Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Plant Species (Biological Materials)
2.2. Requisition of Rhizbacterium
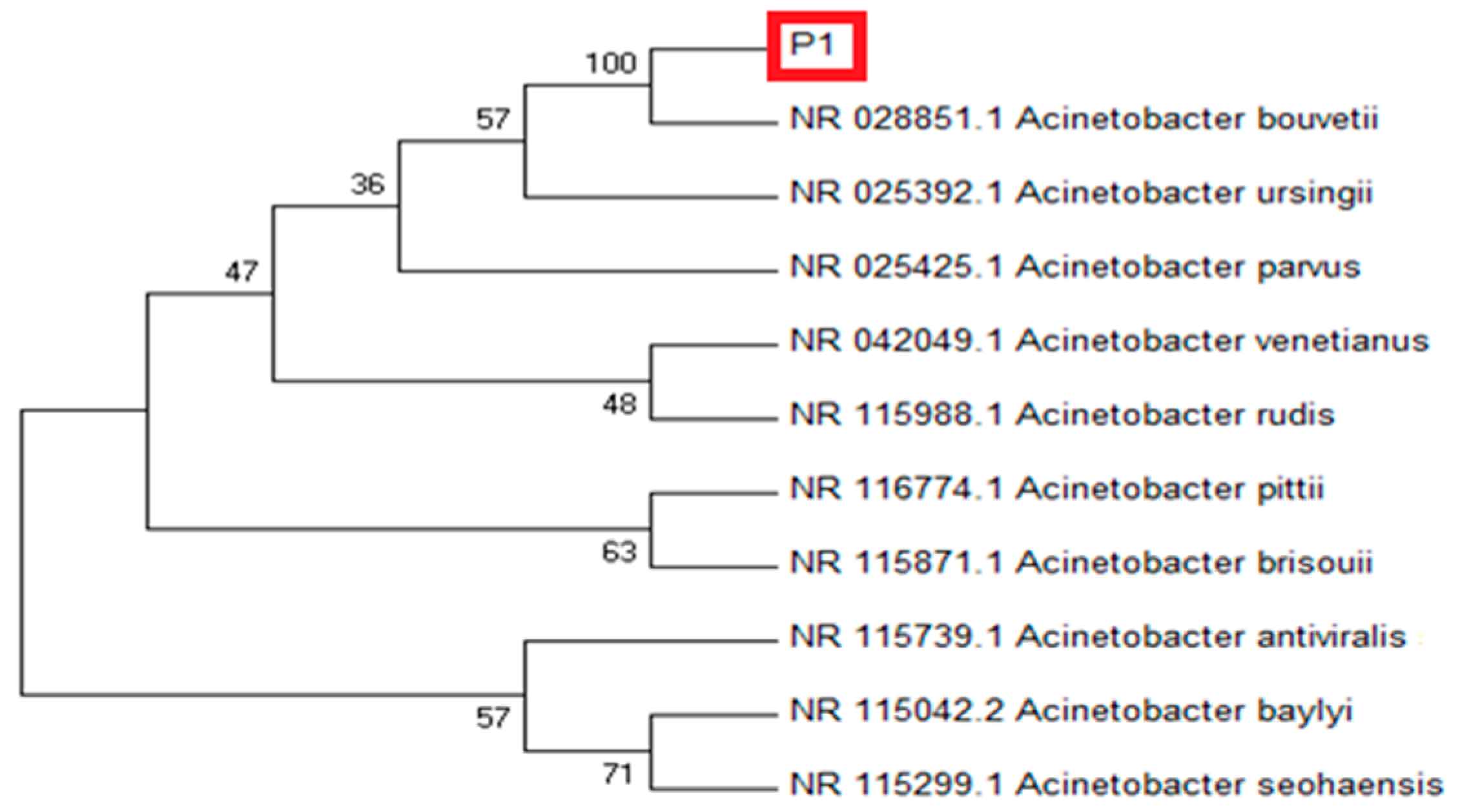
2.3. Molecular Identification of Strain
2.4. Screening the Rhizobacterium
2.4.1. Chromate Tolerance
2.4.2. Phosphate Solubilization Index
2.4.3. Potential of the Isolate to Mitigate Chromate stress in Sunflower
- Treatment 1 = 0 µg mL−1 of Cr+6
- Treatment 2 = 100 µg mL−1 of Cr+6
- Treatment 3 = 300 µg mL−1 of Cr+6
- Treatment 4 = Bacterial inoculum.
