Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Supplements
2.2. Animals and Study Design
2.3. Unilateral 6-OHDA Lesions
2.4. Evaluation of Neuroprotective Effects
2.4.1. Immunohistochemical Staining and Image Analysis
2.4.2. Behavioral Tests
2.5. Evaluation of Oxidative Stress and Inflammation Markers in Serum
2.5.1. ROS Assay
2.5.2. Quantification of Antioxidant Enzyme Activity
2.5.3. Quantification of Inflammatory Cytokines
2.6. Evaluation of Microbiota Composition and Microbial Metabolites
2.6.1. Bacterial Genomic DNA Isolation
2.6.2. 16S rRNA Amplicon Sequencing and Data Analysis
2.6.3. High-Performance Liquid Chromatography (HPLC)
2.7. Statistical Analyses
3. Results
3.1. Long-Term Probiotic and Prebiotic Supplementation Ameliorated General Motor Symptoms and Confered Neuroprotective Effects in Rats with 6-OHDA-Induced PD
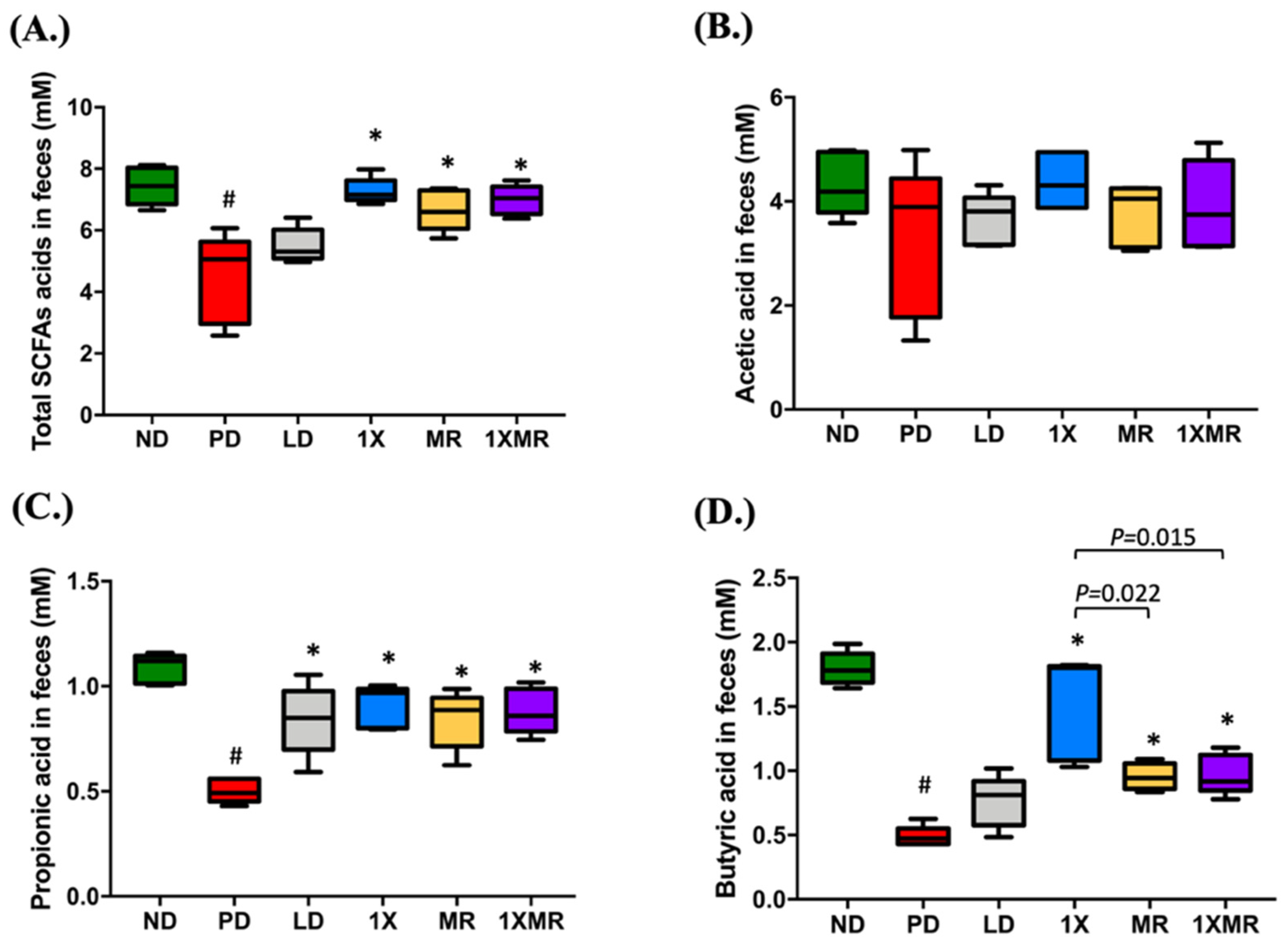
3.2. Probiotic and Prebiotic Supplementation Reduced Inflammation, Promoted Antioxidant Activity, and Increased SCFA Production
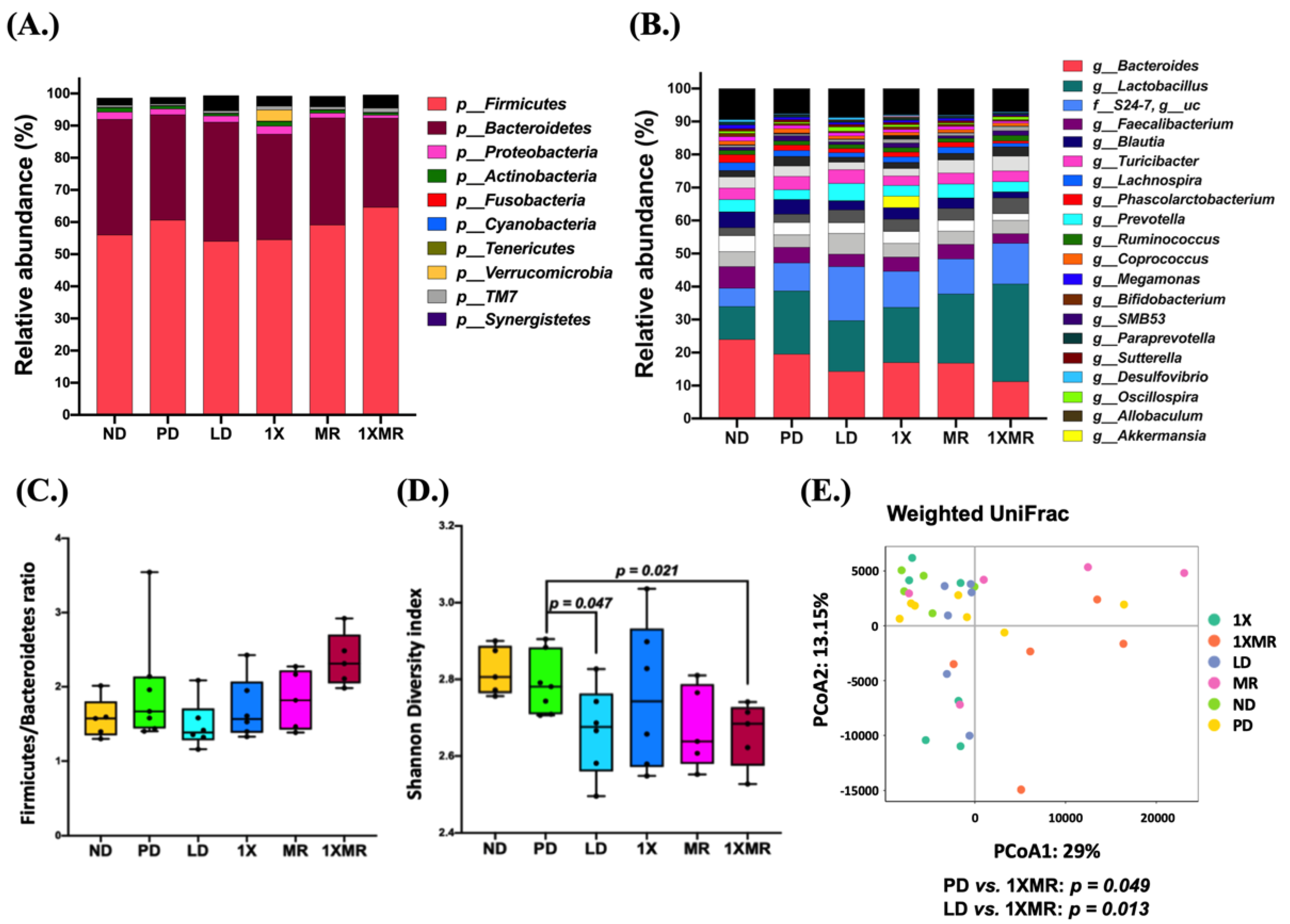
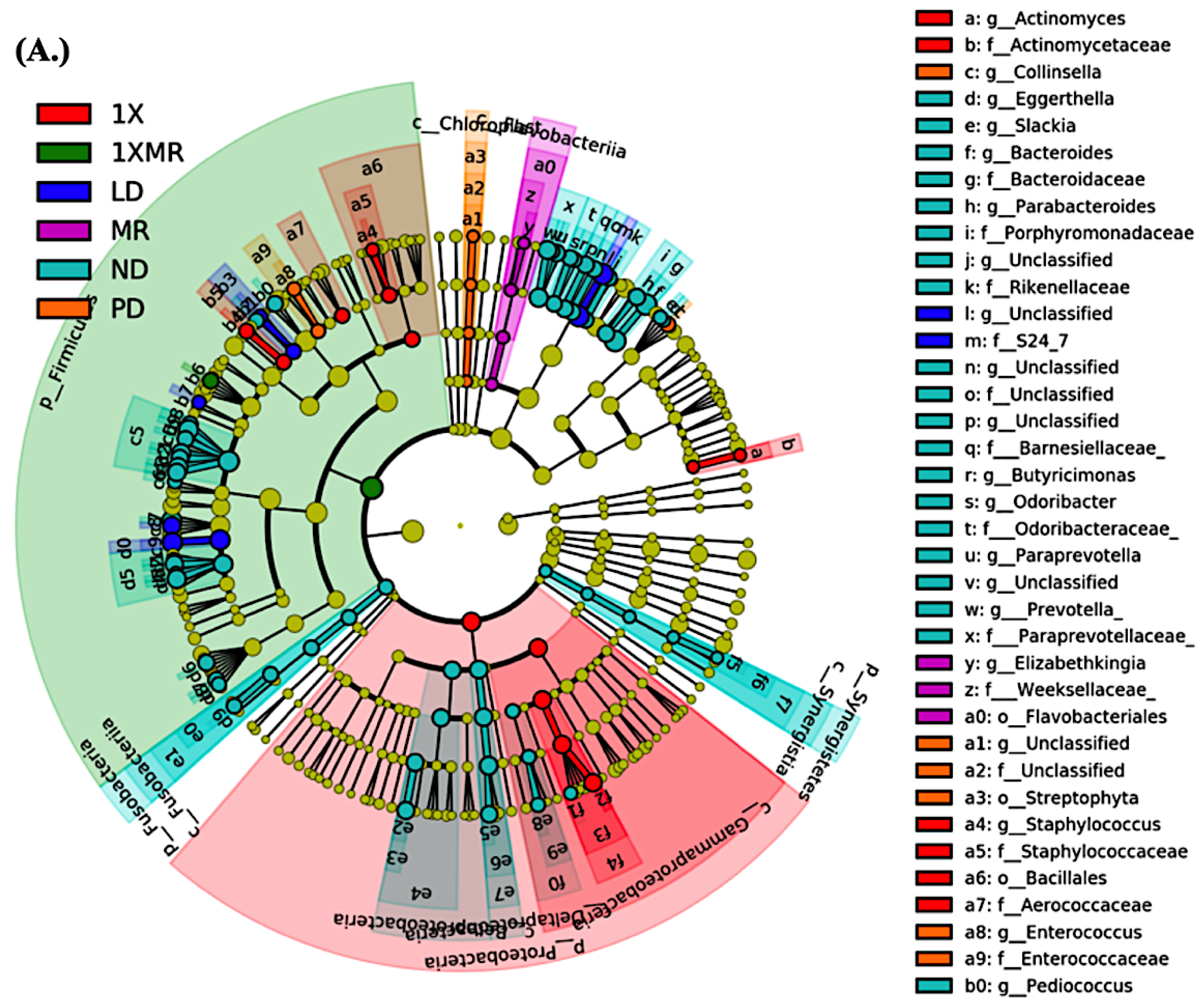
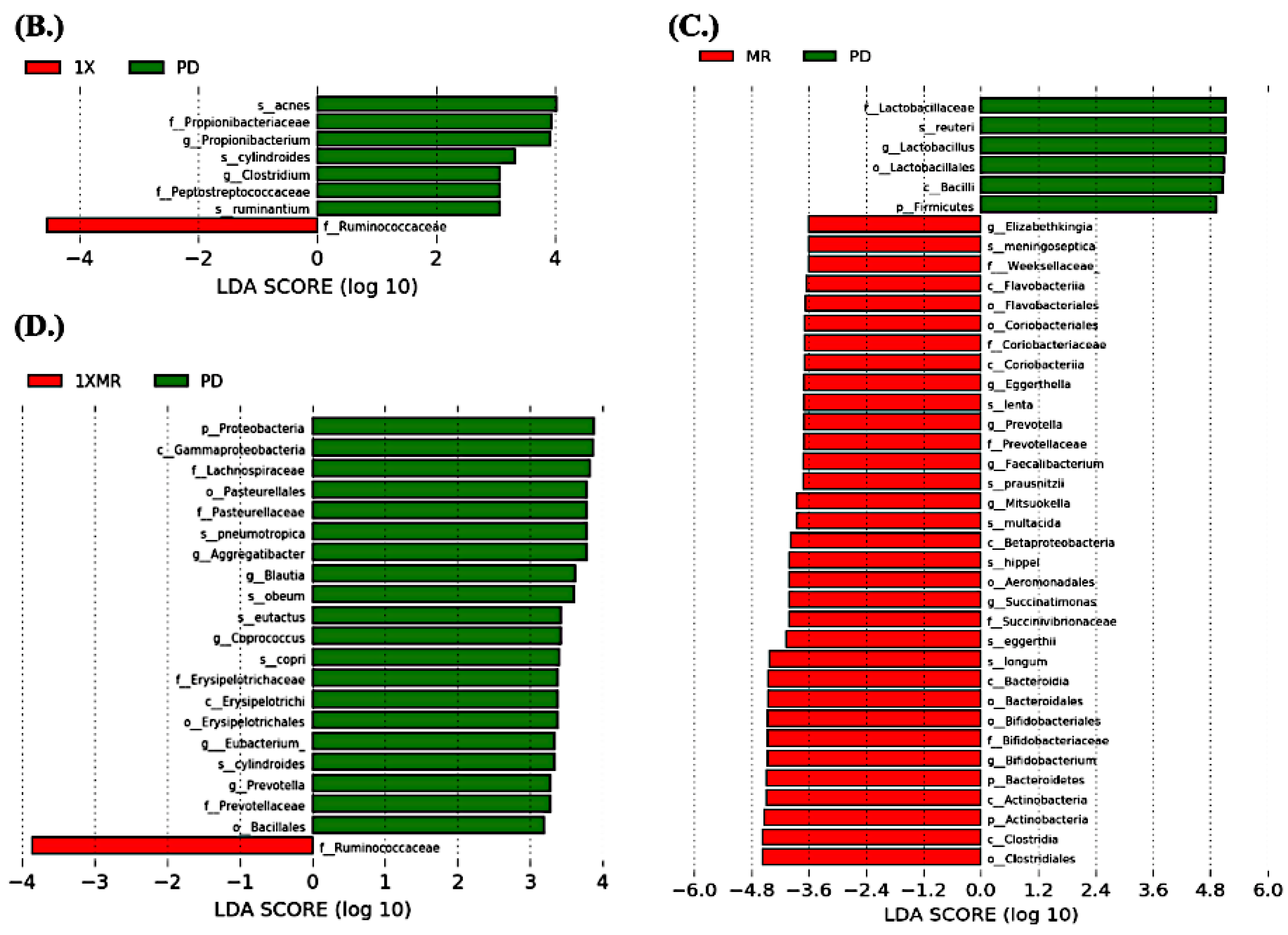
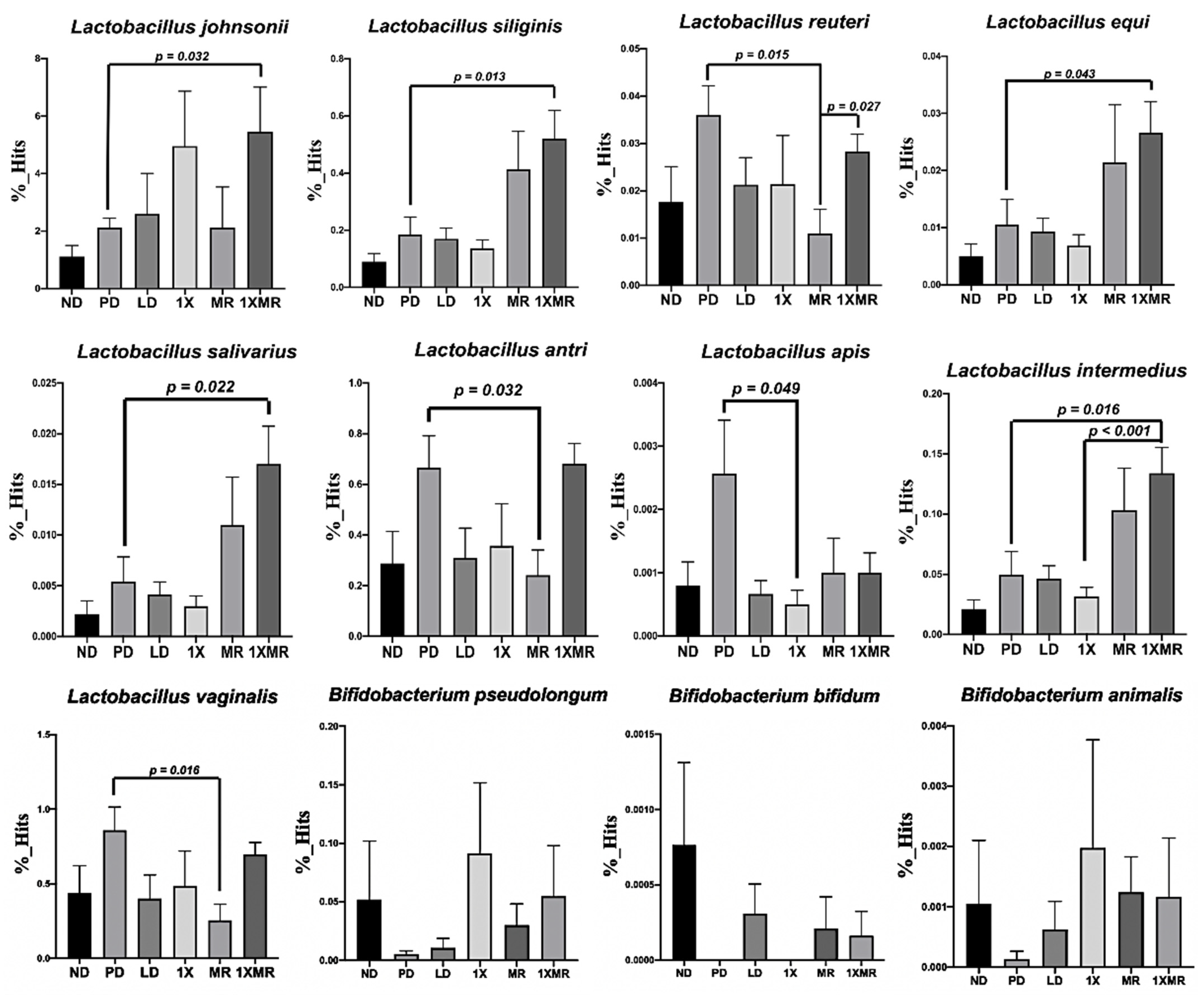
3.3. Long-Term Probiotic and Prebiotic Supplementation Alter 6-OHDA-Induced Gut Dysbiosis by Remodeling the Composition of the Fecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinson’s Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Martinez-Martin, P. The importance of non-motor disturbances to quality of life in Parkinson’s disease. J. Neurol. Sci. 2011, 310, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Del Tredici, K.; Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013, 9, 13–24. [Google Scholar] [CrossRef]
- Hemmerle, A.M.; Herman, J.P.; Seroogy, K.B. Stress, depression and Parkinson’s disease. Exp. Neurol. 2012, 233, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebouvier, T.; Neunlist, M.; des Varannes, S.B.; Coron, E.; Drouard, A.; N’Guyen, J.M.; Chaumette, T.; Tasselli, M.; Paillusson, S.; Flamand, M.; et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS ONE 2010, 5, e12728. [Google Scholar] [CrossRef] [Green Version]
