Anemopsis californica Attenuates Photoaging by Regulating MAPK, NRF2, and NFATc1 Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Total Phenolic, Flavonoid, and Tannin Contents
2.4. HPLC Analysis
2.5. 2,2-Diphenyl-1-Picrylhdrazyl Radical Scavenging Activity
- OD0: Optical density of negative control
- ODx: Optical density of the sample
2.6. 2,2’-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Radical Scavenging Activity
- OD0: Optical density of negative control
- ODx: Optical density of the sample
2.7. Cell Culture and Treatment
2.8. MTT Assay
2.9. NO Assay
2.10. ROS Assay
2.11. Enzyme-Linked Immunosorbent Assay
2.12. Reverse Transcriptase (RT)-PCR
2.13. Western Blot
2.14. Statistical Analysis
3. Results
3.1. Analysis of Chemical Contents of AC Extract
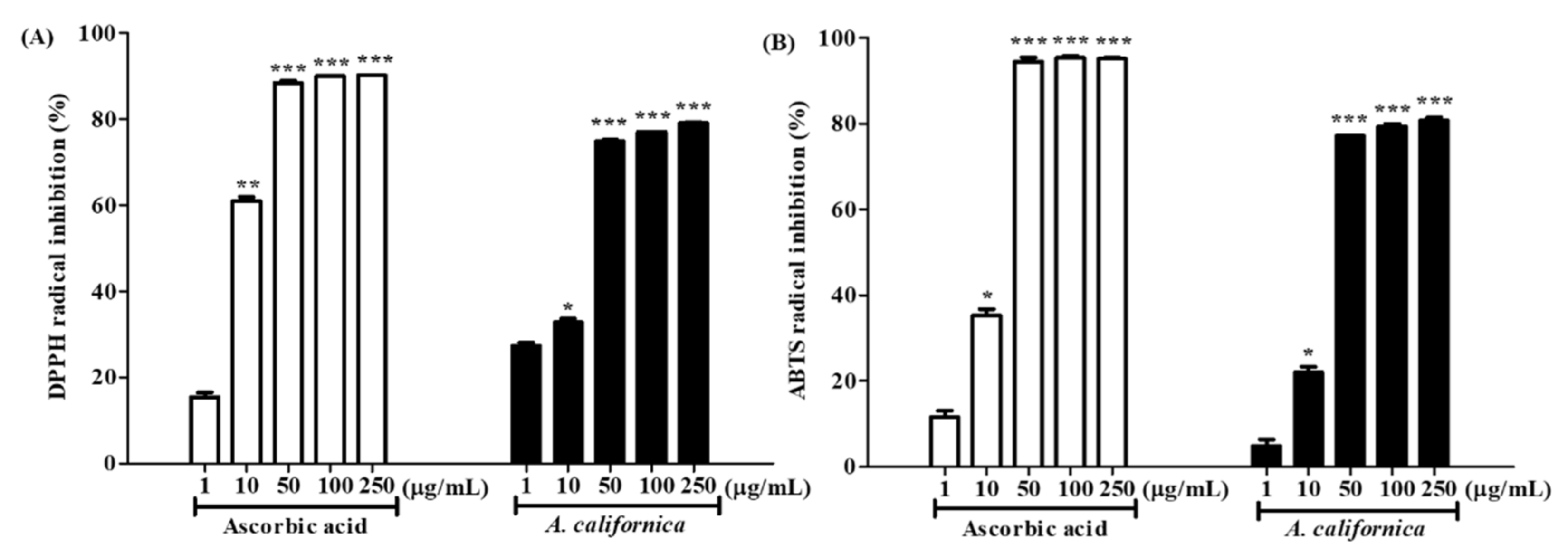
3.2. Antioxidative Activities of AC Extract
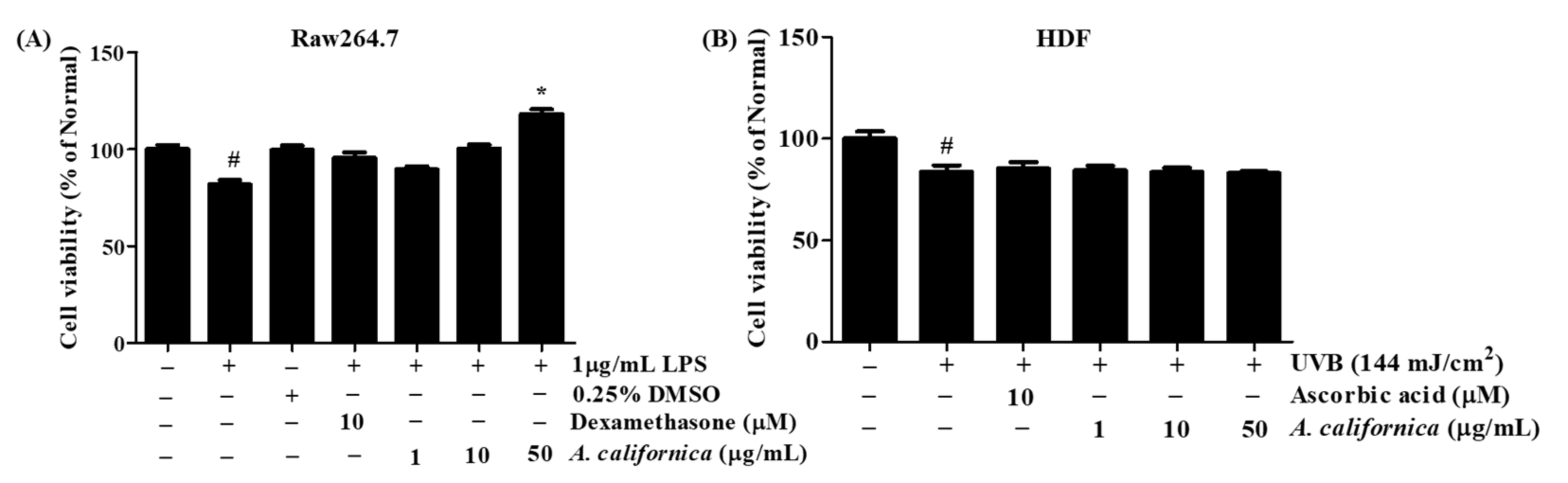
3.3. Cytotoxicity of AC Extract
3.4. AC Extract Regulates Inflammatory Response in Raw264.7 Cells
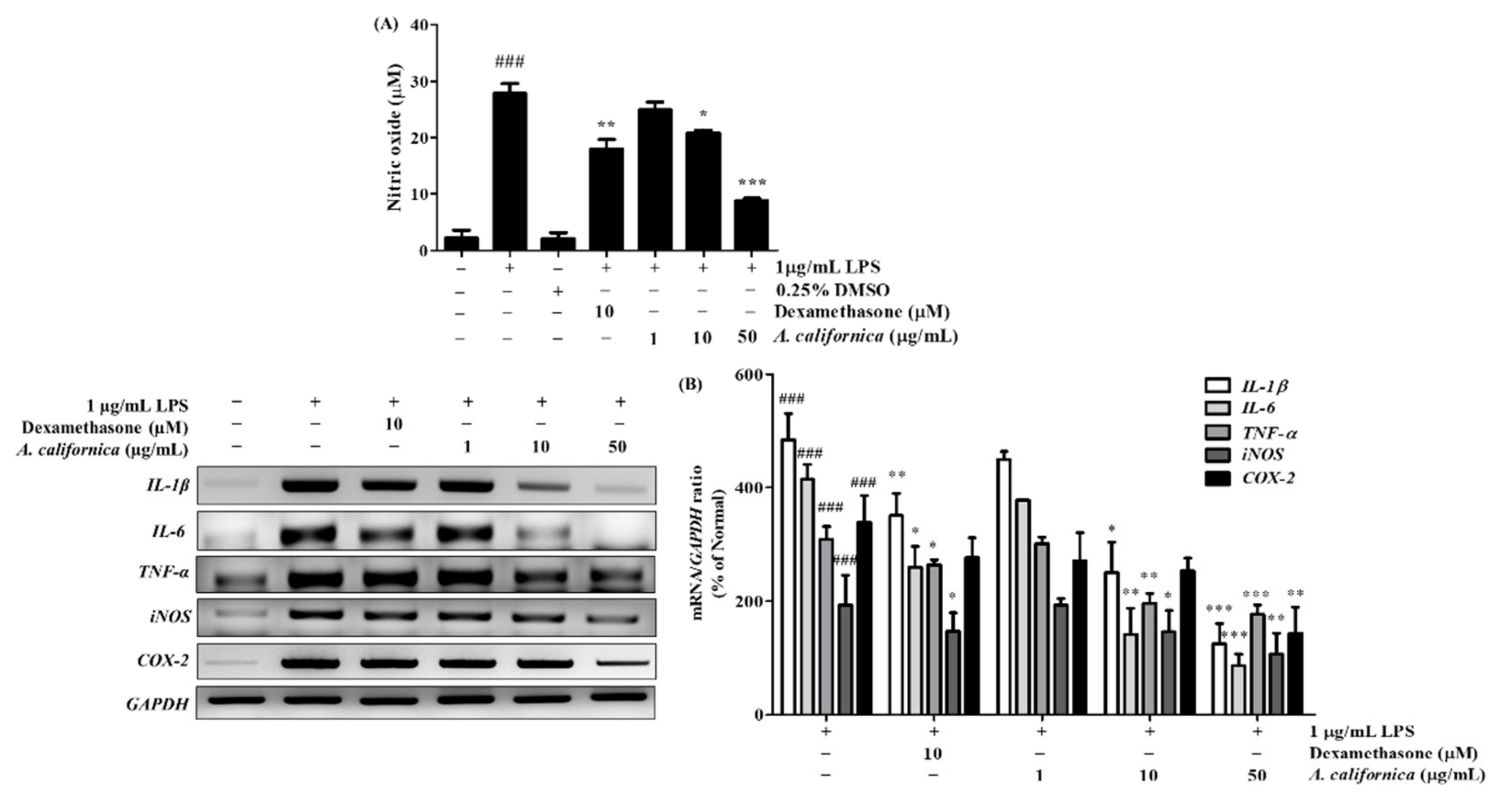
3.4.1. Effect of AC Extract on NO Production in LPS-Induced Raw264.7 Cells
3.4.2. Effect of AC Extract on the mRNA Expression of IL-1β, IL-6, TNF-α, iNOS, and COX-2 in LPS-Induced Raw264.7 Cells
3.5. AC Extract Protects HDF Cells from UVB Irradiation
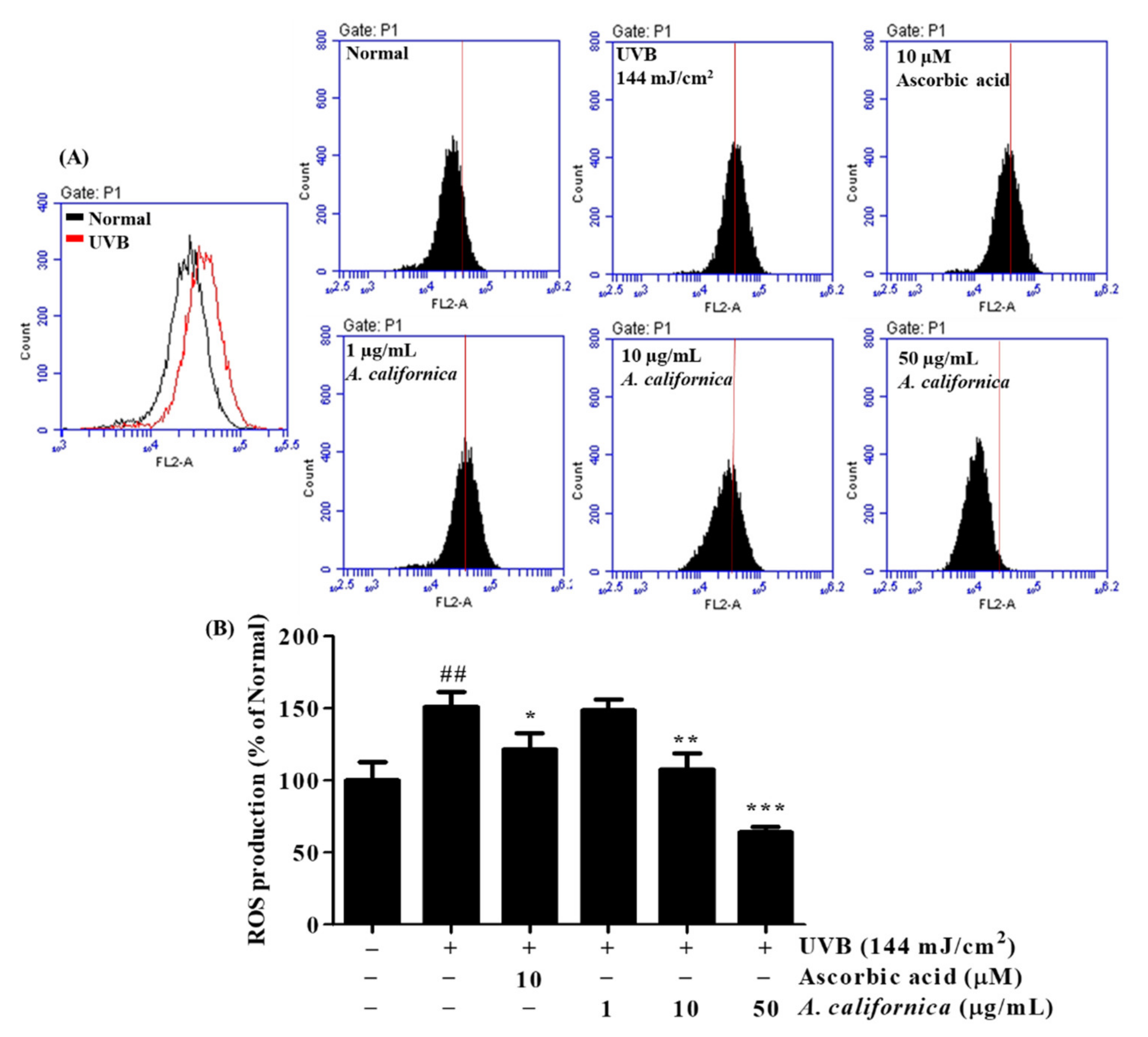
3.5.1. Effect of AC Extract on ROS Production in UVB-Irradiated HDF Cells
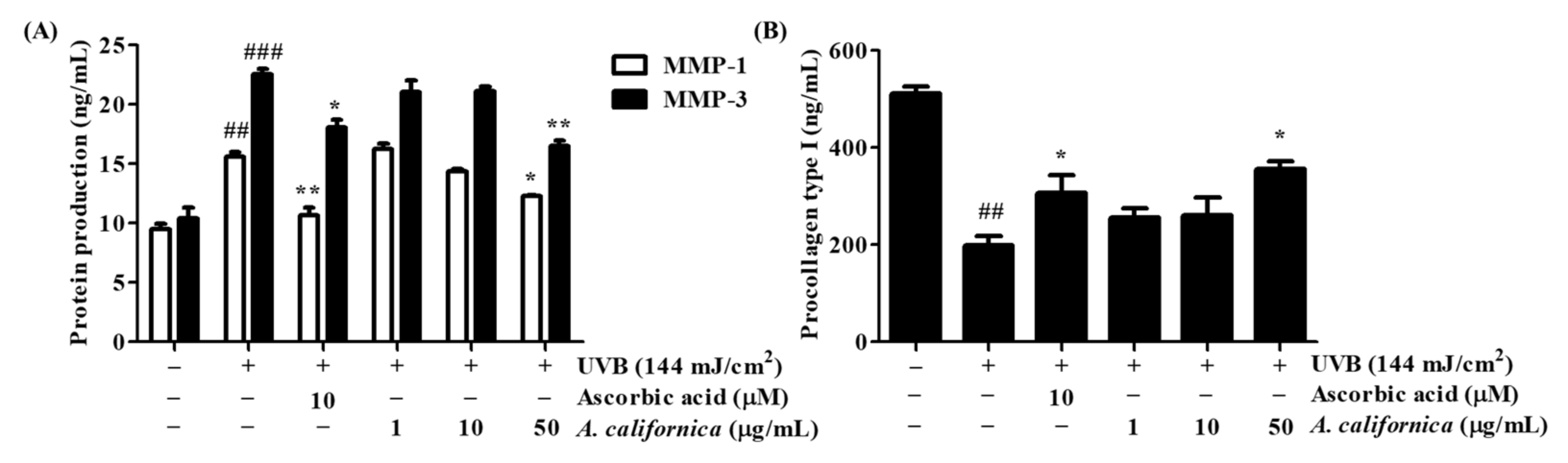
3.5.2. Effect of AC Extract on the Protein Secretion of MMP-1, MMP-3, and Procollagen Type I in UVB-Irradiated HDF Cells
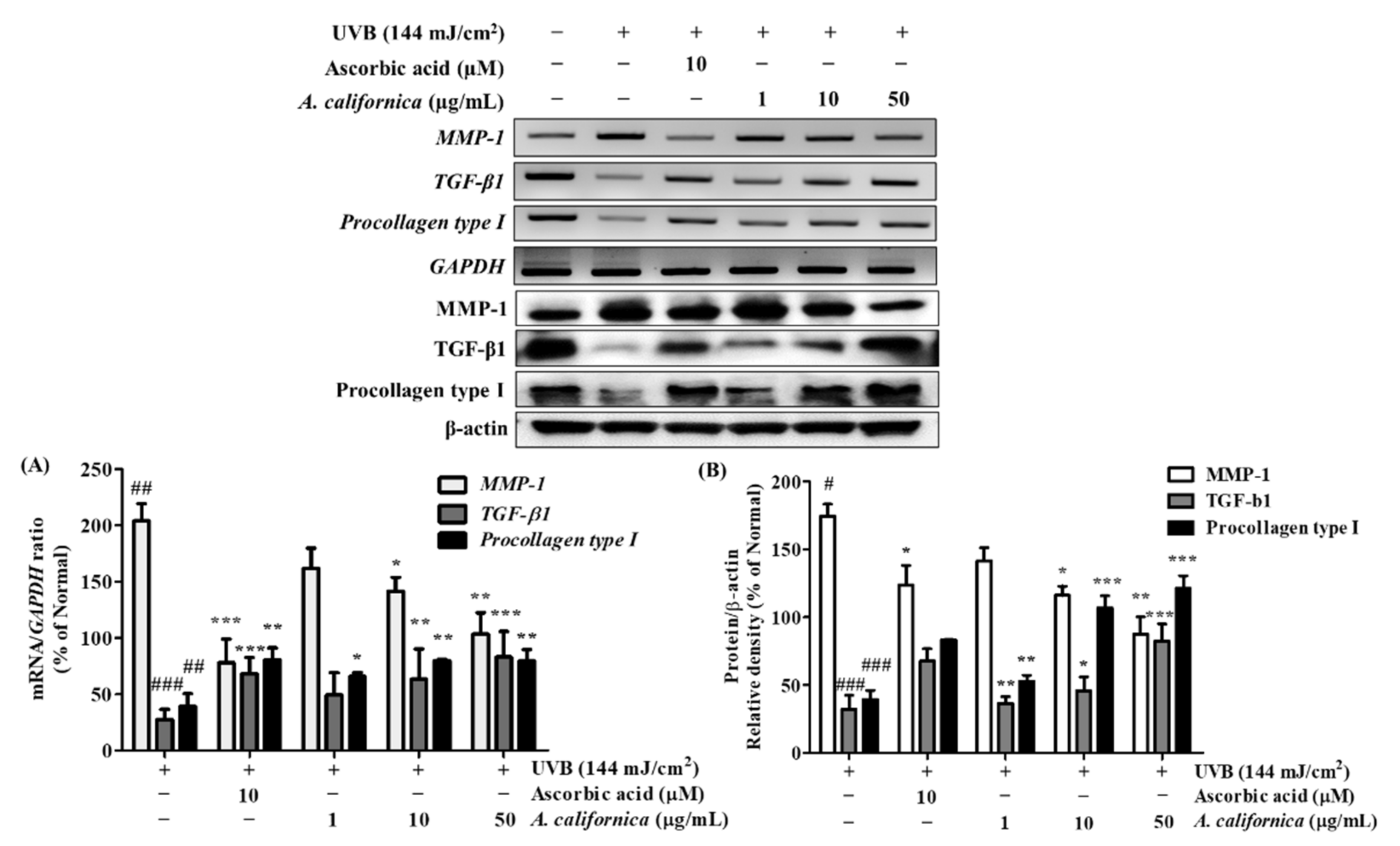
3.5.3. Effect of AC Extract on the mRNA and Protein Expression of MMP-1, TGF-β1, and Procollagen Type I in UVB-Irradiated HDF Cells