- Treatment 5 = Bacterial inoculum + 100 µg mL−1 of Cr+6
- Treatment 6 = Bacterial inoculum + 300 µg mL−1 of Cr+6.
2.5. Plant Growth Related Metabolites in Bacterial Culture
2.5.1. Determination of Phytohormones
2.5.2. Flavonoids, Phenol, and Proline Determination
2.6. Microbial Interaction with Host; a Strategy to Cop Excess Chromate
2.7. Determination of Plant’s Metabolites
2.7.1. Estimation of Indole Acetic Acid
2.7.2. Estimation of Total Flavonoids, Total Phenols, and Proline
2.7.3. Malondialdehyde (MDA) Determination
2.8. Estimation of Electrolyte Leakage
2.9. Lignin Concentration in the Root
2.10. Visualization of ROS and Their Accumulation in Leaves
2.11. Antioxidant System of Sunflower
2.11.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.11.2. Catalase and Ascorbate Peroxidases Activity Determination
2.11.3. Peroxidase and Superoxide Dismutase Activity
2.11.4. Estimation of Reduced Glutathione
2.12. Estimation of Heavy Metals
2.12.1. Colorimetric Determination as Preliminary Test
2.12.2. BCR Sequential Extraction Method
Stage 1: Exchangeable or Acid-Soluble
Stage 2: Cr+6 Extraction
Stage 3: Cr+3 Extraction
2.12.3. Atomic Absorption Spectroscopy (AAS)
2.13. Bioconcentration Factor
2.14. P1 Colonization with Host Roots
2.15. Data Analysis
3. Results
3.1. P1 Molecular Identification
3.2. Characterization of the Selected Strains
3.2.1. Impact of Chromate Rhizobacterial Growth
3.2.2. Production of Bioactive Compounds
3.2.3. Reduction of Cr+6 to Cr+3
3.3. Net Assimilation Rate (NAR) and Relative Growth Rate (RGR)
3.4. Effects of P1 and Chromate Stress on Metabolites of Host Plants
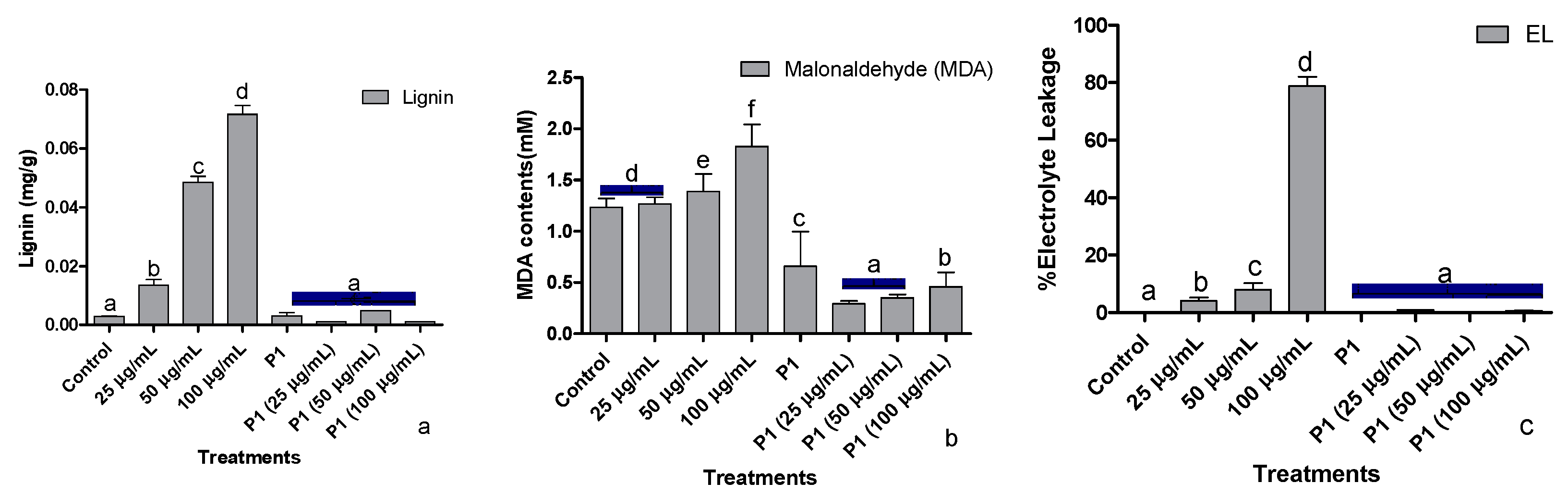
3.5. Root lignification, Electrolyte Leakage, and Malonaldehyde Concentration
3.6. Antioxidant
3.7. 3,3′-Diaminobenzidine (DAB) Stain Assay
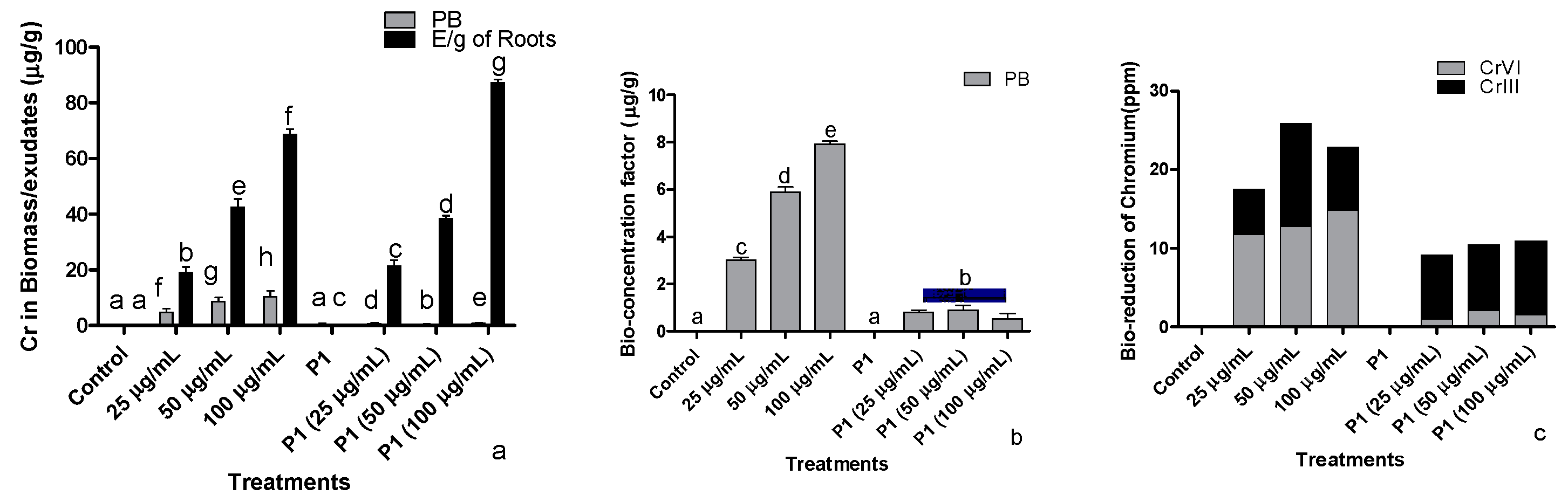
3.8. Determination of Uptake and Accumulation of Cr+6 by Host Plants
3.8.1. By Colorimetric Method
3.8.2. Bioconcentration of Cr+6 in Host
3.8.3. Determination of Cr Species by BCR Extraction
3.8.4. Assessing Root Colonization Potential of P1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [Green Version]
- Topalidis, V.; Harris, A.; Hardaway, C.J.; Benipal, G.; Douvris, C. Investigation of selected metals in soil samples exposed to agricultural and automobile activities in Macedonia, Greece using inductively coupled plasma-optical emission spectrometry. Microchem. J. 2017, 130, 213–220. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493. [Google Scholar] [CrossRef]
- Chang, L.W.; Magos, L.; Suzuki, T. Toxicology of Metals; Taylor & Francis: Boca Raton, FL, USA, 1996. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: New York, NY, USA, 2012; pp. 133–164. [Google Scholar]
- Bayramoğlu, G.; Çelik, G.; Yalçın, E.; Yılmaz, M.; Arıca, M.Y. Modification of surface properties of Lentinus sajor-caju mycelia by physical and chemical methods: Evaluation of their Cr6+ removal efficiencies from aqueous medium. J. Hazard. Mater. 2005, 119, 219–229. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M.J.T. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Mertz, W. Chromium in human nutrition: A review. J. Nutr. 1993, 123, 626–633. [Google Scholar] [CrossRef]