- Schaffernicht, G.; Shang, Q.; Stievenard, A.; Btzel, K.; Dening, Y.; Kempe, R.; Toussaint, M.; Gndel, D.; Kranz, M.; Reichmann, H.; et al. Pathophysiological changes in the enteric nervous system of rotenone-exposed mice as early radiological markers for Parkinson’s disease. Front. Neurol. 2021, 12, 642604. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.D. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjrkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Caso, J.; Balanz-Martnez, V.; Palomo, T.; Garca-Bueno, B. The microbiota and gut-brain axis: Contributions to the immunopathogenesis of schizophrenia. Curr. Pharm. Des. 2016, 22, 6122–6133. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Neufeld, K.-A.M. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Brmann, J.; Fabender, K.; Schwiertz, A.; Schfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Sun, M.F.; Shen, Y.Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Solans, M.R.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef]
- Ochoa-Repraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Suzuki, T.; Kaji, R.; Serata, M.; Nagata, T.; Ando, M.; Iizuka, R.; Tsujibe, S.; Murakami, J.; Kiyoshima-Shibata, J.; et al. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacol. Immunotoxicol. 2012, 34, 423–433. [Google Scholar] [CrossRef]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Frida, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Westrm, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Ling, Z.; Yu, X.; Chen, W.; Li, H.; Jin, J.; Pang, M.; Zhang, H.; Yu, J.; et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016, 1642, 180–188. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Doron, S.; Gorbach, S.L. Probiotics: Their role in the treatment and prevention of disease. Expert Rev. Anti Infect. Ther. 2006, 4, 261–275. [Google Scholar] [CrossRef]
- Alvarez-Olmos, M.I.; Oberhelman, R.A. Probiotic agents and infectious diseases: A modern perspective on a traditional therapy. Clin. Infect. Dis. 2001, 32, 1567–1576. [Google Scholar] [CrossRef] [Green Version]