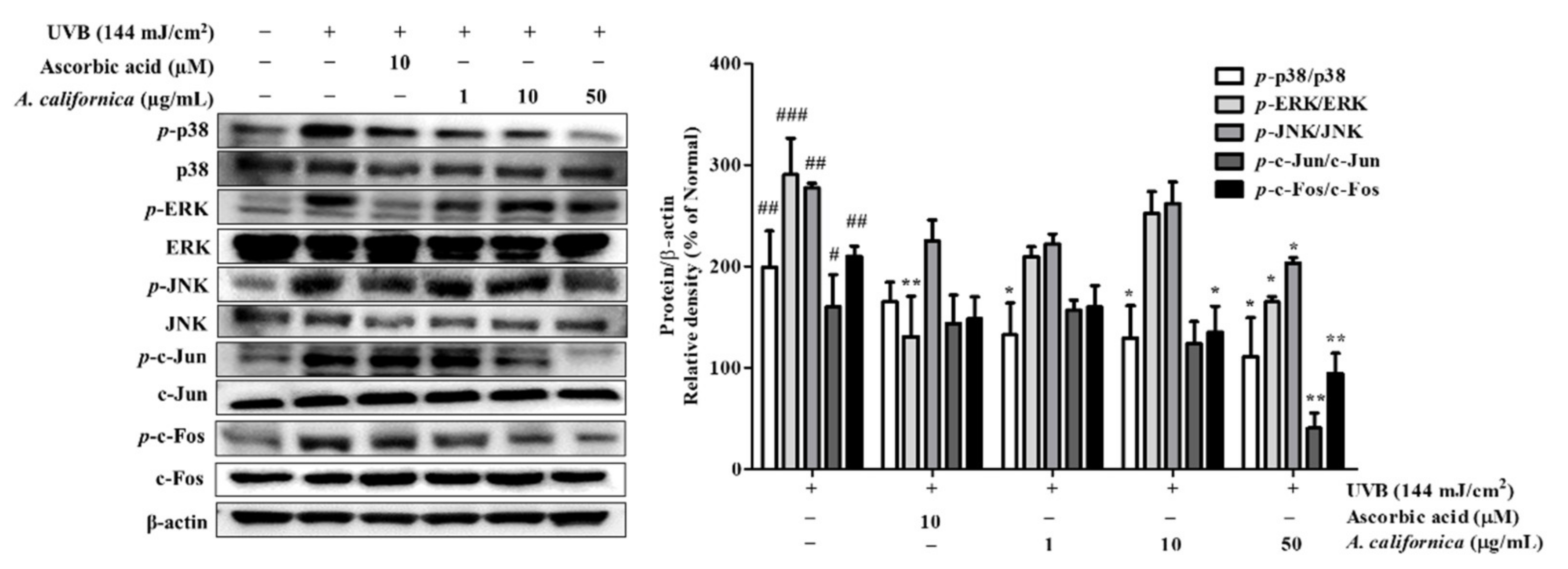
3.5.4. Effect of AC Extract on MAPK/AP-1 Activation in UVB-Irradiated HDF Cells
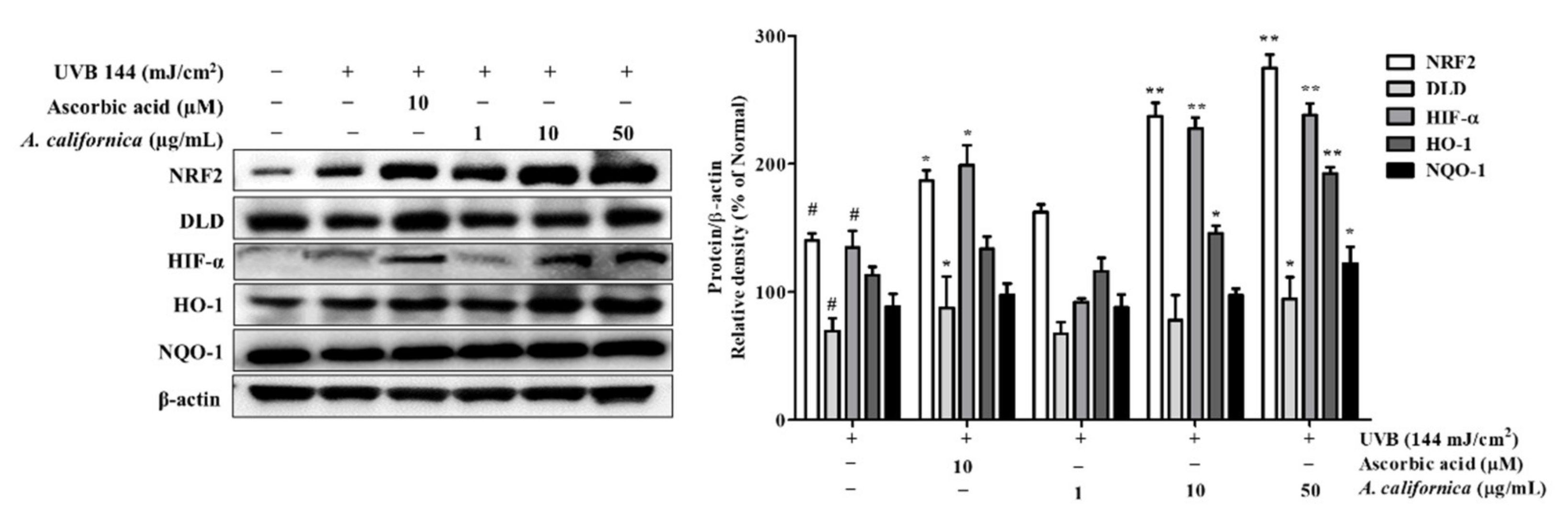
3.5.5. Effect of AC Extract on NRF2 Activation in UVB-Irradiated HDF Cells
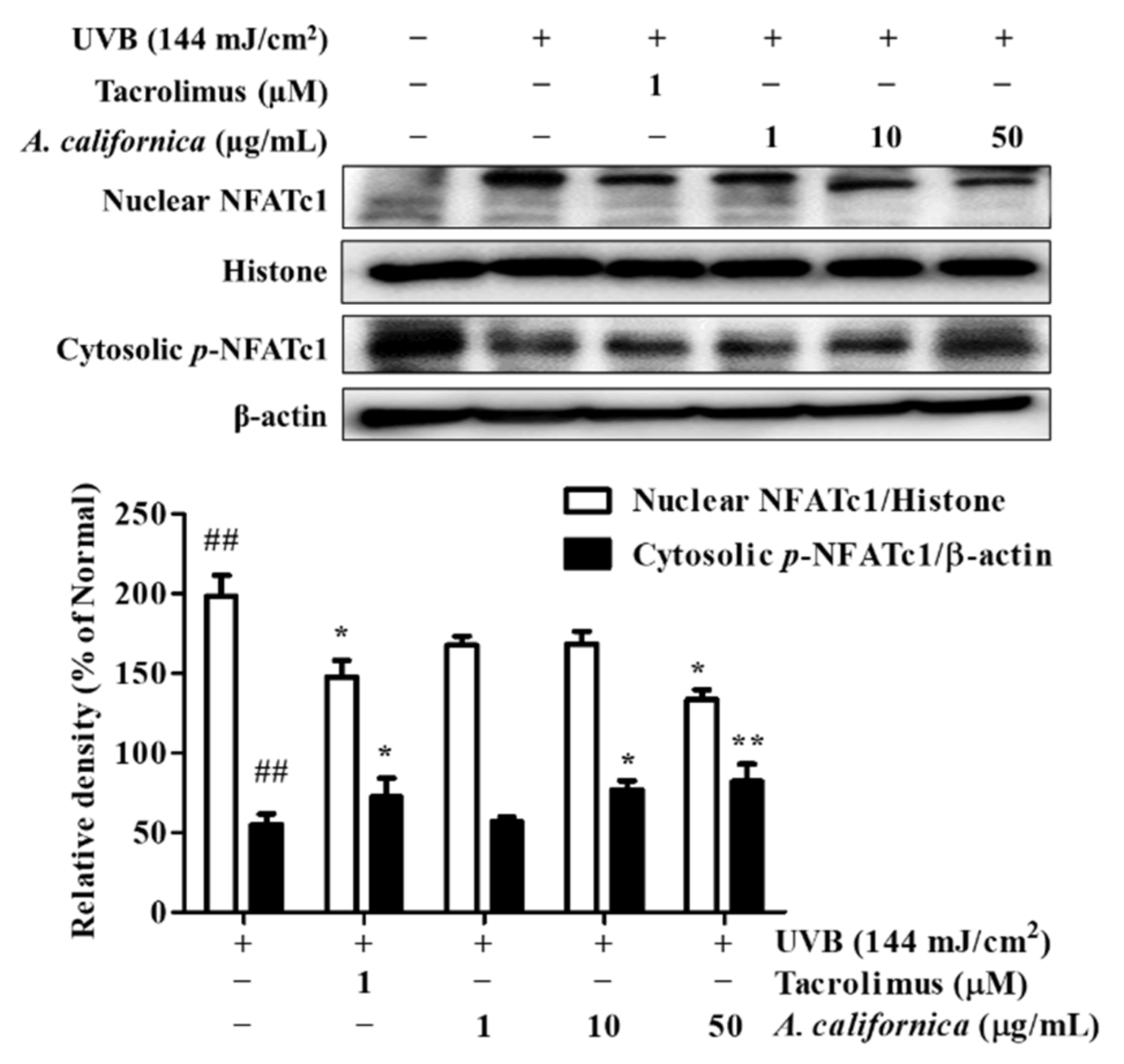
3.5.6. Effect of AC Extract on NFATc1 Nuclear Translocation in UVB-Irradiated HDF Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosch, R.; Philips, N.; Suárez-Pérez, J.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-W.; Hung, Y.-C.; Lin, T.-Y.; Fang, J.-Y.; Yang, P.-M.; Chen, M.-H.; Pan, T.-L. Comparison of the Biological Impact of UVA and UVB upon the Skin with Functional Proteomics and Immunohistochemistry. Antioxidants 2019, 8, 569. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lyga, J. Inflammaging in Skin and Other Tissues—The Roles of Complement System and Macrophage. IADT 2014, 13, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xin, Y.; Zhang, Z.; Zou, X.; Xue, K.; Zhang, H.; Zhang, W.; Liu, K. Extracellular Vesicles from Adipose-Derived Stem Cells Ameliorate Ultraviolet B-Induced Skin Photoaging by Attenuating Reactive Oxygen Species Production and Inflammation. Stem Cell Res. Ther. 2020, 11, 264. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ponnappan, U. Aging and Immune Function: Molecular Mechanisms to Interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Dennler, S.; Prunier, C.; Ferrand, N.; Gauthier, J.-M.; Atfi, A. C-Jun Inhibits Transforming Growth Factor β-Mediated Transcription by Repressing Smad3 Transcriptional Activity. J. Biol. Chem. 2000, 275, 28858–28865. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.; Park, H.M.; Lee, C.H.; Do, S.