- Vincent, J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef] [Green Version]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.; Tchobanoglous, G. MWH′s Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O.J.W. Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in eastern cape province, south africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P.J.E.S.; Research, P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef]
- Santana Martinez, J.C. The Impact of Reclamation and Vegetation Removal on Compositional and Functional Attributes of Soil Microbial Communities in the Athabasca Oil Sands Region. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2021. [Google Scholar]
- Reyed, R.M. Biotherapeutic Approaches: Bioremediation of Industrial Heavy Metals from Ecosphere. In Rhizobiont in Bioremediation of Hazardous Waste; Springer: New York, NY, USA, 2021; pp. 565–592. [Google Scholar]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [Green Version]
- Tekere, M. Biological Strategies for Heavy Metal Remediation. In Methods for Bioremediation of Water and Wastewater Pollution; Springer: New York, NY, USA, 2020; pp. 393–413. [Google Scholar]
- Spain, O.; Plöhn, M.; Funk, C. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef]
- Gupta, P.; Rani, R.; Chandra, A.; Varjani, S.J.; Kumar, V. Effectiveness of plant growth-promoting Rhizobacteria in phytoremediation of chromium stressed soils. In Waste Bioremediation; Springer: New York, NY, USA, 2018; pp. 301–312. [Google Scholar]
- Mitra, S.; Pramanik, K.; Ghosh, P.K.; Soren, T.; Sarkar, A.; Dey, R.S.; Pandey, S.; Maiti, T.K. Characterization of Cd-resistant Klebsiella michiganensis MCC3089 and its potential for rice seedling growth promotion under Cd stress. Microbiol. Res. 2018, 210, 12–25. [Google Scholar] [CrossRef]
- Ke, T.; Guo, G.; Liu, J.; Zhang, C.; Tao, Y.; Wang, P.; Xu, Y.; Chen, L. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ. Pollut. 2021, 271, 116314. [Google Scholar] [CrossRef]
- Afsal, F.; Majumdar, A.; Kumar, J.S.; Bose, S. Microbial Inoculation to Alleviate the Metal Toxicity in Crop Plants and Subsequent Growth Promotion. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Springer: New York, NY, USA, 2020; pp. 451–479. [Google Scholar]
- Pandey, N.; Chandrakar, V.; Keshavkant, S. Mitigating arsenic toxicity in plants: Role of microbiota. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Springer: New York, NY, USA, 2018; pp. 191–218. [Google Scholar]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Sarma, H.; Prasad, M.N.V. Metabolic engineering of rhizobacteria associated with plants for remediation of toxic metals and metalloids. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Elsevier: New York, NY, USA, 2019; pp. 299–318. [Google Scholar]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 1–14. [Google Scholar] [CrossRef]
- Singh, B.P. Screening and characterization of plant growth promoting rhizobacteria (PGPR): An overview. Bull. Environ. Sci. Res. 2015, 4, 1–2. [Google Scholar]