- Alander, M.; Korpela, R.; Saxelin, M.; Vilpponen-Salmela, T.; Mattila-Sandholm, T.; Von Wright, A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett. Appl. Microbiol. 1997, 24, 361–364. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gbel, R.; Michaelsen, K.F.; Al-Soud, W.A.; Srensen, S.J.; Hansen, L.H.; Jakobsen, M. Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp. lactis Bi-07. FEMS Microbiol. Ecol. 2011, 75, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Rutten, N.B.M.M.; Gorissen, D.M.W.; Eck, A.; Niers, L.E.M.; Vlieger, A.M.; Besseling-van der Vaart, I.; Budding, A.E.; Savelkoul, P.H.M.; van der Ent, C.K.; Rijkers, G.T. Long term development of gut microbiota composition in atopic children: Impact of probiotics. PLoS ONE 2015, 10, e0137681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, M.F.; Laursen, R.P.; Larnkjr, A.; Michaelsen, K.F.; Bahl, M.I.; Licht, T.R. Administration of two probiotic strains during early childhood does not affect the endogenous gut microbiota composition despite probiotic proliferation. BMC Microbiol. 2017, 17, 175. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [Green Version]
- Nurrahma, B.A.; Tsao, S.-P.; Wu, C.-H.; Yeh, T.-H.; Hsieh, P.-S.; Panunggal, B.; Huang, H.-Y. Probiotic supplementation facilitates recovery of 6-OHDA-induced motor deficit via improving mitochondrial function and energy metabolism. Front. Aging Neurosci. 2021, 13, 668775. [Google Scholar] [CrossRef]
- Sgroi, S.; Kaelin-Lang, A.; Capper-Loup, C. Spontaneous locomotor activity and L-DOPA-induced dyskinesia are not linked in 6-OHDA parkinsonian rats. Front. Behav. Neurosci. 2014, 8, 331. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 1, 41. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high- throughput community sequencing data. Nat Methods. 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Hwang, K.-T.; Chung, M.-Y.; Cho, D.-H.; Park, C.-S. Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): Role for a metal ion chelating effect. J. Food Sci. 2005, 70, m388–m391. [Google Scholar] [CrossRef]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Castelli, V.; d’Angelo, M.; Lombardi, F.; Alfonsetti, M.; Antonosante, A.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Cifone, M.G.; Giordano, A.; et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging 2020, 12, 4641–4659. [Google Scholar] [CrossRef]
