B.A.; Rahman, R.M.; Appleton, I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef]
- Okello, E.J.; Mather, J. Comparative kinetics of acetyl-and butyryl-cholinesterase inhibition by green tea catechins| relevance to the symptomatic treatment of Alzheimer’s disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, X.; Li, W.; Niu, B.; Li, J.; Sun, J.; Qin, M.; Zhou, Z. Mitochondrial dysfunction in endothelial cells induced by airborne fine particulate matter (<2.5 μm). J. Appl. Toxicol. 2019, 39, 1424–1432. [Google Scholar] [PubMed]
- Gualtieri, M.; Longhin, E.; Mattioli, M.; Mantecca, P.; Tinaglia, V.; Mangano, E.; Proverbio, M.C.; Bestetti, G.; Camatini, M.; Battaglia, C. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicol. Lett. 2012, 209, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Ren, L.; Wei, J.; Duan, J.; Zhang, L.; Sun, Z. PM2.5 induces male reproductive toxicity via mitochondrial dysfunction, DNA damage and RIPK1 mediated apoptotic signaling pathway. Sci. Total Environ. 2018, 634, 1435–1444. [Google Scholar] [CrossRef]
- Abib, R.T.; Peres, K.C.; Barbosa, A.M.; Peres, T.V.; Bernardes, A.; Zimmermann, L.M.; Quincozes-Santos, A.; Fiedler, H.D.; Leal, R.B.; Farina, M.; et al. Epigallocatechin-3-gallate protects rat brain mitochondria against cadmium-induced damage. Food Chem. Toxicol. 2011, 49, 2618–2623. [Google Scholar] [CrossRef]
- Wang, X.; Xi, Y.; Zeng, X.; Zhao, H.; Cao, J.; Jiang, W. Effects of chlorogenic acid against aluminium neurotoxicity in ICR mice through chelation and antioxidant actions. J. Funct. Food. 2018, 40, 365–376. [Google Scholar] [CrossRef]
- Ben, P.; Zhang, Z.; Xuan, C.; Sun, S.; Shen, L.; Gao, Y.; Cao, X.; Zhou, Y.; Lan, L.; Yin, Z.; et al. Protective effect of L-theanine on cadmium-induced apoptosis in PC12 cells by inhibiting the mitochondria-mediated pathway. Neurochem. Res. 2015, 40, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Tong, L.; Shen, D.; Yu, J.E.; Hu, Z.Q.; Li, Y.J.; Xue, E.F.; Tang, H.F. Airborne bacteria enriched PM2.5 enhances the inflammation in an allergic adolescent mouse model induced by ovalbumin. Inflammation 2020, 43, 32–43. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Brito, J.M.; Toledo, A.C.; Nakagawa, N.K.; Piccin, V.S.; Junqueira, M.S.; Macchione, M. Subchronic effects of nasally instilled diesel exhaust particulates on the nasal and airway epithelia in mice. Inhal. Toxicol. 2010, 22, 610–617. [Google Scholar] [CrossRef]
- Xing, W.J.; Kong, F.J.; Li, G.W.; Qiao, K.; Zhang, W.H.; Zhang, L.; Bai, S.Z.; Xi, Y.H.; Li, H.X.; Tian, Y.; et al. Calcium-sensing receptors induce apoptosis during simulated ischaemia–reperfusion in Buffalo rat liver cells. Clin. Exp. Pharmacol. Physiol. 2011, 38, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, X.; Nan, A.; Zhang, N.; Chen, L.; Zhou, H.; Qiu, Q.; Zhu, J.; Ling, Y.; Jiang, Y. Circular RNA 406961 interacts with ILF2 to regulate PM2.5-induced inflammatory responses in human bronchial epithelial cells via activation of STAT3/JNK pathways. Environ. Int. 2020, 141, 105755. [Google Scholar] [CrossRef]
- Jin, X.; Su, R.; Li, R.; Song, L.; Chen, M.; Cheng, L.; Li, Z. Amelioration of particulate matter-induced oxidative damage by vitamin c and quercetin in human bronchial epithelial cells. Chemosphere 2016, 144, 459–466. [Google Scholar] [CrossRef]
- Fernando, I.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2016, 172, 150–158. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, J.; Chu, W.; Wang, S.; Yang, T.; Tao, Y.; Wang, G. Involvement of TLR2 and TLR4 and Th1/Th2 shift in inflammatory responses induced by fine ambient particulate matter in mice. Inhal. Toxicol. 2012, 24, 918–927. [Google Scholar] [CrossRef]
- Peng, F.; Tsuji, G.; Zhang, J.Z.; Chen, Z.; Furue, M. Potential role of PM2.5 in melanogenesis. Environ. Int. 2019, 132, 105063. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Wu, T.; Lu, R.; Shi, B. Caffeic acid phenethyl ester attenuates lipopolysaccharide-stimulated proinflammatory responses in human gingival fibroblasts via NF-κB and PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017, 794, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell. Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (–)-epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-κB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef]