- Qadir, M.; Hussain, A.; Shah, M.; Lee, I.J.; Iqbal, A.; Irshad, M.; Ismail; Sayyed, A.; Husna; Ahmad, A.; et al. Comparative assessment of chromate bioremediation potential of Pantoea conspicua and Aspergillus niger. J. Hazard. Mater. 2022, 424, 127314. [Google Scholar] [CrossRef]
- Oves, M.; Khan, M.S.; Zaidi, A. Chromium reducing and plant growth promoting novel strain Pseudomonas aeruginosa OSG41 enhance chickpea growth in chromium amended soils. Eur. J. Soil Biol. 2013, 56, 72–83. [Google Scholar] [CrossRef]
- Danish, S.; Kiran, S.; Fahad, S.; Ahmad, N.; Ali, M.A.; Tahir, F.A.; Rasheed, M.K.; Shahzad, K.; Li, X.; Wang, D.; et al. Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2019, 185, 109706. [Google Scholar] [CrossRef]
- Oshundiya, F.O.; Olowe, V.; Sowemimo, F.; Odedina, J.N. Seed yield and quality of sunflower (Helianthus annuus L.) as influenced by staggered sowing and organic fertilizer application in the humid tropics. Helia 2014, 37, 237–255. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Murad, W. Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef]
- Rokhbakhsh-Zamin, F.; Sachdev, D.; Kazemi-Pour, N.; Engineer, A.; Pardesi, K.R.; Zinjarde, S.; Dhakephalkar, P.K.; Chopade, B.A. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. Microbiol. Biotechnol. 2011, 21, 556–566. [Google Scholar] [CrossRef]
- Iman, M. Effect of phosphate solubilizing fungi on growth and nutrient uptake of soyabean (Glycine max L.) plants. J. Appl. Sci. Res. 2008, 4, 592–598. [Google Scholar]
- Hussain, A.; Hasnain, S. Interactions of bacterial cytokinins and IAA in the rhizosphere may alter phytostimulatory efficiency of rhizobacteria. World J. Microbiol. Biotechnol. 2011, 27, 2645. [Google Scholar] [CrossRef]
- Coombe, B.; Cohen, D.; Paleg, L.G. Barley endosperm bioassay for gibberellins. I. Parameters of the response system. Plant Physiol. 1967, 42, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Warrier, R.; Paul, M.; Vineetha, M. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 2013, 3, 90–97. [Google Scholar]
- El Far, M.; Taie, H.A. Antioxidant activities, total anthocyanins, phenolics and flavonoids contents of some sweetpotato genotypes under stress of different concentrations of sucrose and sorbitol. Aust. J. Basic Appl. Sci. 2009, 3, 3609–3616. [Google Scholar]
- Prabhavathi, R.; Prasad, M.; Jayaramu, M. Studies on qualitative and quantitative phytochemical analysis of Cissus quadrangularis. Adv. Appl. Sci. Res. 2016, 7, 11–17. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, P.; Latha, P.; Reddy, P.V. Chapter 4—Photosynthetic rates. In Phenotyping Crop Plants for Physiological and Biochemical Traits; Sudhakar, P., Latha, P., Reddy, P.V., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 33–39. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Schmedes, A.; Hølmer, G. A new thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J. Am. Oil Chem. Soc. 1989, 66, 813–817. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Moreira-Vilar, F.C.; de Cássia Siqueira-Soares, R.; Finger-Teixeira, A.; de Oliveira, D.M.; Ferro, A.P.; da Rocha, G.J.; Maria de Lourdes, L.F.; dos Santos, W.D.; Ferrarese-Filho, O. The acetyl bromide method is faster, simpler and presents best recovery of lignin in different herbaceous tissues than Klason and thioglycolic acid methods. PLoS ONE 2014, 9, e110000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Ahmad, I.; Abbasi, B.H. Free radical scavenging (DPPH) potential in nine Mentha species. J. Toxicol. Ind. Health 2012, 28, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Lee, I.-J. Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acid changes in soybean pods and seeds. Acta Physiol. Plant. 2013, 35, 263–269. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Janknegt, P.J.; Rijstenbil, J.W.; Van de Poll, W.H.; Gechev, T.S.; Buma, A.G.J. A comparison of quantitative and qualitative superoxide dismutase assays for application to low temperature microalgae. J. Photochem. Photobiol. B Biol. 2007, 87, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Mavis, R.D.; Stellwagen, E. Purification and subunit structure of glutathione reductase from bakers’ yeast. J. Biol. Chem. 1968, 243, 809–814. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
- Zahoor, M.; Irshad, M.; Rahman, H.; Qasim, M.; Afridi, S.G.; Qadir, M.; Hussain, A. Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol. Environ. Saf. 2017, 142, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kazi, T.; Jamali, M.; Kazi, G.; Arain, M.; Afridi, H.; Siddiqui, A.J.A. Evaluating the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential extraction procedure and a leaching test. Anal. Bioanal. Chem. 2005, 383, 297–304. [Google Scholar] [CrossRef]
- WHO. Chromium in Drinking-Water; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ismaila, A.H.; Qadira, M.; Husnaa, M.I.; Ahmadb, A.; Hamayuna, M. Endophytic Fungi Isolated from Citrullus Colocynthesl. Leaves and Their Potential for Secretion of Indole Acetic Acid and Gibberellin. J. Appl. Environ. Biol. Sci. 2018, 8, 80–84. [Google Scholar]
- Lugtenberg, B.J.; Malfanova, N.; Kamilova, F.; Berg, G. Plant growth promotion by microbes. Mol. Microb. Ecol. Rhizosphere 2013, 2, 561–573. [Google Scholar]
- Pál, M.; Szalai, G.; Kovács, V.; Gondor, O.; Janda, T. Salicylic acid-mediated abiotic stress tolerance. In Salicylic Acid; Springer: New York, NY, USA, 2013; pp. 183–247. [Google Scholar]
- Luo, J.; Xia, W.; Cao, P.; Xiao, Z.a.; Zhang, Y.; Liu, M.; Zhan, C.; Wang, N. Integrated transcriptome analysis reveals plant hormones jasmonic acid and salicylic acid coordinate growth and defense responses upon fungal infection in poplar. Biomolecules 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Li, W.; Lv, J.; Jia, Y.; Wang, M.; Xia, G. Ectopic expression of a wheat MYB transcription factor gene, TaMYB73, improves salinity stress tolerance in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 1511–1522. [Google Scholar]
- Hamayun, M.; Khan, N.; Khan, M.N.; Qadir, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Rehman, K.U.; Lee, I.-J. Antimicrobial and plant growth-promoting activities of bacterial endophytes isolated from Calotropis procera (Ait.) WT Aiton. Biocell 2021, 45, 363–369. [Google Scholar] [CrossRef]