-G.; Park, J.-M.; Han, N.-Y.; Do, M.H.; Lee, J.H.; Lee, H.; Kim, S.Y. Dihydrolipoyl Dehydrogenase as a Potential UVB Target in Skin Epidermis; Using an Integrated Approach of Label-Free Quantitative Proteomics and Targeted Metabolite Analysis. J. Proteom. 2015, 117, 70–85. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Hwang, E.; Yi, T.-H. Paeonol Extracted from Paeonia Suffruticosa Andr. Ameliorated UVB-Induced Skin Photoaging via DLD/Nrf2/ARE and MAPK/AP-1 Pathway: Paeonol Ameliorated UVB-Induced Skin Photoaging. Phytother. Res. 2018, 32, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Faßbender, S.; Sondenheimer, K.; Majora, M.; Schindler, J.; Opitz, F.V.; Pollet, M.; Haarmann-Stemmann, T.; Krutmann, J.; Weighardt, H. Keratinocytes Counteract UVB-Induced Immunosuppression in Mice Via HIF-1a Signaling. J. Investig. Dermatol. 2021, S0022202X2102217X. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A Mutual Interplay. Redox Biol 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Fric, J.; Zelante, T.; Wong, A.Y.W.; Mertes, A.; Yu, H.-B.; Ricciardi-Castagnoli, P. NFAT Control of Innate Immunity. Blood 2012, 120, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Izutsu, Y.; Yahagi, S.; Okano, Y. Reactive Oxygen Species in HaCaT Keratinocytes After UVB Irradiation Are Triggered by Intracellular Ca2+ Levels. J. Investig. Dermatol. Symp. Proc. 2009, 14, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Mazière, C.; Morlière, P.; Massy, Z.; Kamel, S.; Louandre, C.; Conte, M.-A.; Mazière, J.