- Morgan, T.E.; Davis, D.A.; Iwata, N.; Tanner, J.A.; Snyder, D.; Ning, Z.; Finch, C.E. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ. Health Perspect. 2011, 119, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, S.; Zhang, W.; Wu, C.; Liu, H.; Zhang, F.; Ding, W. Nrf2 deficiency exacerbates PM2.5-induced olfactory bulb injury. Biochem. Biophys. Res. Commun. 2018, 505, 1154–1160. [Google Scholar] [CrossRef]
- Hu, W.; Xie, G.; Zhou, T.; Tu, J.; Zhang, J.; Lin, Z.; Gao, L. Intranasal administration of white tea alleviates the olfactory function deficit induced by chronic unpredictable mild stress. Pharm. Biol. 2020, 58, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.; Tashev, R.E.; Valcheva-Kuzmanova, S.V. Chlorogenic acid, gallic acid and ferulic acid prevent the development of hyperactivity and anxiety in olfactory bulbectomized rats. Bulg. Chem. Commun. 2020, 52, 125–130. [Google Scholar]
- Li, Q.; Zheng, J.; Xu, S.; Zhang, J.; Cao, Y.; Qin, Z.; Jiang, C. The neurotoxicity induced by PM2.5 might be strongly related to changes of the hippocampal tissue structure and neurotransmitter levels. Toxicol. Res. 2019, 7, 1144–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Li, B.; Sang, N. Particulate matter (PM2.5) exposure season-dependently induces neuronal apoptosis and synaptic injuries. J. Environ. Sci. 2017, 54, 336–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiyazahan, D.B.; Thenmozhi, A.J.; Manivasagam, T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]


| Al | Fe | Mg | Mn | Ba | Zn | Cu | Pb | Li | Cr | Co | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30.36 ± 6.41 | 16.91 ± 3.12 | 6.26 ± 1.20 | 0.58 ± 0.14 | 0.22 ± 0.06 | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Antibody | Catalog | Concentration | Manufacturer |
|---|---|---|---|
| β-actin | sc-69879 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| p-JNK | sc-6254 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| p-IκB-α | sc-8404 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| BAX | sc-7480 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| Caspase-1 | sc-392736 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| COX-2 | sc-376861 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| iNOS | sc-7271 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| APP/Aβ | sc-28365 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| p-tau | sc-12952 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| AChE | sc-373901 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| TLR2 | sc-21759 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| TLR4 | sc-52962 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| BCl-2 | sc-509 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| AChR-α3 | sc-365479 | 1:1000 | Santa Cruz Biotech. (Dallas, TX, USA) |
| TNF-α | 5178SC | 1:1000 | Cell Signaling Tech. (Danvers, MA, USA) |
| ChAT | 20747-1AP | 1:1000 | Bioneer (Daejeon, Korea) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Kang, J.Y.; Park, S.K.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Kim, J.C.; Heo, H.J. Powdered Green Tea (Matcha) Attenuates the Cognitive Dysfunction via the Regulation of Systemic Inflammation in Chronic PM2.5-Exposed BALB/c Mice. Antioxidants 2021, 10, 1932. https://doi.org/10.3390/antiox10121932
Kim JM, Kang JY, Park SK, Moon JH, Kim MJ, Lee HL, Jeong HR, Kim JC, Heo HJ. Powdered Green Tea (Matcha) Attenuates the Cognitive Dysfunction via the Regulation of Systemic Inflammation in Chronic PM2.5-Exposed BALB/c Mice. Antioxidants. 2021; 10(12):1932. https://doi.org/10.3390/antiox10121932
Chicago/Turabian StyleKim, Jong Min, Jin Yong Kang, Seon Kyeong Park, Jong Hyun Moon, Min Ji Kim, Hyo Lim Lee, Hye Rin Jeong, Jong Cheol Kim, and Ho Jin Heo. 2021. "Powdered Green Tea (Matcha) Attenuates the Cognitive Dysfunction via the Regulation of Systemic Inflammation in Chronic PM2.5-Exposed BALB/c Mice" Antioxidants 10, no. 12: 1932. https://doi.org/10.3390/antiox10121932
APA StyleKim, J. M., Kang, J. Y., Park, S. K., Moon, J. H., Kim, M. J., Lee, H. L., Jeong, H. R., Kim, J. C., & Heo, H. J. (2021). Powdered Green Tea (Matcha) Attenuates the Cognitive Dysfunction via the Regulation of Systemic Inflammation in Chronic PM2.5-Exposed BALB/c Mice. Antioxidants, 10(12), 1932. https://doi.org/10.3390/antiox10121932