- Lutgendorff, F.; Trulsson, L.M.; van Minnen, L.P.; Rijkers, G.T.; Timmerman, H.M.; Franzén, L.E.; Gooszen, H.G.; Akkermans, L.M.A.; Söderholm, J.D.; Sandström, P.A. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1111–G1121. [Google Scholar] [CrossRef] [Green Version]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef] [Green Version]
- Ahire, J.J.; Mokashe, N.U.; Patil, H.J.; Chaudhari, B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2013, 50, 26–34. [Google Scholar] [CrossRef]
- de Farias, C.C.; Maes, M.; Bonifácio, K.L.; Bortolasci, C.C.; de Souza Nogueira, A.; Brinholi, F.F.; Matsumoto, A.K.; do Nascimento, M.A.; de Melo, L.B.; Nixdorf, S.L.; et al. Highly specific changes in antioxidant levels and lipid peroxidation in Parkinson’s disease and its progression: Disease and staging biomarkers and new drug targets. Neurosci. Lett. 2016, 617, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Romo-Araiza, A.; Gutirrez-Salmen, G.; Galvn, E.J.; Hernndez-Frausto, M.; Herrera-Lpez, G.; Romo-Parra, H.; Garca-Contreras, V.; Fernndez-Presas, A.M.; Jasso-Chvez, R.; Borlongan, C.V.; et al. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front. Aging Neurosci. 2018, 10, 416. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and butyrate improve β-cell metabolism and mitochondrial respiration under oxidative stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarczyk, M.; Miller, M.; Freund, G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.M.; Neish, A.S. Redox signaling mediated by the gut microbiota. Free Radic. Biol. Med. 2017, 105, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, A.; Malik, R.K.; Chauhan, P. Functional and safety aspects of enterococci in dairy foods. Indian J. Microbiol. 