-C. Oxidized Low-Density Lipoprotein Elicits an Intracellular Calcium Rise and Increases the Binding Activity of the Transcription Factor NFAT. Free. Radic. Biol. Med. 2005, 38, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, R.J.; Armstrong, J.L.; Reynolds, N.J.; Lovat, P.E. NFAT Signalling Is a Novel Target of Oncogenic BRAF in Metastatic Melanoma. Br. J. Cancer 2009, 101, 1448–1455. [Google Scholar] [CrossRef][Green Version]
- Yan, Y.; Li, J.; Ouyang, W.; Ma, Q.; Hu, Y.; Zhang, D.; Ding, J.; Qu, Q.; Subbaramaiah, K.; Huang, C. NFAT3 Is Specifically Required for TNF-α-Induced Cyclooxygenase-2 (COX-2) Expression and Transformation of Cl41 Cells. J. Cell Sci. 2006, 119, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Granucci, F. Regulation and Dysregulation of Innate Immunity by NFAT Signaling Downstream of Pattern Recognition Receptors (PRRs). Eur. J. Immunol. 2012, 42, 1924–1931. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Li, J.; Zhang, W.; Ren, F.; Yue, W. NFATc1 Activation Promotes the Invasion of U251 Human Glioblastoma Multiforme Cells through COX-2. Int. J. Mol. Med. 2015, 35, 1333–1340. [Google Scholar] [CrossRef]
- Bussey, R.; Sy-Cordero, A.; Figueroa, M.; Carter, F.; Falkinham, J.; Oberlies, N.; Cech, N. Antimycobacterial Furofuran Lignans from the Roots of Anemopsis Californica. Planta Med. 2014, 80, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.L.; Lucero, M.E.; Holguin, F.O.; Estell, R.E.; Posakony, J.J.; Simon, J.; O’Connell, M.A. Composition and Antimicrobial Activity of Anemopsis Californica Leaf Oil. J. Agric. Food Chem. 2005, 53, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Del Toro Sánchez, C.L.; Lomelí, M.G.; Sánchez García, E.R.; Guerrero Medina, P.J.; Del Río, J.A.M.; Castellanos Hernández, O.A. Phytochemical Composistion and Total Antioxidant Capacity of Anemopsis Californica; University of Guadalajara: Queretaro, Mexico, 2011. [Google Scholar]