- Rolli, E.; Vergani, L.; Ghitti, E.; Patania, G.; Mapelli, F.; Borin, S. “Cry-for-help” in contaminated soil: A dialogue among plants and soil microbiome to survive in hostile conditions. Environ. Microbiol. 2021, 23, 5690–5703. [Google Scholar]
- Hassan, S.; Mathesius, U. The role of flavonoids in root–rhizosphere signalling: Opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Mishra, J.; Fatima, T.; Arora, N.K. Role of secondary metabolites from plant growth-promoting rhizobacteria in combating salinity stress. In Plant Microbiome: Stress Response; Springer: New York, NY, USA, 2018; pp. 127–163. [Google Scholar]
- Bahadur, A.; Ahmad, R.; Afzal, A.; Feng, H.; Suthar, V.; Batool, A.; Khan, A.; Mahmood-ul-Hassan, M.J.C. The influences of Cr-tolerant rhizobacteria in phytoremediation and attenuation of Cr (VI) stress in agronomic sunflower (Helianthus annuus L.). Chemosphere 2017, 179, 112–119. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Iqbal, R.; Manevski, K. Investigating the effect of Azospirillum brasilense and Rhizobium pisi on agronomic traits of wheat (Triticum aestivum L.). Arch. Agron. Soil Sci. 2019, 65, 1554–1564. [Google Scholar] [CrossRef]
- Karthik, C.; Elangovan, N.; Kumar, T.S.; Govindharaju, S.; Barathi, S.; Oves, M.; Arulselvi, P.I. Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol. Res. 2017, 204, 65–71. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: New York, NY, USA, 2018; pp. 253–268. [Google Scholar]
- Mukhopadyay, M.; Bantawa, P.; Das, A.; Sarkar, B.; Bera, B.; Ghosh, P.; Mondal, T.K. Changes of growth, photosynthesis and alteration of leaf antioxidative defence system of tea [Camellia sinensis (L.) O. Kuntze] seedlings under aluminum stress. Biometals 2012, 25, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.S.; Schat, H.; Vooijs, R. In Vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry 1998, 49, 1531–1535. [Google Scholar] [CrossRef]
- Finger-Teixeira, A.; de Lourdes Lucio Ferrarese, M.; Soares, A.R.; da Silva, D.; Ferrarese-Filho, O. Cadmium-induced lignification restricts soybean root growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.-F. Target or barrier? The cell wall of early-and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lux, A. Does diversity in root structure affect the diversity in cadmium uptake by plants? Opinion paper. Agrochimica 2010, 54, 342–352. [Google Scholar]
- Ashraf, M.; Ozturk, M.; Ahmad, M.S.A. Plant Adaptation and Phytoremediation; Springer: New York, NY, USA, 2010. [Google Scholar]
- Jha, Y.; Subramanian, R. Effect of root-associated bacteria on soluble sugar metabolism in plant under environmental stress. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–240. [Google Scholar]
- Hussain, A.; Shah, M.; Hamayun, M.; Qadir, M.; Iqbal, A. Heavy metal tolerant endophytic fungiAspergillus welwitschiaeimproves growth, ceasing metal uptake and strengthening antioxidant system in Glycine max L. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Ganesh, M.; Sricharan, S.; Rajesh, N. Extractive separation and determination of chromium in tannery effluents and electroplating waste water using tribenzylamine as the extractant. J. Hazard. Mater. 2009, 165, 886–892. [Google Scholar] [CrossRef]