2008, 48, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, A.; Kaur, G.; Gupta, H.; Vij, S.; Malik, R.K. Interspecies diversity, safety and probiotic potential of bacteriocinogenic Enterococcus faecium isolated from dairy food and human faeces. World J. Microbiol. Biotechnol. 2011, 27, 591–602. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef] [Green Version]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gøbel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin. Nutr. 2013, 32, 935–940. [Google Scholar] [CrossRef]
- Rajkumar, H.; Kumar, M.; Das, N.; Kumar, S.N.; Challa, H.R.; Nagpal, R. Effect of probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: A randomized controlled single-blind pilot study. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 289–298. [Google Scholar] [CrossRef]
- Laar, T.V.; Boertien, J.M.; Herranz, A.H. Faecal transplantation, pro- and prebiotics in Parkinson’s disease; hope or hype? J. Parkinsons Dis. 2019, 9, S371–S379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Gu, R.; Li, W.; Zhou, W.; Cong, Z.; Xue, J.; Liu, Y.; Wei, Q.; Zhou, Y. Lactobacillus rhamnosus GG attenuates tenofovir disoproxil fumarate-induced bone loss in male mice via gut-microbiota-dependent anti-inflammation. Ther. Adv. Chronic Dis. 2019, 10, 2040622319860653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyman-Lindn, L.; Kotowska, D.; Sand, E.; Bjursell, M.; Plaza, M.; Turner, C.; Holm, C.; Frida, F.; Berger, K. Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr. Res. 2016, 60, 29993. [Google Scholar] [CrossRef] [Green Version]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- van den Munckhof, I.C.L.; Kurilshikov, A.; Horst, R.T.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; de Graaf, J.; et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 2018, 19, 1719–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sabatino, A.; Cazzola, P.; Ciccocioppo, R.; Morera, R.; Biancheri, P.; Rovedatti, L.; Cantoro, L.; Vanoli, A.; Tinozzi, F.P.; Tinozzi, S.; et al. Efficacy of butyrate in the treatment of mild to moderate Crohn’s disease. Dig. Liver Dis. Suppl. 2007, 1, 31–35. [Google Scholar] [CrossRef]