- Han, S.; Park, K.-K.; Chung, W.-Y.; Lee, S.K.; Kim, J.; Hwang, J.-K. Anti-Photoaging Effects of 2-Methoxy-5-(2-Methyl Propyl) Pyrazine Isolated from Peach (Prunus Persica (L.) Batsch). Food Sci. Biotechnol. 2010, 19, 1667–1671. [Google Scholar] [CrossRef]
- Gogoi, R.; Loying, R.; Sarma, N.; Begum, T.; Pandey, S.K.; Lal, M. Comparative Analysis of In-Vitro Biological Activities of Methyl Eugenol Rich Cymbopogon Khasianus Hack., Leaf Essential Oil with Pure Methyl Eugenol Compound. Curr. Pharm. Biotechnol. 2020, 21, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Potential Protective Effects of Alpha-Pinene against Cytotoxicity Caused by Aspirin in the IEC-6 Cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Bautista-Bautista, N.; Blasco-Cabal, J.L.; Gonzalez-Ávila, M.; Gutiérrez-Lomelí, M.; Arriaga-Alba, M. Antimutagenicity of Methanolic Extracts from Anemopsis Californica in Relation to Their Antioxidant Activity. Evid.-Based Complement. Altern. Med. 2014, 2014, 273878. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, C.N.; Ferrey, S.L.; Lowrey, T.; Guerra, L.; Van Slambrouck, S.; Steelant, W.F.A. In Vitro Anticancer Activity of Anemopsis Californica. Oncol. Lett. 2010, 1, 711–715. [Google Scholar] [CrossRef]
- Lacher, S.E.; Levings, D.C.; Freeman, S.; Slattery, M. Identification of a Functional Antioxidant Response Element at the HIF1A Locus. Redox Biol. 2018, 19, 401–411. [Google Scholar] [CrossRef]
- Poetker, D.M.; Reh, D.D. A Comprehensive Review of the Adverse Effects of Systemic Corticosteroids. Otolaryngol. Clin. N. Am. 2010, 43, 753–768. [Google Scholar] [CrossRef]