- Kazi, T.G.; Afridi, H.I.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Kandhro, G.A. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol. Trace Elem. Res. 2008, 122, 1–18. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Z.-T.; Liu, Z.-W. Chemically modified chicken feather as sorbent for removing toxic chromium (VI) ions. Ind. Eng. Chem. Res. 2009, 48, 6882–6889. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Tuzen, M.; Soylak, M. Speciation and separation of Cr (VI) and Cr (III) using coprecipitation with Ni2+/2-Nitroso-1-naphthol-4-sulfonic acid and determination by FAAS in water and food samples. Food Chem. Toxicol. 2009, 47, 2601–2605. [Google Scholar] [CrossRef]
- Paiva, L.B.; de Oliveira, J.G.; Azevedo, R.A.; Ribeiro, D.R.; da Silva, M.G.; Vitória, A.P. Ecophysiological responses of water hyacinth exposed to Cr3+ and Cr6+. Environ. Exp. Bot. 2009, 65, 403–409. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Husna, H.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Murad, W.; Irshad, M.; Qadir, M.; Kim, H.-Y. Pseudocitrobacter Anthropi Reduces Heavy Metal Uptake and Improves Phytohormones and Antioxidant System in Glycine Max L. World J. Microbiol. Biotechnol. 2021, 37, 195. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, S.S.; Duarte, A.C.; Pereira, E. Oxidative stress biomarkers and antioxidant defense in plants exposed to metallic nanoparticles. In Nanomaterials and Plant Potential; Springer: New York, NY, USA, 2019; pp. 427–439. [Google Scholar]
- Panda, S.; Choudhury, S. Chromium stress in plants. Braz. J. Plant Physiol. 2005, 17, 95–102. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Liu, S.; Hao, H.; Lu, X.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Wang, R. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Rahman, I.U.; Iqbal, A.; Hamayun, M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE 2018, 13, e0208150. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Irshad, M.; Ahmad, A.; Lodhi, M.A.; Lee, I.-J. Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response. Antioxidants 2021, 10, 1868. https://doi.org/10.3390/antiox10121868
Qadir M, Hussain A, Hamayun M, Shah M, Iqbal A, Irshad M, Ahmad A, Lodhi MA, Lee I-J. Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response. Antioxidants. 2021; 10(12):1868. https://doi.org/10.3390/antiox10121868
Chicago/Turabian StyleQadir, Muhammad, Anwar Hussain, Muhammad Hamayun, Mohib Shah, Amjad Iqbal, Muhammad Irshad, Ayaz Ahmad, Muhammad Arif Lodhi, and In-Jung Lee. 2021. "Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response" Antioxidants 10, no. 12: 1868. https://doi.org/10.3390/antiox10121868
APA StyleQadir, M., Hussain, A., Hamayun, M., Shah, M., Iqbal, A., Irshad, M., Ahmad, A., Lodhi, M. A., & Lee, I.-J. (2021). Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response. Antioxidants, 10(12), 1868. https://doi.org/10.3390/antiox10121868