- Furet, J.; Kong, L.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediat. Inflamm. 2014, 2014, 348959. [Google Scholar] [CrossRef] [Green Version]
- Iljazovic, A.; Roy, U.; Glvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Precup, G.; Vodnar, D.-C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabrok, H.B.; Klopfleisch, R.; Ghanem, K.Z.; Clavel, T.; Blaut, M.; Loh, G. Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis 2012, 33, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Bilen, M.; Fonkou, M.D.M.; Tomei, E.; Armstrong, N.; Bittar, F.; Lagier, J.C.; Daoud, Z.; Fournier, P.E.; Raoult, D.; Cadoret, F. Eggerthella timonensis sp. nov, a new species isolated from the stool sample of a pygmy female. MicrobiologyOpen 2018, 7, e00575. [Google Scholar] [CrossRef] [PubMed]
- Landis, G.N.; Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 2005, 126, 365–379. [Google Scholar] [CrossRef]
- Ho, Y.S.; Xiong, Y.; Ma, W.; Spector, A.; Ho, D.S. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J. Biol. Chem. 2004, 279, 32804–32812. [Google Scholar] [CrossRef] [Green Version]
- Chriett, S.; Dbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent action of butyrate over $\beta$-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, C.; Liu, G.; Wang, P.; Shi, M.; Yang, M.; Zhong, Z.; Ding, S.; Li, Y.; Liu, B.; et al. Sodium butyrate protects against oxidative stress in human nucleus pulposus cells via elevating PPARγ-regulated Klotho expression. Int. Immunopharmacol. 2020, 85, 106657. [Google Scholar] [CrossRef]
- Choi, J.G.; Huh, E.; Kim, N.; Kim, D.-H.; Oh, M.S. High-throughput 16S rRNA gene sequencing reveals that 6-hydroxydopamine affects gut microbial environment. PLoS ONE 2019, 14, e0217194. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Halder, C.V.; Faria, A.V.d.