- Röck, K.; Fischer, J.W. Rolle der extrazellulären Matrix bei der extrinsischen Hautalterung. Hautarzt 2011, 62, 591–597. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, S.-S.; Bang, M.-H.; Jo, S.K.; Jhee, K.-H.; Yang, S.-A. Protection against UVB-Induced Damages in Human Dermal Fibroblasts: Efficacy of Tricin Isolated from Enzyme-Treated Zizania Latifolia Extract. Biosci. Biotechnol. Biochem. 2019, 83, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Del-Toro-Sánchez, C.L.; Zurita, F.; Gutiérrez-Lomelí, M.; Solis-Sánchez, B.; Wence-Chávez, L.; Rodríguez-Sahagún, A.; Castellanos-Hernández, O.A.; Vázquez-Armenta, G.; Siller-López, F. Modulation of Antioxidant Defense System after Long Term Arsenic Exposure in Zantedeschia Aethiopica and Anemopsis Californica. Ecotoxicol. Environ. Saf. 2013, 94, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Lohakul, J.; Soontrapa, K.; Sampattavanich, S.; Akarasereenont, P.; Panich, U. Activation of Nrf2 Reduces UVA-Mediated MMP-1 Upregulation via MAPK/AP-1 Signaling Cascades: The Photoprotective Effects of Sulforaphane and Hispidulin. J. Pharmacol. Exp. Ther. 2017, 360, 388–398. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular Mechanisms of the Keap1–Nrf2 Pathway in Stress Response and Cancer Evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Thompson, J.W.; Narayanan, S.V.; Perez-Pinzon, M.A. Redox Signaling Pathways Involved in Neuronal Ischemic Preconditioning. Curr. Neuropharmacol. 2012, 10, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Knatko, E.V.; Ibbotson, S.H.; Zhang, Y.; Higgins, M.; Fahey, J.W.; Talalay, P.; Dawe, R.S.; Ferguson, J.; Huang, J.T.-J.; Clarke, R.; et al. Nrf2 Activation Protects against Solar-Simulated Ultraviolet Radiation in Mice and Humans. Cancer Prev. Res. 2015, 8, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic Acid-Based Nanogel as an Efficient Anti-Inflammatory Agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Daré, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Tannic Acid, a Promising Anti-Photoaging Agent: Evidences of Its Antioxidant and Anti-Wrinkle Potentials, and Its Ability to Prevent Photodamage and MMP-1 Expression in L929 Fibroblasts Exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Abu-Yousif, A.O.; Smith, K.A.; Getsios, S.; Green, K.J.; Van Dross, R.T.; Pelling, J.C. Enhancement of UVB-Induced Apoptosis by Apigenin in Human Keratinocytes and Organotypic Keratinocyte Cultures. Cancer Res. 2008, 68, 3057–3065. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Van Dross, R.T.; Abu-Yousif, A.; Morrison, A.R.; Pelling, J.C. Apigenin Prevents UVB-Induced Cyclooxygenase 2 Expression: Coupled MRNA Stabilization and Translational Inhibition. Mol. Cell. Biol. 2007, 27, 283–296. [Google Scholar] [CrossRef]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The Polyphenol Chlorogenic Acid Attenuates UVB-Mediated Oxidative Stress in Human HaCaT Keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Oxidized Low-Density Lipoprotein Elicits an Intracellular Calcium Rise and Increases the Binding Activity of the Transcription Factor NFAT—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15649649/ (accessed on 2 March 2021).
- Alfonso-Jaume, M.A.; Mahimkar, R.; Lovett, D.H. Co-Operative Interactions between NFAT (Nuclear Factor of Activated T Cells) C1 and the Zinc Finger Transcription Factors Sp1/Sp3 and Egr-1 Regulate MT1-MMP (Membrane Type 1 Matrix Metalloproteinase) Transcription by Glomerular Mesangial Cells. Biochem. J. 2004, 380, 735–747. [Google Scholar] [CrossRef]