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Citronberg, J.S.; Curtis, K.R.; White, E.; Newcomb, P.A.; Newton, K.; Atkinson, C.; Song, X.; Lampe, J.W.; Hullar, M.A.J. Association of gut microbial communities with plasma lipopolysaccharide-binding protein (LBP) in premenopausal women. ISME J. 2018, 12, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, A.S.; Krabbe, K.S.; Krogh-Madsen, R.; Taudorf, S.; Pedersen, B.K.; Møller, K. Human endotoxemia as a model of systemic inflammation. Curr. Med. Chem. 2008, 15, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]


| ND | PD | LD | 1X | MR | 1XMR | |
|---|---|---|---|---|---|---|
| ROS (% normal control) | 100.0 ± 5.8 | 187.5 ± 11.0 a | 172.4 ± 19.0 | 105.0 ± 21.3 b | 113.7 ± 10.3 b | 107.4 ± 12.1 b |
| TNF-α (pg/mL) | 43.9 ± 1.5 | 64.9 ± 1.6 a | 59.9 ± 2.4 ab | 51.2 ± 1.7 ab | 53.7 ± 2.9 ab | 52.0 ± 2.3 ab |
| SOD activity (mU/mL) | 68.7 ± 8.3 | 43.7 ± 1.6 a | 47.0 ± 1.6 ab | 51.6 ± 2.8 ab | 49.8 ± 1.6 ab | 50.6 ± 1.1 ab |
| GPx activity (mU/mL) | 65.3 ± 4.4 | 53.9 ± 2.1 a | 58.5 ± 1.4 b | 60.5 ± 1.1 b | 58.6 ± 1.0 b | 59.3 ± 1.2 b |
| Catalase activity (mU/mL) | 4.8 ± 0.1 | 3.4 ± 0.3 a | 3.6 ± 0.5 a | 4.4 ± 0.6 b | 3.8 ± 0.3 a | 3.8 ± 0.1 ab |
| Progression Parameters | Correlation | Bacterial Taxa |
|---|---|---|
| Time on the rod(s) | + | Synergistes (r = 0.353; p = 0.040) |
| SOD 1 activity (mU/mL) | + | Eggerthella (r = 0.497; p = 0.003) |
| GPx activity (mU/mL) | + | Eggerthella (r = 0.402; p = 0.018) |
| Catalase activity (mU/mL) | + | Bacteroides (r = 0.404; p = 0.017), Anaerostipes (r = 0.473; p = 0.004), Prevotella (r = 0.391; p = 0.022), Sutterella (r = 0.392; p = 0.021), Catenibacterium (r = 0.503; p = 0.002), Megamonas (r = 0.489; p = 0.003), Citrobacter (r = 0.517; p = 0.001), Synergistes (r = 0.450; p = 0.007), Eggerthella (r = 0.372; p = 0.030) |
| − | Lactobacillus (r = −0.372; p = 0.030) |
| Progression Parameters | Correlation | Bacterial Taxa |
|---|---|---|
| Contralateral rotation (rotations/minute) | + | Lactobacillus reuteri (r = 0.343; p = 0.046), Lactobacillus apis (r = 0.380; p = 0.026) |
| Time on the rod(s) | − | Lactobacillus apis (r = −0.343; p = 0.046), Lactobacillus amylolyticus (r = −0.365; p = 0.033), |