- Saygili, E.; Rana, O.R.; Meyer, C.; Gemein, C.; Andrzejewski, M.G.; Ludwig, A.; Weber, C.; Schotten, U.; Krüttgen, A.; Weis, J.; et al. The Angiotensin-Calcineurin-NFAT Pathway Mediates Stretch-Induced up-Regulation of Matrix Metalloproteinases-2/-9 in Atrial Myocytes. Basic Res. Cardiol. 2009, 104, 435–448. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.T.N.; Fang, M.; Do, N.Q.; Jeong, J.; Oh, S.; Zheng, S.; Kim, M.; Choi, J.; Lim, S.; Yi, T.H. Anemopsis californica Attenuates Photoaging by Regulating MAPK, NRF2, and NFATc1 Signaling Pathways. Antioxidants 2021, 10, 1882. https://doi.org/10.3390/antiox10121882
Nguyen QTN, Fang M, Do NQ, Jeong J, Oh S, Zheng S, Kim M, Choi J, Lim S, Yi TH. Anemopsis californica Attenuates Photoaging by Regulating MAPK, NRF2, and NFATc1 Signaling Pathways. Antioxidants. 2021; 10(12):1882. https://doi.org/10.3390/antiox10121882
Chicago/Turabian StyleNguyen, Quynh T. N., Minzhe Fang, Nhung Quynh Do, Jeehaeng Jeong, Sarang Oh, Shengdao Zheng, Minseon Kim, Junhui Choi, Seojun Lim, and Tae Hoo Yi. 2021. "Anemopsis californica Attenuates Photoaging by Regulating MAPK, NRF2, and NFATc1 Signaling Pathways" Antioxidants 10, no. 12: 1882. https://doi.org/10.3390/antiox10121882
APA StyleNguyen, Q. T. N., Fang, M., Do, N. Q., Jeong, J., Oh, S., Zheng, S., Kim, M., Choi, J., Lim, S., & Yi, T. H. (2021). Anemopsis californica Attenuates Photoaging by Regulating MAPK, NRF2, and NFATc1 Signaling Pathways. Antioxidants, 10(12), 1882. https://doi.org/10.3390/antiox10121882