| SOD 1 activity (mU/mL) | − | Lactobacillus reuteri (r = −0.383; p = 0.025), Lactobacillus antri (r = −0.393; p = 0.035), Lactobacillus frumenti (r = −0.343; p = 0.046), Lactobacillus camelliae (r = −0.385; p = 0.024), Lactobacillus senmaizukei (r = −0.366; p = 0.032), Lactobacillus panis (r = −0.378; p = 0.027), Lactobacillus amylolyticus (r = −0.421; p = 0.013) |
| GPx activity (mU/mL) | − | Lactobacillus reuteri (r = −0.360; p = 0.036), Lactobacillus apis (r = −0.351; p = 0.041), Lactobacillus camelliae (r = −0.376; p = 0.028) |
| Catalase activity (mU/mL) | + | Bifidobacterium adolescentis (r = 0.411; p = 0.015), Bifidobacterium longum (r = 0.341; p = 0.048), Lactobacillus plantarum (r = 0.483; p = 0.030), Lactobacillus pentosus (r = 0.395; p = 0.020), Lactobacillus japonicus (r = 0.381; p = 0.026) |
| − | Lactobacillus taiwanensis (r = −0.371; p = 0.030), Lactobacillus siliginis (r = −0.387; p = 0.023), Lactobacillus intermedius (r = −0.429; p = 0.011), Lactobacillus salivarius (r = −0.399; p = 0.019), Lactobacillus tucceti (r = −0.400; p = 0.018), Lactobacillus panis (r = −0.362; p = 0.035) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, S.-P.; Nurrahma, B.A.; Kumar, R.; Wu, C.-H.; Yeh, T.-H.; Chiu, C.-C.; Lee, Y.-P.; Liao, Y.-C.; Huang, C.-H.; Yeh, Y.-T.; et al. Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants 2021, 10, 1823. https://doi.org/10.3390/antiox10111823
Tsao S-P, Nurrahma BA, Kumar R, Wu C-H, Yeh T-H, Chiu C-C, Lee Y-P, Liao Y-C, Huang C-H, Yeh Y-T, et al. Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants. 2021; 10(11):1823. https://doi.org/10.3390/antiox10111823
Chicago/Turabian StyleTsao, Shu-Ping, Bira Arumndari Nurrahma, Ravi Kumar, Chieh-Hsi Wu, Tu-Hsueh Yeh, Ching-Chi Chiu, Yen-Peng Lee, Yi-Chi Liao, Cheng-Hsieh Huang, Yao-Tsung Yeh, and et al. 2021. "Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats" Antioxidants 10, no. 11: 1823. https://doi.org/10.3390/antiox10111823
APA StyleTsao, S.-P., Nurrahma, B. A., Kumar, R., Wu, C.-H., Yeh, T.-H., Chiu, C.-C., Lee, Y.-P., Liao, Y.-C., Huang, C.-H., Yeh, Y.-T., & Huang, H.-Y. (2021). Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants, 10(11), 1823. https://doi.org/10.3390/antiox10111823







